Loss of Nitric Oxide Induces Fibrogenic Response in Organotypic 3D Co-Culture of Mammary Epithelia and Fibroblasts—An Indicator for Breast Carcinogenesis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies
2.2. Cell Lines
2.3. Cell Culture
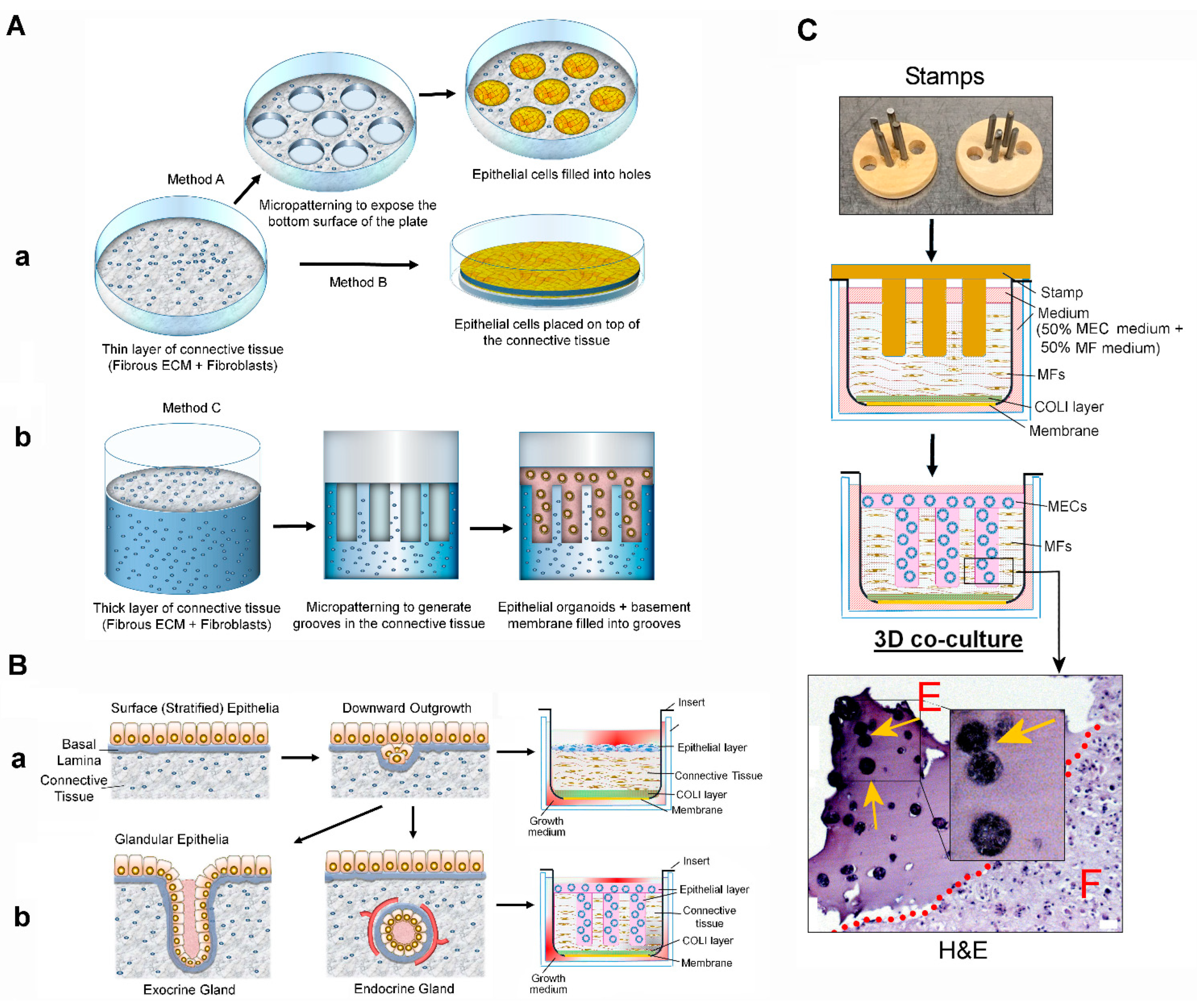
2.4. D Mono-Culture of Mammary Epithelial Cells and Mammary Fibroblasts
2.5. D Organotypic Co-Culture
2.6. Animal Studies
2.7. Immunohistochemistry
2.8. Immunofluorescence Staining and Imaging
2.9. Image Analysis
2.10. Statistics
3. Results
3.1. Novel Organotypic 3D Co-Culture of the Mammary Gland
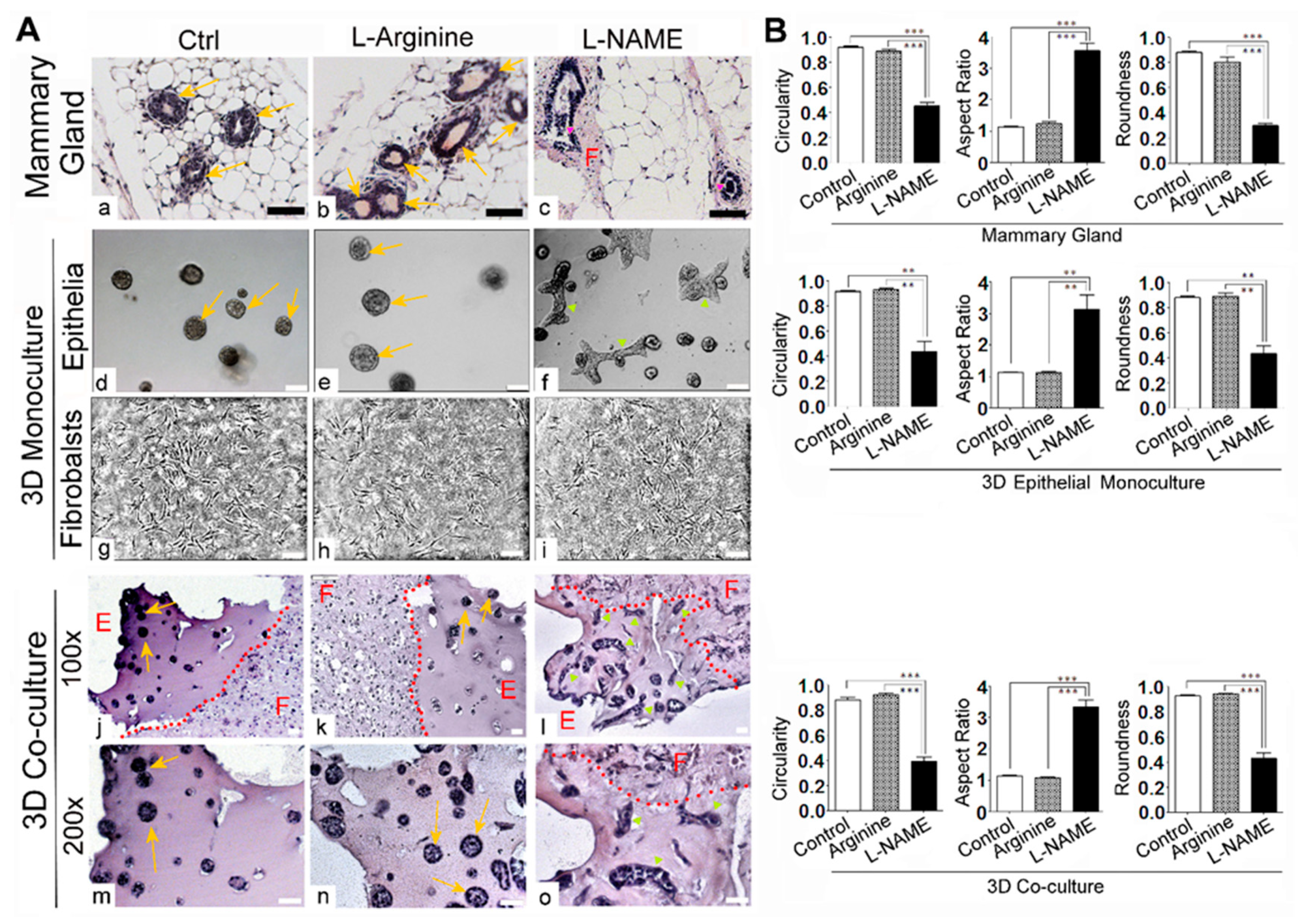
3.2. Deprivation of NO Influences the Gross Phenotype of 3D Co-Cultures of Mammary Epithelial Cells and Fibroblasts in a Manner that Resembles the In Vivo Response
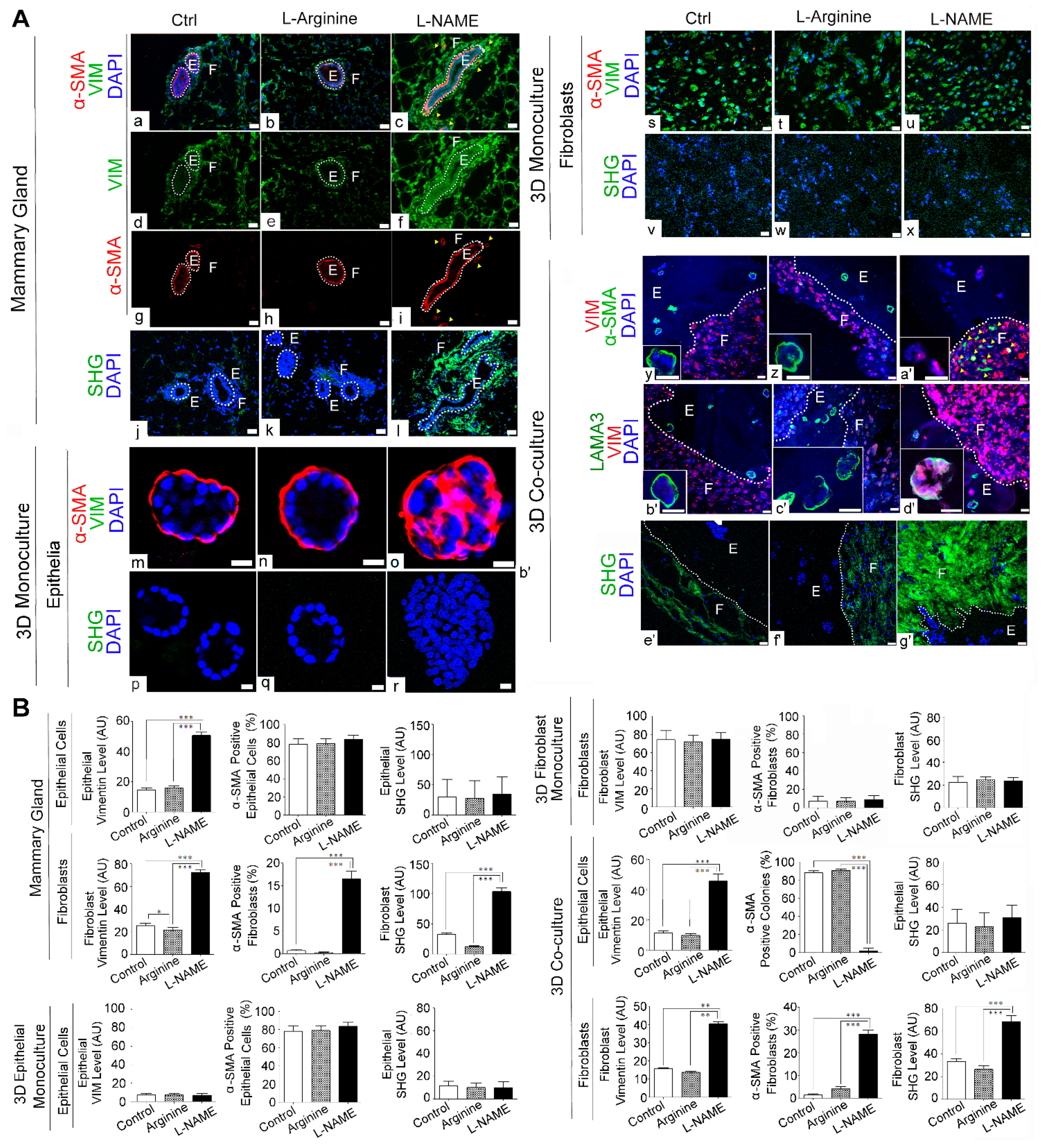
3.3. NO Deprivation Induces Fibrogenic Signals in 3D Co-Cultures of Mammary Epithelial Cells and Fibroblasts in a Manner that Resembles the In Vivo Response
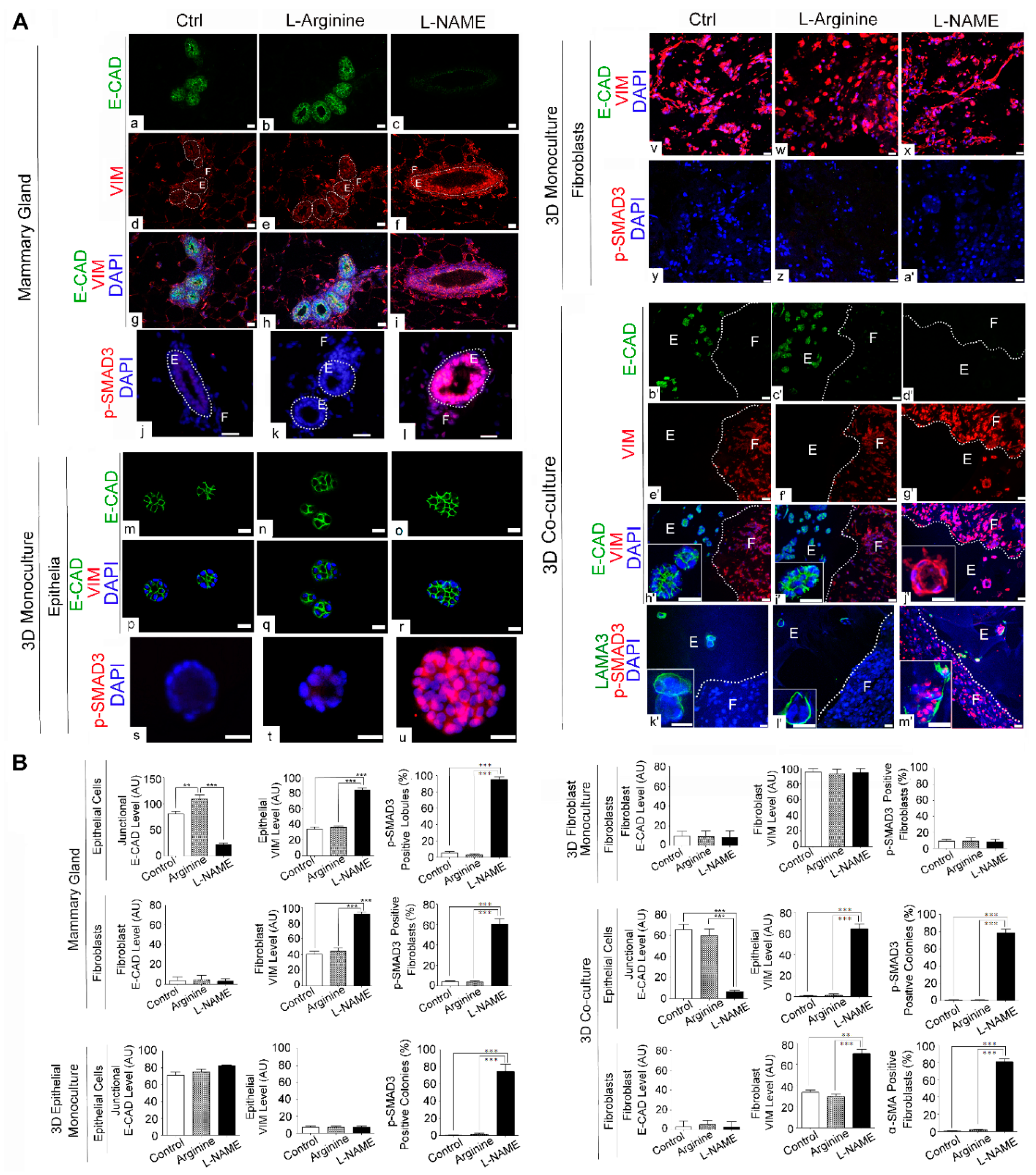
3.4. NO Deprivation Induces Epithelial-to-Mesenchymal Transition upon TGF-β Activation in 3D Co-Cultures of Mammary Epithelial Cells and Fibroblasts in a Manner that Resembles the In Vivo Response
3.5. NO Deprivation Impairs the Apico–Basal Polarity and Induces Invasive Phenotype of Mammary Epithelia in 3D Co-Cultures of Mammary Epithelial Cells and Fibroblasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, Z.-M.; Nguyen, M.; Barsky, S.H. Human breast carcinoma desmoplasia is PDGF initiated. Oncogene 2000, 19, 4337–4345. [Google Scholar] [CrossRef] [Green Version]
- Jackson, J.G.; Orr, J. The ducts of carcinomatous breasts with particular reference to connective tissue changes. J. Pathol. Bacteriol. 1957, 74, 265–273. [Google Scholar] [CrossRef]
- Barsky, S.H.; Rao, C.N.; Grotendorst, G.R.; Liotta, L.A. Increased content of Type V Collagen in desmoplasia of human breast carcinoma. Am. J. Pathol. 1982, 108, 276–283. [Google Scholar]
- Lagacé, R.; Grimaud, J.A.; Schürch, W.; Seemayer, T.A. Myofibroblastic stromal reaction in carcinoma of the breast: Variations of col-lagenous matrix and structural glycoproteins. Virchows Arch. 1985, 408, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Littlepage, L.; Werb, Z. The Fibroblastic Coconspirator in Cancer Progression. Cold Spring Harb. Symp. Quant. Biol. 2005, 70, 383–388. [Google Scholar] [CrossRef]
- Hinz, B.; Phan, S.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.-L.; Gabbiani, G. The Myofibroblast: One Function, Multiple Origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Otranto, M.; Sarrazy, V.; Bonté, F.; Hinz, B.; Gabbiani, G.; Desmoulière, A. The role of the myofibroblast in tumor stroma re-modeling. Cell Adh. Migr. 2012, 6, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Ohmichi, H.; Koshimizu, U.; Matsumoto, K.; Nakamura, T. Hepatocyte growth factor (HGF) acts as a mesenchyme-derived morphogenic factor during fetal lung development. Development 1998, 125, 1315–1324. [Google Scholar] [CrossRef]
- De Wever, O.; Mareel, M. Role of tissue stroma in cancer cell invasion. J. Pathol. 2003, 200, 429–447. [Google Scholar] [CrossRef]
- Flier, J.S.; Underhill, L.H.; Dvorak, H.F. Tumors: Wounds That Do Not Heal. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Dickersin, G.R.; Dvorak, A.M.; Manseau, E.J.; Pyne, K. Human breast carcinoma: Fibrin deposits and desmoplasia. Inflammatory cell type and distribution. Microvasculature and infarction. J. Natl. Cancer Inst. 1981, 67, 335–345. [Google Scholar] [PubMed]
- Peres, R.; Betsholtz, C.; Westermark, B.; Heldin, C.H. Frequent expression of growth factors for mesenchymal cells in human mammary carcinoma cell lines. Cancer Res. 1987, 47, 3425–3429. [Google Scholar]
- Ethier, S.P. Growth Factor Synthesis and Human Breast Cancer Progression. J. Natl. Cancer Inst. 1995, 87, 964–973. [Google Scholar] [CrossRef]
- Ronnov-Jessin, L.; Petersen, O.W. Induction of α-smooth muscle actin by transforming growth factor-β1 in quiescent human breast gland fibroblasts. Lab. Investig. 1993, 68, 696–707. [Google Scholar]
- Bronzert, D.A.; Pantazis, P.; Antoniades, H.N.; Kasid, A.; Davidson, N.; Dickson, R.B.; Lippman, M.E. Synthesis and secretion of platelet-derived growth factor by human breast cancer cell lines. Proc. Natl. Acad. Sci. USA 1987, 84, 5763–5767. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-Y.L.; Kim, J.H.; Park, C.K. VEGF Induces TGF-β1 Expression and Myofibroblast Transformation after Glaucoma Surgery. Am. J. Pathol. 2013, 182, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, R.J.; Oh, S.Y. Breast cancer-associated fibroblasts: Where we are and where we need to go. Cancers 2016, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, S.; Pickup, M.W.; Weaver, V.M. From transformation to metastasis: Deconstructing the extracellular matrix in breast cancer. Cancer Metastasis Rev. 2016, 35, 655–667. [Google Scholar] [CrossRef]
- Yoshida, G.J.; Azuma, A.; Miura, Y.; Orimo, A. Activated Fibroblast Program Orchestrates Tumor Initiation and Progression; Molecular Mechanisms and the Associated Therapeutic Strategies. Int. J. Mol. Sci. 2019, 20, 2256. [Google Scholar] [CrossRef] [Green Version]
- Kuperwasser, C.; Chavarria, T.; Wu, M.; Magrane, G.; Gray, J.W.; Carey, L.; Richardson, A.; Weinberg, R.A. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc. Natl. Acad. Sci. USA 2004, 101, 4966–4971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.; Yao, J.; Carroll, D.K.; Weremowicz, S.; Chen, H.; Carrasco, D.; Richardson, A.; Violette, S.; Nikolskaya, T.; Nikolsky, Y.; et al. Regulation of In Situ to Invasive Breast Carcinoma Transition. Cancer Cell 2008, 13, 394–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Zheng, X.; Bommarito, M.; Metzger, S.; Walia, Y.; Letson, J.; Schroering, A.; Kalinoski, A.; Weaver, D.; Figy, C.; et al. Reduced Basal Nitric Oxide Production Induces Precancerous Mammary Lesions via ERBB2 and TGFβ. Sci. Rep. 2019, 9, 6688. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Sharma, V.; Letson, J.; Walia, Y.; Fernando, V.; Furuta, S. Reconstituting breast tissue with organotypic three-dimensional co-culture of epithelial and stromal cells in discontinuous extracellular matrices. Bio-Protocol 2019, 9, e3392. [Google Scholar] [CrossRef] [PubMed]
- Arendt, L.M.; Keller, P.J.; Skibinski, A.; Gonçalves, A.; Naber, S.P.; Buchsbaum, R.J.; Gilmore, H.; Come, S.E.; Kuperwasser, C. Anatomical localization of progenitor cells in human breast tissue reveals enrichment of uncommitted cells within immature lobules. Breast Cancer Res. 2014, 16, 453. [Google Scholar] [CrossRef] [Green Version]
- Jayadev, R.; Sherwood, D.R. Basement membranes. Curr. Biol. 2017, 27, R207–R211. [Google Scholar] [CrossRef] [Green Version]
- March, S.; Ramanan, V.; Trehan, K.; Ng, S.; Galstian, A.; Gural, N.; Scull, M.A.; Shlomai, A.; Mota, M.M.; Fleming, H.E.; et al. Micropatterned coculture of primary human hepatocytes and supportive cells for the study of hepatotropic pathogens. Nat. Protoc. 2015, 10, 2027–2053. [Google Scholar] [CrossRef] [Green Version]
- Stark, H.J.; Willhauck, M.J.; Mirancea, N.; Boehnke, K.; Nord, I.; Breitkreutz, D.; Pavesio, A.; Boukamp, P.; Fusenig, N.E. Au-thentic fibroblast matrix in dermal equivalents normalises epidermal histogenesis and dermoepidermal junction in organotypic co-culture. Eur. J. Cell Biol. 2004, 83, 631–645. [Google Scholar] [CrossRef]
- Kalabis, J.; Wong, G.S.; Vega, M.E.; Natsuizaka, M.; Robertson, E.S.; Herlyn, M.; Nakagawa, H.; Rustgi, A.K. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat. Protoc. 2012, 7, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koledova, Z.; Lu, P. A 3D Fibroblast-Epithelium Co-culture Model for Understanding Microenvironmental Role in Branching Morphogenesis of the Mammary Gland. In Methods in Molecular Biology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2017; Volume 1501, pp. 217–231. [Google Scholar]
- Chatterjee, S.; Bhat, V.; Berdnikov, A.; Liu, J.; Zhang, G.; Buchel, E.; Safneck, J.; Marshall, A.J.; Murphy, L.C.; Postovit, L.-M.; et al. Paracrine Crosstalk between Fibroblasts and ER+ Breast Cancer Cells Creates an IL1β-Enriched Niche that Promotes Tumor Growth. iScience 2019, 19, 388–401. [Google Scholar] [CrossRef] [Green Version]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Debnath, J.; Muthuswamy, S.K.; Brugge, J.S. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 2003, 30, 256–268. [Google Scholar] [CrossRef]
- Furuta, S.; Ren, G.; Mao, J.-H.; Bissell, M.J. Laminin signals initiate the reciprocal loop that informs breast-specific gene expression and homeostasis by activating NO, p53 and microRNAs. eLife 2018, 7, e26148. [Google Scholar] [CrossRef]
- Theodossiou, T.A.; Thrasivoulou, C.; Ekwobi, C.; Becker, D.L. Second Harmonic Generation Confocal Microscopy of Collagen Type I from Rat Tendon Cryosections. Biophys. J. 2006, 91, 4665–4677. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, S.; Leopold, E.; Schmidt, K.; Brunner, F.; Mayer, B. Inhibition of nitric oxide synthesis by NG-nitro-L-arginine methyl ester (L-NAME): Requirement for bioactivation to the free acid, NG-nitro-L-arginine. Br. J. Pharmacol. 1996, 118, 1433–1440. [Google Scholar] [CrossRef]
- Desmouliere, A.; Darby, I.A.; Laverdet, B.; Bonté, F. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.; Duffy, H.S. Fibroblasts and Myofibroblasts: What Are We Talking About? J. Cardiovasc. Pharmacol. 2011, 57, 376–379. [Google Scholar] [CrossRef]
- Adriance, M.C.; Inman, J.L.; Petersen, O.W.; Bissell, M.J. Myoepithelial cells: Good fences make good neighbors. Breast Cancer Res. 2005, 7, 190–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Weinberg, R.A. Epithelial–Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015, 25, 675–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Willis, B.C.; Borok, Z. TGF-β-induced EMT: Mechanisms and implications for fibrotic lung disease. Am. J. Physiol. Cell. Mol. Physiol. 2007, 293, L525–L534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Wang, Y.; Li, Y.; Xia, X.; Zhao, S.; Che, Y.; Sun, Y.; Lei, L. TGF-β1 promotes bovine mammary fibroblast proliferation through the ERK 1/2 signalling pathway. Cell Biol. Int. 2016, 40, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Flanders, K.C.; Bertolette, D.; Lyakh, L.A.; Wurthner, J.U.; Parks, W.T.; Letterio, J.J.; Ruscetti, F.W.; Roberts, A.B. Levels of phospho-Smad2/3 are sensors of the interplay between effects of TGF-β and retinoic acid on monocytic and granulocytic differentiation of HL-60 cells. Blood 2003, 101, 498–507. [Google Scholar] [CrossRef] [Green Version]
- Whiteman, E.L.; Liu, C.-J.; Fearon, E.R.; Margolis, B. The transcription factor snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene 2008, 27, 3875–3879. [Google Scholar] [CrossRef] [Green Version]
- Royer, C.; Lu, X. Epithelial cell polarity: A major gatekeeper against cancer? Cell Death Differ. 2011, 18, 1470–1477. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Norgard, R.J.; Stanger, B.Z. Cellular Plasticity in Cancer. Cancer Discov. 2019, 9, 837–851. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Vasioukhin, V. Cell polarity and cancer—cell and tissue polarity as a non-canonical tumor suppressor. J. Cell Sci. 2008, 121, 1141–1150. [Google Scholar] [CrossRef] [Green Version]
- Hinck, L.; Näthke, I. Changes in cell and tissue organization in cancer of the breast and colon. Curr. Opin. Cell Biol. 2014, 26, 87–95. [Google Scholar] [CrossRef] [Green Version]
- O’Hare, M.J.; Ormerod, M.G.; Monaghan, P.; Lane, E.B.; Gusterson, B.A. Characterization in vitro of luminal and myoepithelial cells isolated from the human mammary gland by cell sorting. Differentiation 1991, 46, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Kenny, P.A.; Lee, E.H.; Bissell, M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 2007, 4, 359–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidi, P.-A.; Bissell, M.J.; Lelièvre, S.A. Three-Dimensional Culture of Human Breast Epithelial Cells: The How and the Why. Methods in Molecular Biology 2012, 945, 193–219. [Google Scholar] [CrossRef] [Green Version]
- DeClerck, Y.A. Desmoplasia: A Response or a Niche? Cancer Discov. 2012, 2, 772–774. [Google Scholar] [CrossRef] [Green Version]
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature 2004, 432, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Chandler, C.; Liu, T.; Buckanovich, R.; Coffman, L.G. The double edge sword of fibrosis in cancer. Transl. Res. 2019, 209, 55–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef] [Green Version]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.-L.; et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397–1410.e14. [Google Scholar] [CrossRef]
- Noguchi, S.; Saito, A.; Nagase, T. YAP/TAZ Signaling as a Molecular Link between Fibrosis and Cancer. Int. J. Mol. Sci. 2018, 19, 3674. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Cheresh, P.; Kamp, D.W. Molecular Basis of Asbestos-Induced Lung Disease. Annu. Rev. Pathol. Mech. Dis. 2013, 8, 161–187. [Google Scholar] [CrossRef] [Green Version]
- Steenland, K.; Stayner, L. Silica, asbestos, man-made mineral fibers, and cancer. Cancer Causes Control. 1997, 8, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P.E. Fibrosis and Adipose Tissue Dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Talakatta, G.; Sarikhani, M.; Muhamed, J.; Dhanya, K.; Somashekar, B.S.; Mahesh, P.A.; Sundaresan, N.; Ravindra, P.V. Dia-betes induces fibrotic changes in the lung through the activation of TGF-β signaling pathways. Sci. Rep. 2018, 8, 11920. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Gabazza, E.C. Links between Fibrogenesis and Cancer: Mechanistic and Therapeutic Challenges. Int. J. Mol. Sci. 2019, 20, 4313. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Wu, G. Regulatory role for amino acids in mammary gland growth and milk synthesis. Amino Acids 2008, 37, 89–95. [Google Scholar] [CrossRef]
- Iizuka, T.; Sasaki, M.; Oishi, K.; Uemura, S.; Koike, M. The Presence of Nitric Oxide Synthase in the Mammary Glands of Lactating Rats. Pediatr. Res. 1998, 44, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Tezer, M.; Ozluk, Y.; Asoglu, O.; Kadioglu, A.; Sanli, O. Nitric Oxide May Mediate Nipple Erection. J. Androl. 2011, 33, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Hord, N.G.; Ghannam, J.S.; Garg, H.K.; Berens, P.D.; Bryan, N.S. Nitrate and Nitrite Content of Human, Formula, Bovine, and Soy Milks: Implications for Dietary Nitrite and Nitrate Recommendations. Breastfeed. Med. 2011, 6, 393–399. [Google Scholar] [CrossRef]
- Torregrossa, A.C.; Aranke, M.; Bryan, N.S. Nitric oxide and geriatrics: Implications in diagnostics and treatment of the elderly. J. Geriatr. Cardiol. 2011, 8, 230–242. [Google Scholar] [PubMed] [Green Version]
- Hoang, H.H.; Padgham, S.V.; Meininger, C. L-arginine, tetrahydrobiopterin, nitric oxide and diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 76–82. [Google Scholar] [CrossRef]
- Gámez-Méndez, A.M.; Vargas-Robles, H.; Arellano-Mendoza, M.; Cruz-Laguna, E.; Rios, A.; Escalante, B. Early stage of obesity potentiates nitric oxide reduction during the development of renal failure. J. Nephrol. 2014, 27, 281–287. [Google Scholar] [CrossRef]
- Moens, A.L.; Kass, D.A. Tetrahydrobiopterin and Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2006, 26, 2439–2444. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Dikalov, S.; Price, S.R.; McCann, L.; Fukai, T.; Holland, S.M.; Mitch, W.E.; Harrison, D.G. Oxidation of tet-rahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Investig. 2003, 111, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Rabender, C.S.; Alam, A.; Sundaresan, G.; Cardnell, R.J.; Yakovlev, V.; Mukhopadhyay, N.D.; Graves, P.; Zweit, J.; Mikkelsen, R.B. The Role of Nitric Oxide Synthase Uncoupling in Tumor Progression. Mol. Cancer Res. 2015, 13, 1034–1043. [Google Scholar] [CrossRef] [Green Version]
- Kolluru, G.; Bir, S.C.; Kevil, C.G. Endothelial Dysfunction and Diabetes: Effects on Angiogenesis, Vascular Remodeling, and Wound Healing. Int. J. Vasc. Med. 2012, 2012, 918267 . [Google Scholar] [CrossRef] [Green Version]
- Leopold, J.A. Cellular and molecular mechanisms of arterial stiffness associated with obesity. Hypertension 2013, 62, 1003–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Cardnell, R.J.; Rabender, C.S.; Ross, G.R.; Guo, C.; Howlett, E.L.; Alam, A.; Wang, X.Y.; Akbarali, H.; Mikkelsen, R.B. Se-piapterin ameliorates chemically induced murine colitis and azoxymethane-induced colon cancer. J. Pharmacol. Exp. Ther. 2013, 347, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Fernando, V.; Zheng, X.; Walia, Y.; Sharma, V.; Letson, J.; Furuta, S. S-Nitrosylation: An Emerging Paradigm of Redox Signaling. Antioxidants 2019, 8, 404. [Google Scholar] [CrossRef] [Green Version]
- Edgar, K.S.; Matesanz, N.; Gardiner, T.A.; Katusic, Z.S.; McDonald, D.M. Hyperoxia depletes (6R)-5,6,7,8-tetrahydrobiopterin levels in the neonatal retina: Implications for nitric oxide synthase function in retinopathy. Am. J. Pathol. 2015, 185, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Kuzkaya, N.; Weissmann, N.; Harrison, D.G.; Dikalov, S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003, 278, 22546–22554. [Google Scholar] [CrossRef] [Green Version]
- Saini, H.; Eliato, K.R.; Veldhuizen, J.; Zare, A.; Allam, M.; Silva, C.; Kratz, A.; Truong, D.; Mouneimne, G.; LaBaer, J.; et al. The role of tumor-stroma interactions on desmoplasia and tumorigenicity within a microengineered 3D platform. Biomaterials 2020, 247, 119975. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.M.; VanDuijn, M.M.; Inman, J.L.; Fletcher, D.A.; Bissell, M.J. Tissue Geometry Determines Sites of Mammary Branching Morphogenesis in Organotypic Cultures. Science 2006, 314, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Ray, L.A.; Thakuri, P.S.; Tran, S.; Konopka, M.C.; Luker, G.D.; Tavana, H. Organotypic breast tumor model elucidates dynamic remodeling of tumor microenvironment. Biomaterials 2020, 238, 119853. [Google Scholar] [CrossRef]
- Truong, D.D.; Kratz, A.; Park, J.G.; Barrientos, E.S.; Saini, H.; Nguyen, T.; Pockaj, B.; Mouneimne, G.; LaBaer, J.; Nikkhah, M. A Human Organotypic Microfluidic Tumor Model Permits Investigation of the Interplay between Patient-Derived Fibroblasts and Breast Cancer Cells. Cancer Res. 2019, 79, 3139–3151. [Google Scholar] [CrossRef] [Green Version]
- Shatanawi, A.; Qasrawi, H. Arginase Inhibition Suppresses Breast Cancer Cell Proliferation. FASEB J. 2017, 31, lb529. [Google Scholar]
- Reisser, D.; Onier-Cherix, N.; Jeannin, J.-F. Arginase Activity is Inhibited by l -NAME, both In Vitro and In Vivo. J. Enzym. Inhib. Med. Chem. 2002, 17, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.A.; Green, B.G.; Niedzwiecki, L.; Harrison, R.K.; Grant, S.K. Effect of nitric oxide synthase substrate analog in-hibitors on rat liver arginase. Biochem. Biophys. Res. Commun. 1993, 197, 523–528. [Google Scholar] [CrossRef]
- Pershing, N.L.; Yang, C.-F.J.; Xu, M.; Counter, C.M. Treatment with the nitric oxide synthase inhibitor L-NAME provides a survival advantage in a mouse model of Kras mutation-positive, non-small cell lung cancer. Oncotarget 2016, 7, 42385–42392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.-B.; Dong, X.-S.; Sun, W.-Z.; Zhao, D.-L.; Yang, Y. Effect of a nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester on invasion of human colorectal cancer cell line SL-174T. World J. Gastroenterol. 2005, 11, 6385–6388. [Google Scholar] [CrossRef]
- Caneba, C.; Yang, L.; Baddour, J.; Curtis, R.; Win, J.; Hartig, S.M.; Marini, J.C.; Nagrath, D. Nitric oxide is a positive regulator of the Warburg effect in ovarian cancer cells. Cell Death Dis. 2014, 5, e1302. [Google Scholar] [CrossRef]
- Vyas-Read, S.; Shaul, P.W.; Yuhanna, I.S.; Willis, B.C. Nitric oxide attenuates epithelial-mesenchymal transition in alveolar epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2007, 293, L212–L221. [Google Scholar] [CrossRef]
- Le, X.; Wei, D.; Huang, S.; Lancaster, J.R.; Xie, K. Nitric oxide synthase II suppresses the growth and metastasis of human cancer regardless of its up-regulation of protumor factors. Proc. Natl. Acad. Sci. USA 2005, 102, 8758–8763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirakawa, M.; Oike, M.; Masuda, K.; Ito, Y. Tumor cell apoptosis by irradiation-induced nitric oxide production in vascular endothelium. Cancer Res. 2002, 62, 1450–1457. [Google Scholar] [PubMed]
- Reis, O.T.G.; Raini, J.C.; Coradi, S.T.; Constantino, D.H.J. Effect of L-Arginine and L-NAME treatments on polymorphonuclear leukocytes and mononuclear cells influx during tumor growth. Acta Cir. Bras. 2009, 24, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrila, J.; Yang, J.; Crabbé, A.; Sarker, S.F.; Liu, Y.; Ott, C.M.; Nelman-Gonzalez, M.A.; Clemett, S.J.; Nydam, S.D.; Forsyth, R.J.; et al. Three-dimensional organotypic co-culture model of intestinal epithelial cells and macrophages to study Salmonella enterica colonization patterns. NPJ Microgravity 2017, 3, 10. [Google Scholar] [CrossRef]
- Kook, Y.-M.; Kim, H.; Kim, S.; Heo, C.Y.; Park, M.H.; Lee, K.; Koh, W.-G. Promotion of Vascular Morphogenesis of Endothelial Cells Co-Cultured with Human Adipose-Derived Mesenchymal Stem Cells Using Polycaprolactone/Gelatin Nanofibrous Scaffolds. Nanomaterials 2018, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Laczkó, D.; Wang, F.; Johnson, F.B.; Jhala, N.; Rosztóczy, A.; Ginsberg, G.G.; Falk, G.W.; Rustgi, A.K.; Lynch, J.P. Modeling Esophagitis Using Human Three-Dimensional Organotypic Culture System. Am. J. Pathol. 2017, 187, 1787–1799. [Google Scholar] [CrossRef] [Green Version]
- Harms, M.J.; Li, Q.; Lee, S.; Zhang, C.; Kull, B.; Hallen, S.; Thorell, A.; Alexandersson, I.; Hagberg, C.E.; Peng, X.-R.; et al. Mature Human White Adipocytes Cultured under Membranes Maintain Identity, Function, and Can Transdifferentiate into Brown-like Adipocytes. Cell Rep. 2019, 27, 213–225.e5. [Google Scholar] [CrossRef] [Green Version]
- Muller, S.; Ader, I.; Creff, J.; Leménager, H.; Achard, P.; Casteilla, L.; Sensebé, L.; Carrière, A.; Deschaseaux, F. Human adipose stromal-vascular fraction self-organizes to form vascularized adipose tissue in 3D cultures. Sci. Rep. 2019, 9, 7250. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, G.; Zheng, X.; Sharma, V.; Letson, J.; Nestor-Kalinoski, A.L.; Furuta, S. Loss of Nitric Oxide Induces Fibrogenic Response in Organotypic 3D Co-Culture of Mammary Epithelia and Fibroblasts—An Indicator for Breast Carcinogenesis. Cancers 2021, 13, 2815. https://doi.org/10.3390/cancers13112815
Ren G, Zheng X, Sharma V, Letson J, Nestor-Kalinoski AL, Furuta S. Loss of Nitric Oxide Induces Fibrogenic Response in Organotypic 3D Co-Culture of Mammary Epithelia and Fibroblasts—An Indicator for Breast Carcinogenesis. Cancers. 2021; 13(11):2815. https://doi.org/10.3390/cancers13112815
Chicago/Turabian StyleRen, Gang, Xunzhen Zheng, Vandana Sharma, Joshua Letson, Andrea L. Nestor-Kalinoski, and Saori Furuta. 2021. "Loss of Nitric Oxide Induces Fibrogenic Response in Organotypic 3D Co-Culture of Mammary Epithelia and Fibroblasts—An Indicator for Breast Carcinogenesis" Cancers 13, no. 11: 2815. https://doi.org/10.3390/cancers13112815
APA StyleRen, G., Zheng, X., Sharma, V., Letson, J., Nestor-Kalinoski, A. L., & Furuta, S. (2021). Loss of Nitric Oxide Induces Fibrogenic Response in Organotypic 3D Co-Culture of Mammary Epithelia and Fibroblasts—An Indicator for Breast Carcinogenesis. Cancers, 13(11), 2815. https://doi.org/10.3390/cancers13112815







