Podocalyxin in Normal Tissue and Epithelial Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
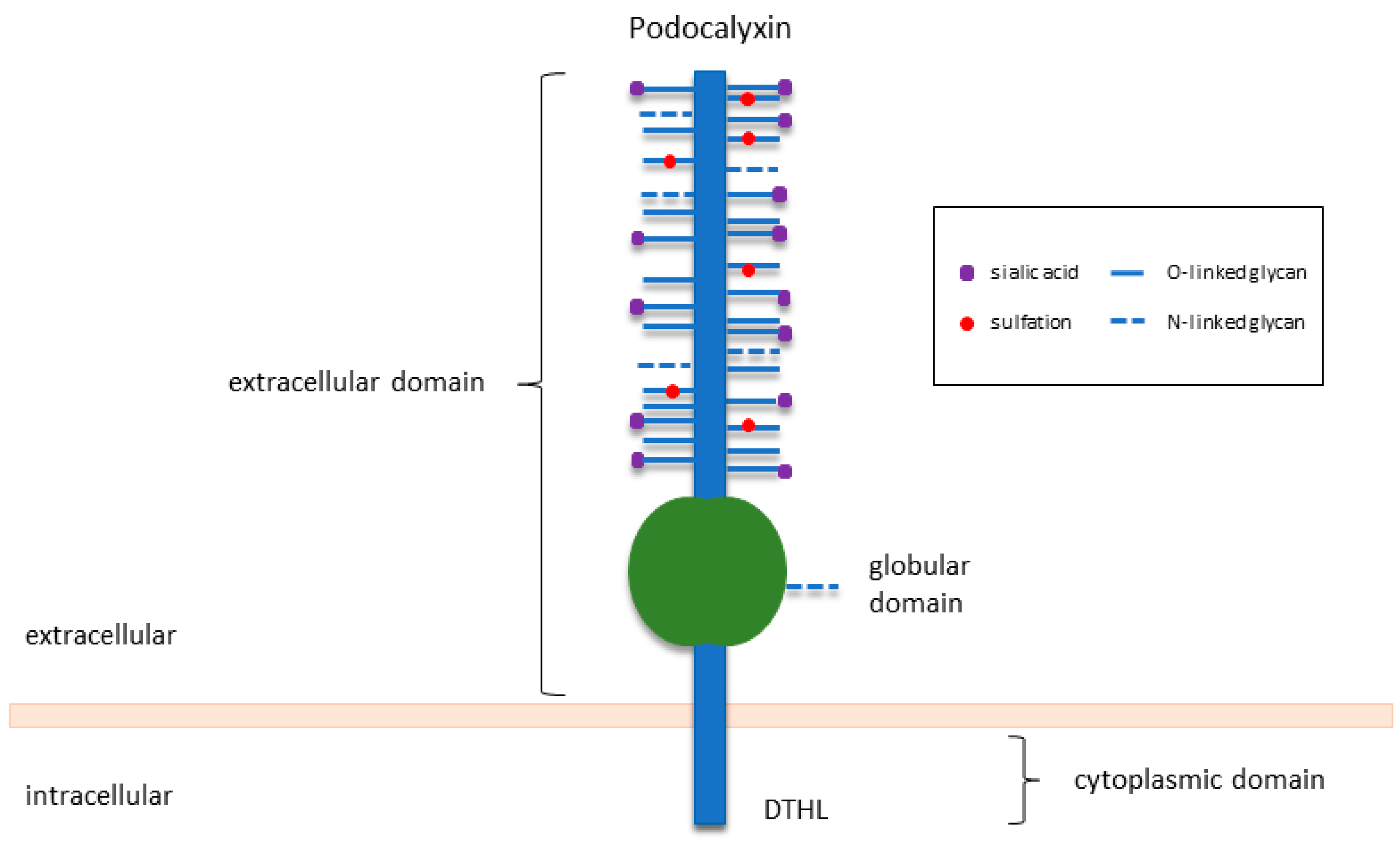
2. An Overview of PODXL in Normal Development and Function
2.1. PODXL Is Essential for Kidney Development and Function
2.2. PODXL Also Plays Subtle Roles in Other Tissues
3. PODXL in Human Malignancies, Specifically in Epithelial Cancers
3.1. PODXL Activates Intracellular Signalling Pathways to Facilitate Cancer Metastasis
3.2. PODXL Mediates the Transforming Growth Factor β (TGFβ)-Induced EMT in Cancer
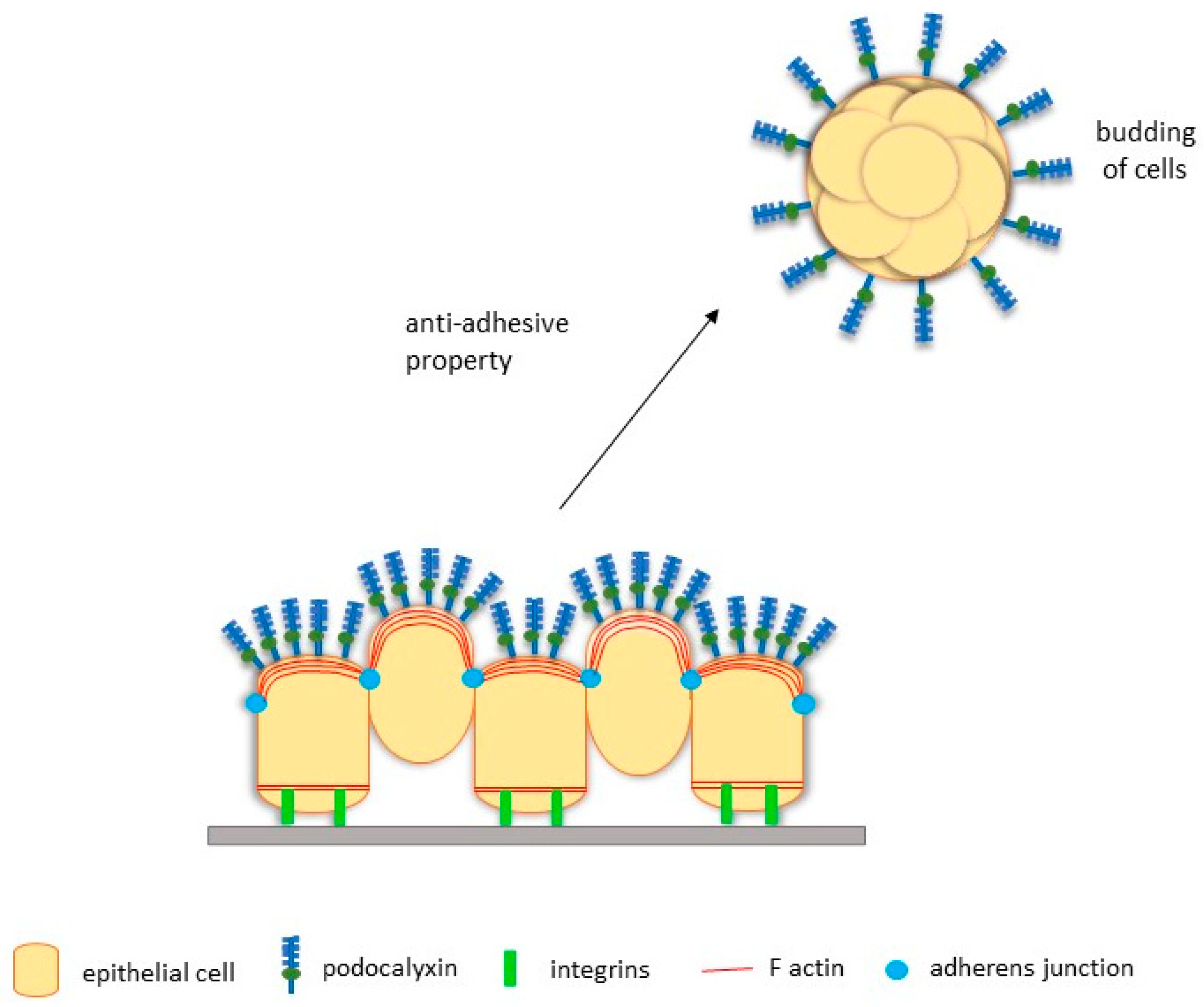
3.3. PODXL Facilitates EMT-Independent Tumour Budding
3.4. PODXL Promotes Migration and Invasion of Cancer Cells
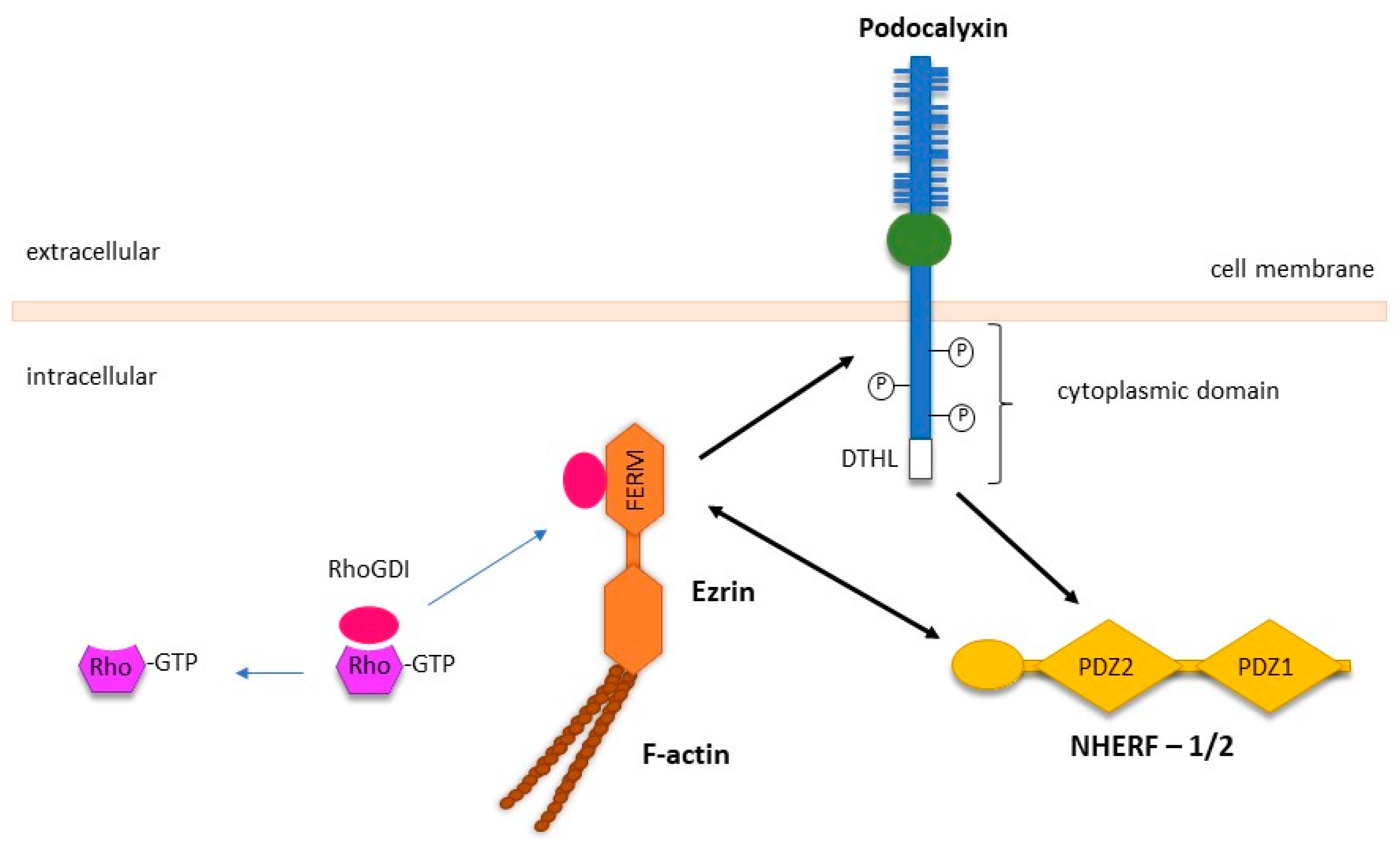
3.5. PODXL Mediates Extravasation during Metastasis via Binding to E- and L-Selectins, and Ezrin
3.6. PODXL May Play an Important Role in Immune Evasion
3.7. PODXL May Participate in Chemotherapy Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ganz, T. Epithelia: Not just physical barriers. Proc. Natl. Acad. Sci. USA 2002, 99, 3357–3358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinck, L.; Nathke, I. Changes in cell and tissue organization in cancer of the breast and colon. Curr. Opin. Cell Biol. 2014, 26, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigelt, B.; Peterse, J.L.; van’t Veer, L.J. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Sharkey, D.J.; Farquhar, M.G. Identification and characterization of podocalyxin—The major sialoprotein of the renal glomerular epithelial cell. J. Cell Biol. 1984, 98, 1591–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvat, R.; Hovorka, A.; Dekan, G.; Poczewski, H.; Kerjaschki, D. Endothelial cell membranes contain podocalyxin—The major sialoprotein of visceral glomerular epithelial cells. J. Cell Biol. 1986, 102, 484–491. [Google Scholar] [CrossRef] [Green Version]
- Miettinen, A.; Solin, M.L.; Reivinen, J.; Juvonen, E.; Väisänen, R.; Holthöfer, H. Podocalyxin in rat platelets and megakaryocytes. Am. J. Pathol. 1999, 154, 813–822. [Google Scholar] [CrossRef] [Green Version]
- Doyonnas, R.; Nielsen, J.S.; Chelliah, S.; Drew, E.; Hara, T.; Miyajima, A.; McNagny, K.M. Podocalyxin is a CD34-related marker of murine hematopoietic stem cells and embryonic erythroid cells. Blood 2005, 105, 4170–4178. [Google Scholar] [CrossRef] [PubMed]
- Vitureira, N.; McNagny, K.; Soriano, E.; Burgaya, F. Pattern of expression of the podocalyxin gene in the mouse brain during development. Gene Expr. Patterns 2005, 5, 349–354. [Google Scholar] [CrossRef]
- Weinman, E.J. New functions for the NHERF family of proteins. J. Clin. Investig. 2001, 108, 185–186. [Google Scholar] [CrossRef]
- Voltz, J.W.; Weinman, E.J.; Shenolikar, S. Expanding the role of NHERF, a PDZ-domain containing protein adapter, to growth regulation. Oncogene 2001, 20, 6309–6314. [Google Scholar] [CrossRef] [Green Version]
- Doyonnas, R.; Kershaw, D.B.; Duhme, C.; Merkens, H.; Chelliah, S.; Graf, T.; McNagny, K.M. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J. Exp. Med. 2001, 194, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Paule, S.G.; Heng, S.; Samarajeewa, N.; Li, Y.; Mansilla, M.; Webb, A.I.; Nebl, T.; Young, S.L.; Lessey, B.A.; Hull, M.L.; et al. Podocalyxin is a key negative regulator of human endometrial epithelial receptivity for embryo implantation. Hum. Reprod. 2021, 36, 1353–1366. [Google Scholar] [CrossRef]
- Sizemore, S.; Cicek, M.; Sizemore, N.; Ng, K.P.; Casey, G. Podocalyxin increases the aggressive phenotype of breast and prostate cancer cells in vitro through its interaction with ezrin. Cancer Res. 2007, 67, 6183–6191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipollone, J.A.; Graves, M.L.; Kobel, M.; Kalloger, S.E.; Poon, T.; Gilks, C.B.; McNagny, K.M.; Roskelley, C.D. The anti-adhesive mucin podocalyxin may help initiate the transperitoneal metastasis of high grade serous ovarian carcinoma. Clin. Exp. Metastasis 2012, 29, 239–252. [Google Scholar] [CrossRef]
- Somasiri, A.; Nielsen, J.S.; Makretsov, N.; McCoy, M.L.; Prentice, L.; Gilks, C.B.; Chia, S.K.; Gelmon, K.A.; Kershaw, D.B.; Huntsman, D.G.; et al. Overexpression of the anti-adhesin podocalyxin is an independent predictor of breast cancer progression. Cancer Res. 2004, 64, 5068–5073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, G.; Neville, P.J.; Liu, X.; Plummer, S.J.; Cicek, M.S.; Krumroy, L.M.; Curran, A.P.; McGreevy, M.R.; Catalona, W.J.; Klein, E.A.; et al. Podocalyxin variants and risk of prostate cancer and tumor aggressiveness. Hum. Mol. Genet. 2006, 15, 735–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amo, L.; Tamayo-Orbegozo, E.; Maruri, N.; Buqué, A.; Solaun, M.; Riñón, M.; Arrieta, A.; Larrucea, S. Podocalyxin-like protein 1 functions as an immunomodulatory molecule in breast cancer cells. Cancer Lett. 2015, 368, 26–35. [Google Scholar] [CrossRef]
- Wong, B.S.; Shea, D.J.; Mistriotis, P.; Tuntithavornwat, S.; Law, R.A.; Bieber, J.M.; Zheng, L.; Konstantopoulos, K. A Direct Podocalyxin-Dynamin-2 Interaction Regulates Cytoskeletal Dynamics to Promote Migration and Metastasis in Pancreatic Cancer Cells. Cancer Res. 2019, 79, 2878–2891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, A.; Johansson, M.E.; Wangefjord, S.; Gaber, A.; Nodin, B.; Kucharzewska, P.; Welinder, C.; Belting, M.; Eberhard, J.; Johnsson, A.; et al. Overexpression of podocalyxin-like protein is an independent factor of poor prognosis in colorectal cancer. Br. J. Cancer 2011, 105, 666–672. [Google Scholar] [CrossRef] [PubMed]
- McNagny, K.M.; Hughes, M.R.; Graves, M.L.; DeBruin, E.J.; Snyder, K.; Cipollone, J.; Turvey, M.; Tan, P.C.; McColl, S.; Roskelley, C.D. Podocalyxin in the Diagnosis and Treatment of Cancer. Adv. Cancer Manag. 2012, 155–194. [Google Scholar] [CrossRef] [Green Version]
- Kershaw, D.B.; Beck, S.G.; Wharram, B.L.; Wiggins, J.E.; Goyal, M.; Thomas, P.E.; Wiggins, R.C. Molecular cloning, expression, and characterization of podocalyxin-like protein 1 from rabbit as a transmembrane protein of glomerular podocytes and vascular endothelium. J. Biol. Chem. 1995, 270, 29439–29446. [Google Scholar] [CrossRef] [Green Version]
- Toyoda, H.; Nagai, Y.; Kojima, A.; Kinoshita-Toyoda, A. Podocalyxin as a major pluripotent marker and novel keratan sulfate proteoglycan in human embryonic and induced pluripotent stem cells. Glycoconj. J. 2017, 34, 817–823. [Google Scholar] [CrossRef]
- Nielsen, J.S.; McNagny, K.M. The role of podocalyxin in health and disease. J. Am. Soc. Nephrol. 2009, 20, 1669–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, R.E.; Kotsianti, A.; Cadman, B.; Boyd, T.; Gerald, W.; Haber, D.A. WT1 regulates the expression of the major glomerular podocyte membrane protein Podocalyxin. Curr. Biol. 2001, 11, 1805–1809. [Google Scholar] [CrossRef] [Green Version]
- Butta, N.; Larrucea, S.; Alonso, S.; Rodriguez, R.B.; Arias-Salgado, E.G.; Ayuso, M.S.; Gonzalez-Manchon, C.; Parrilla, R. Role of transcription factor Sp1 and CpG methylation on the regulation of the human podocalyxin gene promoter. BMC Mol. Biol. 2006, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Stanhope-Baker, P.; Kessler, P.M.; Li, W.; Agarwal, M.L.; Williams, B.R. The Wilms tumor suppressor-1 target gene podocalyxin is transcriptionally repressed by p53. J. Biol. Chem. 2004, 279, 33575–33585. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Li, Y.; Wu, C.; Liu, Y. PINCH1 is transcriptional regulator in podocytes that interacts with WT1 and represses podocalyxin expression. PLoS ONE 2011, 6, e17048. [Google Scholar] [CrossRef] [Green Version]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [Green Version]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, P.M. Glomerular epithelial alterations resulting from sialic acid surface coat removal. Kidney Int. 1979, 15, 376–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Maiti, S.; Sun, G.; Ordonez, N.G.; Udtha, M.; Deng, J.M.; Behringer, R.R.; Huff, V. The Wt1+/R394W mouse displays glomerulosclerosis and early-onset renal failure characteristic of human Denys-Drash syndrome. Mol. Cell. Biol. 2004, 24, 9899–9910. [Google Scholar] [CrossRef] [Green Version]
- Michael, A.F.; Blau, E.; Vernier, R.L. Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab. Investig. 1970, 23, 649–657. [Google Scholar]
- Schnabel, E.; Dekan, G.; Miettinen, A.; Farquhar, M.G. Biogenesis of podocalyxin--the major glomerular sialoglycoprotein--in the newborn rat kidney. Eur. J. Cell Biol. 1989, 48, 313–326. [Google Scholar] [PubMed]
- Takeda, T.; Go, W.Y.; Orlando, R.A.; Farquhar, M.G. Expression of podocalyxin inhibits cell-cell adhesion and modifies junctional properties in Madin-Darby canine kidney cells. Mol. Biol. Cell 2000, 11, 3219–3232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiler, M.W.; Rennke, H.G.; Venkatachalam, M.A.; Cotran, R.S. Pathogenesis of polycation-induced alterations (“fusion”) of glomerular epithelium. Lab. Investig. 1977, 36, 48–61. [Google Scholar]
- Economou, C.G.; Kitsiou, P.V.; Tzinia, A.K.; Panagopoulou, E.; Marinos, E.; Kershaw, D.B.; Kerjaschki, D.; Tsilibary, E.C. Enhanced podocalyxin expression alters the structure of podocyte basal surface. J. Cell Sci. 2004, 117 Pt 15, 3281–3294. [Google Scholar] [CrossRef] [Green Version]
- Hara, M.; Yamagata, K.; Tomino, Y.; Saito, A.; Hirayama, Y.; Ogasawara, S.; Kurosawa, H.; Sekine, S.; Yan, K. Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: Establishment of a highly sensitive ELISA to detect urinary podocalyxin. Diabetologia 2012, 55, 2913–2919. [Google Scholar] [CrossRef] [Green Version]
- McNagny, K.M.; Pettersson, I.; Rossi, F.; Flamme, I.; Shevchenko, A.; Mann, M.; Graf, T. Thrombomucin, a novel cell surface protein that defines thrombocytes and multipotent hematopoietic progenitors. J. Cell Biol. 1997, 138, 1395–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerosuo, L.; Juvonen, E.; Alitalo, R.; Gylling, M.; Kerjaschki, D.; Miettinen, A. Podocalyxin in human haematopoietic cells. Br. J. Haematol. 2004, 124, 809–818. [Google Scholar] [CrossRef]
- Zhang, H.; Nieves, J.L.; Fraser, S.T.; Isern, J.; Douvaras, P.; Papatsenko, D.; D’Souza, S.L.; Lemischka, I.R.; Dyer, M.A.; Baron, M.H. Expression of podocalyxin separates the hematopoietic and vascular potentials of mouse embryonic stem cell-derived mesoderm. Stem Cells 2014, 32, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Sathyanarayana, P.; Menon, M.P.; Bogacheva, O.; Bogachev, O.; Niss, K.; Kapelle, W.S.; Houde, E.; Fang, J.; Wojchowski, D.M. Erythropoietin modulation of podocalyxin and a proposed erythroblast niche. Blood 2007, 110, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maltby, S.; Hughes, M.R.; Zbytnuik, L.; Paulson, R.F.; McNagny, K.M. Podocalyxin selectively marks erythroid-committed progenitors during anemic stress but is dispensable for efficient recovery. Exp. Hematol. 2009, 37, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.S.; McNagny, K.M. Novel functions of the CD34 family. J. Cell Sci. 2008, 121 Pt 22, 3683–3692. [Google Scholar] [CrossRef] [Green Version]
- Baumhueter, S.; Dybdal, N.; Kyle, C.; Lasky, L.A. Global vascular expression of murine CD34, a sialomucin-like endothelial ligand for L-selectin. Blood 1994, 84, 2554–2565. [Google Scholar] [CrossRef] [Green Version]
- Cait, J.; Hughes, M.R.; Zeglinski, M.R.; Chan, A.W.; Osterhof, S.; Scott, R.W.; Canals Hernaez, D.; Cait, A.; Vogl, A.W.; Bernatchez, P.; et al. Podocalyxin is required for maintaining blood-brain barrier function during acute inflammation. Proc. Natl. Acad. Sci. USA 2019, 116, 4518–4527. [Google Scholar] [CrossRef] [Green Version]
- Debruin, E.J.; Hughes, M.R.; Sina, C.; Lu, A.; Cait, J.; Jian, Z.; Lopez, M.; Lo, B.; Abraham, T.; McNagny, K.M. Podocalyxin regulates murine lung vascular permeability by altering endothelial cell adhesion. PLoS ONE 2014, 9, e108881. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Wang, Y.; Li, Y.; Zhao, M.; Nie, G. Serum podocalyxin is significantly increased in early-onset preeclampsia and may represent a novel marker of maternal endothelial cell dysfunction. J. Hypertens. 2017, 35, 2287–2294. [Google Scholar] [CrossRef]
- Vitureira, N.; Andrés, R.; Pérez-Martínez, E.; Martínez, A.; Bribián, A.; Blasi, J.; Chelliah, S.; López-Doménech, G.; De Castro, F.; Burgaya, F.; et al. Podocalyxin is a novel polysialylated neural adhesion protein with multiple roles in neural development and synapse formation. PLoS ONE 2010, 5, e12003. [Google Scholar] [CrossRef] [Green Version]
- Yasuoka, H.; Tsujimoto, M.; Hirokawa, M.; Tori, M.; Nakahara, M.; Miyauchi, A.; Kodama, R.; Sanke, T.; Nakamura, Y. Podocalyxin expression in undifferentiated thyroid carcinomas. J. Clin. Pathol. 2008, 61, 1228–1229. [Google Scholar] [CrossRef]
- Schopperle, W.M.; Kershaw, D.B.; DeWolf, W.C. Human embryonal carcinoma tumor antigen, Gp200/GCTM-2, is podocalyxin. Biochem. Biophys. Res. Commun. 2003, 300, 285–290. [Google Scholar] [CrossRef]
- Huang, T.; Jin, X.; He, L.; Zhang, M.; Wu, J.; Wang, Y.; Fang, J. Role of podocalyxin in astrocytoma: Clinicopathological and in vitro evidence. Oncol. Lett. 2013, 6, 1390–1396. [Google Scholar] [CrossRef]
- Kelley, T.W.; Huntsman, D.; McNagny, K.M.; Roskelley, C.D.; Hsi, E.D. Podocalyxin: A marker of blasts in acute leukemia. Am. J. Clin. Pathol. 2005, 124, 134–142. [Google Scholar] [CrossRef]
- Tamayo-Orbegozo, E.; Amo, L.; Rinon, M.; Nieto, N.; Amutio, E.; Maruri, N.; Solaun, M.; Arrieta, A.; Larrucea, S. Podocalyxin promotes proliferation and survival in mature B-cell non-Hodgkin lymphoma cells. Oncotarget 2017, 8, 99722–99739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Tellez, T.N.; Lopez, T.V.; Vasquez Garzon, V.R.; Villa-Trevino, S. Co-Expression of Ezrin-CLIC5-Podocalyxin Is Associated with Migration and Invasiveness in Hepatocellular Carcinoma. PLoS ONE 2015, 10, e0131605. [Google Scholar] [CrossRef] [Green Version]
- Larsson, A.; Fridberg, M.; Gaber, A.; Nodin, B.; Leveen, P.; Jonsson, G.; Uhlen, M.; Birgisson, H.; Jirstrom, K. Validation of podocalyxin-like protein as a biomarker of poor prognosis in colorectal cancer. BMC Cancer 2012, 12, 282. [Google Scholar] [CrossRef]
- Larsson, A.H.; Nodin, B.; Syk, I.; Palmquist, I.; Uhlen, M.; Eberhard, J.; Jirstrom, K. Podocalyxin-like protein expression in primary colorectal cancer and synchronous lymph node metastases. Diagn. Pathol. 2013, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Kaprio, T.; Fermer, C.; Hagstrom, J.; Mustonen, H.; Bockelman, C.; Nilsson, O.; Haglund, C. Podocalyxin is a marker of poor prognosis in colorectal cancer. BMC Cancer 2014, 14, 493. [Google Scholar] [CrossRef] [Green Version]
- Laitinen, A.; Bockelman, C.; Hagstrom, J.; Kokkola, A.; Fermer, C.; Nilsson, O.; Haglund, C. Podocalyxin as a Prognostic Marker in Gastric Cancer. PLoS ONE 2015, 10, e0145079. [Google Scholar] [CrossRef]
- Borg, D.; Hedner, C.; Nodin, B.; Larsson, A.; Johnsson, A.; Eberhard, J.; Jirstrom, K. Expression of podocalyxin-like protein is an independent prognostic biomarker in resected esophageal and gastric adenocarcinoma. BMC Clin. Pathol. 2016, 16, 13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhu, Z.; Wu, H.; Yu, Z.; Rong, Z.; Luo, Z.; Xu, Y.; Huang, K.; Qiu, Z.; Huang, C. PODXL, negatively regulated by KLF4, promotes the EMT and metastasis and serves as a novel prognostic indicator of gastric cancer. Gastric Cancer 2019, 22, 48–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heukamp, L.C.; Fischer, H.P.; Schirmacher, P.; Chen, X.; Breuhahn, K.; Nicolay, C.; Büttner, R.; Gütgemann, I. Podocalyxin-like protein 1 expression in primary hepatic tumours and tumour-like lesions. Histopathology 2006, 49, 242–247. [Google Scholar] [CrossRef]
- Koch, L.K.; Zhou, H.; Ellinger, J.; Biermann, K.; Holler, T.; von Rucker, A.; Buttner, R.; Gutgemann, I. Stem cell marker expression in small cell lung carcinoma and developing lung tissue. Hum. Pathol. 2008, 39, 1597–1605. [Google Scholar] [CrossRef]
- Kusumoto, H.; Shintani, Y.; Kanzaki, R.; Kawamura, T.; Funaki, S.; Minami, M.; Nagatomo, I.; Morii, E.; Okumura, M. Podocalyxin influences malignant potential by controlling epithelial-mesenchymal transition in lung adenocarcinoma. Cancer Sci. 2017, 108, 528–535. [Google Scholar] [CrossRef] [Green Version]
- Itai, S.; Yamada, S.; Kaneko, M.K.; Harada, H.; Kato, Y. Immunohistochemical Analysis Using Antipodocalyxin Monoclonal Antibody PcMab-47 Demonstrates Podocalyxin Expression in Oral Squamous Cell Carcinomas. Monoclon. Antib. Immunodiagn. Immunother. 2017, 36, 220–223. [Google Scholar] [CrossRef]
- Ney, J.T.; Zhou, H.; Sipos, B.; Buttner, R.; Chen, X.; Kloppel, G.; Gutgemann, I. Podocalyxin-like protein 1 expression is useful to differentiate pancreatic ductal adenocarcinomas from adenocarcinomas of the biliary and gastrointestinal tracts. Hum. Pathol. 2007, 38, 359–364. [Google Scholar] [CrossRef]
- Dallas, M.R.; Chen, S.H.; Streppel, M.M.; Sharma, S.; Maitra, A.; Konstantopoulos, K. Sialofucosylated podocalyxin is a functional E- and L-selectin ligand expressed by metastatic pancreatic cancer cells. Am. J. Physiol. Cell Physiol. 2012, 303, C616–C624. [Google Scholar] [CrossRef]
- Heby, M.; Elebro, J.; Nodin, B.; Jirstrom, K.; Eberhard, J. Prognostic and predictive significance of podocalyxin-like protein expression in pancreatic and periampullary adenocarcinoma. BMC Clin. Pathol. 2015, 15, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniuchi, K.; Furihata, M.; Naganuma, S.; Sakaguchi, M.; Saibara, T. Overexpression of PODXL/ITGB1 and BCL7B/ITGB1 accurately predicts unfavorable prognosis compared to the TNM staging system in postoperative pancreatic cancer patients. PLoS ONE 2019, 14, e0217920. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Lin, W.L.; Hou, Y.T.; Pu, Y.S.; Shun, C.T.; Chen, C.L.; Wu, Y.Y.; Chen, J.Y.; Chen, T.H.; Jou, T.S. Podocalyxin EBP50 ezrin molecular complex enhances the metastatic potential of renal cell carcinoma through recruiting Rac1 guanine nucleotide exchange factor ARHGEF7. Am. J. Pathol. 2010, 176, 3050–3061. [Google Scholar] [CrossRef] [PubMed]
- Boman, K.; Larsson, A.H.; Segersten, U.; Kuteeva, E.; Johannesson, H.; Nodin, B.; Eberhard, J.; Uhlen, M.; Malmstrom, P.U.; Jirstrom, K. Membranous expression of podocalyxin-like protein is an independent factor of poor prognosis in urothelial bladder cancer. Br. J. Cancer 2013, 108, 2321–2328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boman, K.; Andersson, G.; Wennersten, C.; Nodin, B.; Ahlgren, G.; Jirstrom, K. Podocalyxin-like and RNA-binding motif protein 3 are prognostic biomarkers in urothelial bladder cancer: A validatory study. Biomark. Res. 2017, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Yasuoka, H.; Tsujimoto, M.; Inagaki, M.; Kodama, R.; Tsuji, H.; Iwahashi, Y.; Mabuchi, Y.; Ino, K.; Sanke, T.; Nakamura, Y. Clinicopathological significance of podocalyxin and phosphorylated ezrin in uterine endometrioid adenocarcinoma. J. Clin. Pathol. 2012, 65, 399–402. [Google Scholar] [CrossRef]
- Taniuchi, K.; Furihata, M.; Naganuma, S.; Dabanaka, K.; Hanazaki, K.; Saibara, T. Podocalyxin-like protein, linked to poor prognosis of pancreatic cancers, promotes cell invasion by binding to gelsolin. Cancer Sci. 2016, 107, 1430–1442. [Google Scholar] [CrossRef]
- Forse, C.L.; Yilmaz, Y.E.; Pinnaduwage, D.; O’Malley, F.P.; Mulligan, A.M.; Bull, S.B.; Andrulis, I.L. Elevated expression of podocalyxin is associated with lymphatic invasion, basal-like phenotype, and clinical outcome in axillary lymph node-negative breast cancer. Breast Cancer Res. Treat. 2013, 137, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Witte, J.S.; Suarez, B.K.; Thiel, B.; Lin, J.; Yu, A.; Banerjee, T.K.; Burmester, J.K.; Casey, G.; Catalona, W.J. Genome-wide scan of brothers: Replication and fine mapping of prostate cancer susceptibility and aggressiveness loci. Prostate 2003, 57, 298–308. [Google Scholar] [CrossRef]
- Paiss, T.; Wörner, S.; Kurtz, F.; Haeussler, J.; Hautmann, R.E.; Gschwend, J.E.; Herkommer, K.; Vogel, W. Linkage of aggressive prostate cancer to chromosome 7q31-33 in German prostate cancer families. Eur. J. Hum. Genet. 2003, 11, 17–22. [Google Scholar] [CrossRef]
- Neville, P.J.; Conti, D.V.; Paris, P.L.; Levin, H.; Catalona, W.J.; Suarez, B.K.; Witte, J.S.; Casey, G. Prostate cancer aggressiveness locus on chromosome 7q32-q33 identified by linkage and allelic imbalance studies. Neoplasia 2002, 4, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Zhi, Q.; Chen, H.; Liu, F.; Han, Y.; Wan, D.; Xu, Z.; Kuang, Y.; Zhou, J. Podocalyxin-like protein promotes gastric cancer progression through interacting with RUN and FYVE domain containing 1 protein. Cancer Sci. 2019, 110, 118–134. [Google Scholar] [CrossRef] [Green Version]
- Saukkonen, K.; Hagstrom, J.; Mustonen, H.; Juuti, A.; Nordling, S.; Fermer, C.; Nilsson, O.; Seppanen, H.; Haglund, C. Podocalyxin Is a Marker of Poor Prognosis in Pancreatic Ductal Adenocarcinoma. PLoS ONE 2015, 10, e0129012. [Google Scholar] [CrossRef] [Green Version]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Ezzati, P.; Wilkins, J.A. Requirement of podocalyxin in TGF-beta induced epithelial mesenchymal transition. PLoS ONE 2011, 6, e18715. [Google Scholar] [CrossRef]
- Lin, C.W.; Sun, M.S.; Liao, M.Y.; Chung, C.H.; Chi, Y.H.; Chiou, L.T.; Yu, J.; Lou, K.L.; Wu, H.C. Podocalyxin-like 1 promotes invadopodia formation and metastasis through activation of Rac1/Cdc42/cortactin signaling in breast cancer cells. Carcinogenesis 2014, 35, 2425–2435. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.W.; Sun, M.S.; Wu, H.C. Podocalyxin-like 1 is associated with tumor aggressiveness and metastatic gene expression in human oral squamous cell carcinoma. Int. J. Oncol. 2014, 45, 710–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, S.N.; Schnaar, R.L.; Konstantopoulos, K. Podocalyxin-like protein is an E-/L-selectin ligand on colon carcinoma cells: Comparative biochemical properties of selectin ligands in host and tumor cells. Am. J. Physiol. Cell Physiol. 2009, 296, C505–C513. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Iioka, H.; Maruyama, S.; Sumardika, I.W.; Sakaguchi, M.; Kondo, E. PODXL1 promotes metastasis of the pancreatic ductal adenocarcinoma by activating the C5aR/C5a axis from the tumor microenvironment. Neoplasia 2019, 21, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Kuo, C.C.; Lin, B.X.; Cheng, C.H.; Chen, K.C.; Lin, C.W. Podocalyxin-Like Protein 1 Regulates TAZ Signaling and Stemness Properties in Colon Cancer. Int. J. Mol. Sci. 2017, 18, 2047. [Google Scholar] [CrossRef] [Green Version]
- Frose, J.; Chen, M.B.; Hebron, K.E.; Reinhardt, F.; Hajal, C.; Zijlstra, A.; Kamm, R.D.; Weinberg, R.A. Epithelial-Mesenchymal Transition Induces Podocalyxin to Promote Extravasation via Ezrin Signaling. Cell Rep. 2018, 24, 962–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Zhang, L.; Pan, H.; Wang, B.; Yan, F.; Fang, X.; Munnee, K.; Tang, Z. Bmi1 essentially mediates podocalyxin-enhanced Cisplatin chemoresistance in oral tongue squamous cell carcinoma. PLoS ONE 2015, 10, e0123208. [Google Scholar]
- Meder, D.; Shevchenko, A.; Simons, K.; Füllekrug, J. Gp135/podocalyxin and NHERF-2 participate in the formation of a preapical domain during polarization of MDCK cells. J. Cell Biol. 2005, 168, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef] [Green Version]
- Hall, A. Rho family GTPases. Biochem. Soc. Trans. 2012, 40, 1378–1382. [Google Scholar] [CrossRef] [Green Version]
- Bustelo, X.R.; Sauzeau, V.; Berenjeno, I.M. GTP-binding proteins of the Rho/Rac family: Regulation, effectors and functions In Vivo. Bioessays 2007, 29, 356–370. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, D.; Horrillo, A.; Alquezar, C.; González-Manchón, C.; Parrilla, R.; Ayuso, M.S. Control of cell adhesion and migration by podocalyxin. Implication of Rac1 and Cdc42. Biochem. Biophys. Res. Commun. 2013, 432, 302–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eddy, R.J.; Weidmann, M.D.; Sharma, V.P.; Condeelis, J.S. Tumor Cell Invadopodia: Invasive Protrusions that Orchestrate Metastasis. Trends Cell Biol. 2017, 27, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Timpson, P.; Horvath, L.G.; Daly, R.J. FAK signaling in human cancer as a target for therapeutics. Pharmacol. Ther. 2015, 146, 132–149. [Google Scholar] [CrossRef]
- De Luca, A.; Maiello, M.R.; D’Alessio, A.; Pergameno, M.; Normanno, N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: Role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin. Ther. Targets 2012, 16 (Suppl. 2), S17–S27. [Google Scholar] [CrossRef]
- O-charoenrat, P.; Wongkajornsilp, A.; Rhys-Evans, P.H.; Eccles, S.A. Signaling pathways required for matrix metalloproteinase-9 induction by betacellulin in head-and-neck squamous carcinoma cells. Int. J. Cancer 2004, 111, 174–183. [Google Scholar] [CrossRef]
- Shapiro, S.D. Matrix metalloproteinase degradation of extracellular matrix: Biological consequences. Curr. Opin. Cell Biol. 1998, 10, 602–608. [Google Scholar] [CrossRef]
- Gautreau, A.; Poullet, P.; Louvard, D.; Arpin, M. Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 7300–7305. [Google Scholar] [CrossRef] [Green Version]
- Chambers, D.N.; Bretscher, A. Ezrin mutants affecting dimerization and activation. Biochemistry 2005, 44, 3926–3932. [Google Scholar] [CrossRef]
- Yang, J.; Kim, O.; Wu, J.; Qiu, Y. Interaction between tyrosine kinase Etk and a RUN domain- and FYVE domain-containing protein RUFY1. A possible role of ETK in regulation of vesicle trafficking. J. Biol. Chem. 2002, 277, 30219–30226. [Google Scholar] [CrossRef] [Green Version]
- Fidler, I.J. The organ microenvironment and cancer metastasis. Differentiation 2002, 70, 498–505. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalluri, R.; Neilson, E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef]
- Chen, X.F.; Zhang, H.J.; Wang, H.B.; Zhu, J.; Zhou, W.Y.; Zhang, H.; Zhao, M.C.; Su, J.M.; Gao, W.; Zhang, L.; et al. Transforming growth factor-beta1 induces epithelial-to-mesenchymal transition in human lung cancer cells via PI3K/Akt and MEK/Erk1/2 signaling pathways. Mol. Biol. Rep. 2012, 39, 3549–3556. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Gao, X.J.; Xu, L.N.; Liu, T.F.; Liu, X.H.; Liu, L.X. Ezrin is required for epithelial-mesenchymal transition induced by TGF-beta1 in A549 cells. Int. J. Oncol. 2014, 45, 1515–1522. [Google Scholar] [CrossRef] [Green Version]
- Hase, K.; Shatney, C.; Johnson, D.; Trollope, M.; Vierra, M. Prognostic value of tumor "budding" in patients with colorectal cancer. Dis. Colon Rectum 1993, 36, 627–635. [Google Scholar] [CrossRef]
- Graves, M.L.; Cipollone, J.A.; Austin, P.; Bell, E.M.; Nielsen, J.S.; Gilks, C.B.; McNagny, K.M.; Roskelley, C.D. The cell surface mucin podocalyxin regulates collective breast tumor budding. Breast Cancer Res. 2016, 18, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, K.A.; Hughes, M.R.; Hedberg, B.; Brandon, J.; Hernaez, D.C.; Bergqvist, P.; Cruz, F.; Po, K.; Graves, M.L.; Turvey, M.E.; et al. Podocalyxin enhances breast tumor growth and metastasis and is a target for monoclonal antibody therapy. Breast Cancer Res. 2015, 17, 46. [Google Scholar] [CrossRef] [Green Version]
- Crum, C.P.; Drapkin, R.; Miron, A.; Ince, T.A.; Muto, M.; Kindelberger, D.W.; Lee, Y. The distal fallopian tube: A new model for pelvic serous carcinogenesis. Curr. Opin. Obstet. Gynecol. 2007, 19, 3–9. [Google Scholar] [CrossRef]
- Friedl, P.; Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2009, 10, 445–457. [Google Scholar] [CrossRef]
- Chou, J.; Stolz, D.B.; Burke, N.A.; Watkins, S.C.; Wells, A. Distribution of gelsolin and phosphoinositol 4,5-bisphosphate in lamellipodia during EGF-induced motility. Int. J. Biochem. Cell Biol. 2002, 34, 776–790. [Google Scholar] [CrossRef]
- Tanabe, K.; Takei, K. Dynamic instability of microtubules requires dynamin 2 and is impaired in a Charcot-Marie-Tooth mutant. J. Cell Biol. 2009, 185, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Dozynkiewicz, M.A.; Jamieson, N.B.; Macpherson, I.; Grindlay, J.; van den Berghe, P.V.; von Thun, A.; Morton, J.P.; Gourley, C.; Timpson, P.; Nixon, C.; et al. Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev. Cell 2012, 22, 131–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yau, C.; Sninsky, J.; Kwok, S.; Wang, A.; Degnim, A.; Ingle, J.N.; Gillett, C.; Tutt, A.; Waldman, F.; Moore, D.; et al. An optimized five-gene multi-platform predictor of hormone receptor negative and triple negative breast cancer metastatic risk. Breast Cancer Res. 2013, 15, R103. [Google Scholar] [CrossRef] [Green Version]
- Supiot, S.; Gouraud, W.; Campion, L.; Jezéquel, P.; Buecher, B.; Charrier, J.; Heymann, M.F.; Mahé, M.A.; Rio, E.; Chérel, M. Early dynamic transcriptomic changes during preoperative radiotherapy in patients with rectal cancer: A feasibility study. World J. Gastroenterol. 2013, 19, 3249–3254. [Google Scholar] [CrossRef] [PubMed]
- Couzens, A.L.; Knight, J.D.; Kean, M.J.; Teo, G.; Weiss, A.; Dunham, W.H.; Lin, Z.Y.; Bagshaw, R.D.; Sicheri, F.; Pawson, T.; et al. Protein interaction network of the mammalian Hippo pathway reveals mechanisms of kinase-phosphatase interactions. Sci. Signal. 2013, 6, rs15. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, Y.; Zhu, Y.; Yuan, C.; Wang, D.; Zhang, W.; Qi, B.; Qiu, J.; Song, X.; Ye, J.; et al. The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol. Oncol. 2015, 9, 1091–1105. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.W.; Lim, C.J.; Guo, K.; Ng, C.P.; Lee, I.; Hunziker, W.; Zeng, Q.; Hong, W. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 2008, 68, 2592–2598. [Google Scholar] [CrossRef] [Green Version]
- Kohn, E.C. Invasion and metastasis: Biology and clinical potential. Pharmacol. Ther. 1991, 52, 235–244. [Google Scholar] [CrossRef]
- Kansas, G.S. Selectins and their ligands: Current concepts and controversies. Blood 1996, 88, 3259–3287. [Google Scholar] [CrossRef] [Green Version]
- McEver, R.P. Selectin-carbohydrate interactions during inflammation and metastasis. Glycoconj. J. 1997, 14, 585–591. [Google Scholar] [CrossRef]
- Brodt, P.; Fallavollita, L.; Bresalier, R.S.; Meterissian, S.; Norton, C.R.; Wolitzky, B.A. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int. J. Cancer 1997, 71, 612–619. [Google Scholar] [CrossRef]
- Elliott, B.E.; Meens, J.A.; SenGupta, S.K.; Louvard, D.; Arpin, M. The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res. 2005, 7, R365–R373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goubran, H.A.; Stakiw, J.; Radosevic, M.; Burnouf, T. Platelet-cancer interactions. Semin. Thromb. Hemost. 2014, 40, 296–305. [Google Scholar]
- Moscoso, I.; Tejados, N.; Barreiro, O.; Sepúlveda, P.; Izarra, A.; Calvo, E.; Dorronsoro, A.; Salcedo, J.M.; Sádaba, R.; Díez-Juan, A.; et al. Podocalyxin-like protein 1 is a relevant marker for human c-kit(pos) cardiac stem cells. J. Tissue Eng. Regen. Med. 2016, 10, 580–590. [Google Scholar] [CrossRef]
- Wu, J.; Lanier, L.L. Natural killer cells and cancer. Adv. Cancer Res. 2003, 90, 127–156. [Google Scholar]
- Li, Z.; Wang, Y.; Yuan, C.; Zhu, Y.; Qiu, J.; Zhang, W.; Qi, B.; Wu, H.; Ye, J.; Jiang, H.; et al. Oncogenic roles of Bmi1 and its therapeutic inhibition by histone deacetylase inhibitor in tongue cancer. Lab. Investig. 2014, 94, 1431–1445. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Huang, Y.; He, H.; Ni, J. Podocalyxin promotes cisplatin chemoresistance in osteosarcoma cells through phosphatidylinositide 3-kinase signaling. Mol. Med. Rep. 2015, 12, 3916–3922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Types of Carcinoma | PODXL Expression | Diagnostic and Prognostic Significance | References |
|---|---|---|---|
| Breast | Overexpressed in a subset of invasive breast carcinomas (6%), and associated with eightfold increase in relative risks of poor outcome (RR: 8.4). Overexpression is predominantly in higher grade, estrogen and progesterone negative, and lymphatic invasive tumours. | Predictor of cancer progression/poor prognosis. Correlated with increased risk of aggressive phenotype, hormone receptor negativity, and high grade | [15,54] |
| Colorectal | Overexpressed in 5–13% of patients. Associated with increased relative risks of death (HR: 1.98–2.0), reduced 5-year overall survival rates (HR: 1.85–2.28), shorter time to recurrence (HR: 2.11–2.93), and disease-free survival (HR: 2.44). Positive expression is also highly concordant between primary and metastatic lesions. | Predictor of metastatic disease and poor prognosis (in subgroups of left hemi-colon and rectum). | [19,55,56,57] |
| Gastric | Positive expression in 36–75% of patients, correlating with advanced tumour stage and metastasis, poor tumour differentiation, reduced time to recurrence (45% vs. 88%), and disease-specific 5-year survival (24% vs. 43%) as well as overall survival rates (40% vs. 55%). | Predictor of metastatic disease, tumour stage, poor differentiation, and prognosis. | [58,59,60,78] |
| Liver (Hepatocellular carcinoma HCC) | Positive expression in 78% of cases. High expression in sinusoidal endothelia and tumour-like lesions but not in normal adjacent tissues. | Diagnostic marker | [54,61] |
| Lung | |||
| Small cell lung carcinoma | Positive expression in 87% of cases. | Diagnostic marker | [62] |
| Lung adenocarcinoma | Positive expression only in invasive lung adenocarcinomas. | Predictor of aggressive and invasive tumours | [63] |
| Esophageal | Positive expression in 85% of patients, associated with shorter time to recurrence (35% vs. 75%) and 5-year overall survival (28% vs. 69%). | Predictor of poor prognosis | [59] |
| Oral squamous cell | Positive expression in 68% of cases | Diagnostic marker to differentiate oral squamous cell carcinoma from other oral cancers | [64] |
| Ovarian | Overexpressed in 87% of high-grade serous carcinomas. Cell surface expression associated with decreases in disease-free survival (HR: 1.73) | Predictor of poor prognosis in high-grade serous subgroup | [14] |
| Pancreatic ductal adenocarcinoma (PDAC) | Positive expression in 34–44% and overexpressed in 29.4% cases of PDAC cases, associated with high-grade tumours. High expression is associated with poor differentiation, perineural and perivascular invasion, and increased relative risks of death (HR: 1.62–2.21). Overexpression of PODXL in combination with ITGB1 associates with poor postoperative outcomes. | Predictor of high-grade tumours, aggressive phenotype and poor prognosis | [65,66,67,68,73,79] |
| Periampullary | Positive PODXL expression in 46% of pancreatobillary- type (PB-type) subgroup, associated with female sex and poor differentiation grade. Positive PODXL expression in 18% of intestinal-type (I-type); associated with reduced recurrence free survival (HR: 2.44) and overall survival (HR: 2.32) | Predictor of poor differentiation in PB-type subgroup. Predictor of aggressive phenotype and poor prognosis in I-type subgroup. | [67] |
| Prostate | PODXL germ-line mutation is associated with increased risks of developing aggressive prostate cancer. | Genetic marker | [16] |
| Renal | Overexpressed in a subset of patients (9.6%), associated with reduced rates of metastasis-free survival (HR: 3.59) and disease-specific survival (HR: 7.46). | Predictor of aggressive phenotype, metastatic disease, and poor prognosis | [69] |
| Thyroid | Identified only in undifferentiated thyroid carcinoma (UTC), and positively expressed in 52% of UTC cases. | Diagnostic marker for UTC | [49] |
| Urothelial (bladder) | Positive expression associated with higher-grade tumours, reduced 5-years of overall survival (HR: 2.05–3.28), disease-specific survival (HR: 2.7), and 2-year progression-free survival (HR: 7.16). | Predictor of high-grade tumours and poor prognosis | [70,71] |
| Uterine endometrioid adenocarcinoma (EA) | Positive expression detected in 36% of uterine EA cases and associated with higher tumour grades. | Predictor of high-grade tumours | [72] |
| Mechanism | Proteins That PODXL Interacts with | Cancer Type |
|---|---|---|
| EMT | Colocalisation and interaction with collagen I, interaction with ezrin, E-cadherin, and vimentin | Lung [63,81] |
| Migration and Invasion | Colocalisation and interaction with gelsolin | Pancreatic [73] |
| Binding to ezrin | Breast [13] | |
| Enhancing the activation of PI3K, Ras/rac1, and MAPK signalling pathway | Pancreatic [18] | |
| Activation of rac1/cdc42/cortactin signalling | Breast [82] | |
| Activation of FAK | Oral squamous cell [83] | |
| Increasing MMP | Breast [13] | |
| Binding to selectins (E- and L-) | Colon [84]Pancreatic [66] | |
| Colocalisation and interaction with CLIC5 | Liver [54] | |
| Binding to dynamin-2 | Pancreatic [18] | |
| Stimulating the C5aR/C5a axis | Pancreatic [85] | |
| Associates with TAZ | Colon [86] | |
| Extravasation from the vasculature | Binding to ezrin | Breast [87] |
| Immune evasion | Inhibiting NK cell receptors Increasing the expression of MHC I molecule (HLA-ABC) | Breast [17] |
| Chemoresistance | Increasing Bmi1 | Oral tongue squamous cell [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Tran, N.; Wang, Y.; Nie, G. Podocalyxin in Normal Tissue and Epithelial Cancer. Cancers 2021, 13, 2863. https://doi.org/10.3390/cancers13122863
Le Tran N, Wang Y, Nie G. Podocalyxin in Normal Tissue and Epithelial Cancer. Cancers. 2021; 13(12):2863. https://doi.org/10.3390/cancers13122863
Chicago/Turabian StyleLe Tran, Ngoc, Yao Wang, and Guiying Nie. 2021. "Podocalyxin in Normal Tissue and Epithelial Cancer" Cancers 13, no. 12: 2863. https://doi.org/10.3390/cancers13122863
APA StyleLe Tran, N., Wang, Y., & Nie, G. (2021). Podocalyxin in Normal Tissue and Epithelial Cancer. Cancers, 13(12), 2863. https://doi.org/10.3390/cancers13122863






