Integrative Transcriptomic Analysis Reveals Distinctive Molecular Traits and Novel Subtypes of Collecting Duct Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Collection of the INT-CDC Cohort
2.2. RNA Extraction and Microarray Profiling
2.3. Retrieval of Public Gene Expression Data
2.4. Data Preprocessing
2.5. Differential Expression Analysis
2.6. Functional Enrichment Analysis and Visualization
2.7. Meta-Analysis of Transcriptomic Datasets and Single-Sample Scoring
2.8. Cell of Origin Analysis
2.9. Unsupervised Analysis of CDC Tumors
2.10. Prioritization of Anticancer Drugs
2.11. Survival Analysis
3. Results
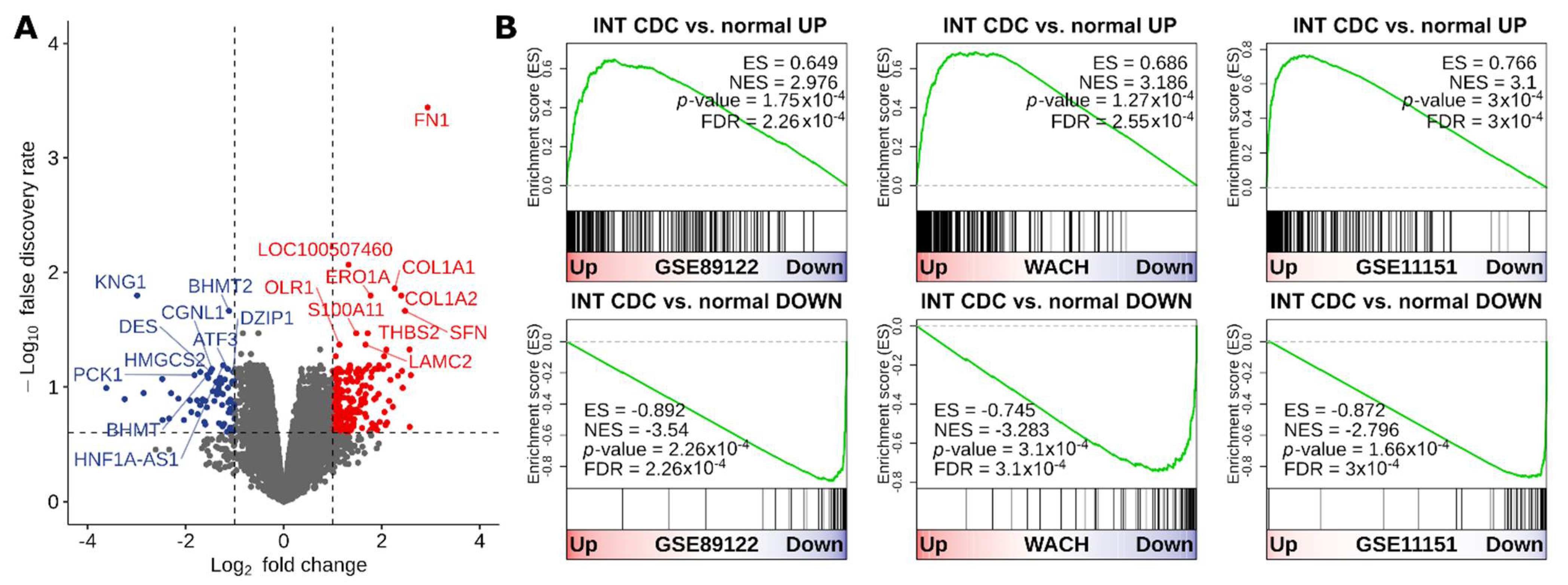
3.1. Transcriptional Divergence of CDC Compared to Normal Kidney and Definition of a Gene Signature Highly Expressed in CDC
3.2. CDC Transcriptional Program Defines Putative Active Drugs
3.3. CDC Arises from the Principal Cells of Collecting Ducts
3.4. CDC Shows Unique Pathway Enrichments
3.5. CDC Shows Intertumor Heterogeneity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Ciszewski, S.; Jakimów, A.; Smolska-Ciszewska, B. Collecting (Bellini) Duct Carcinoma: A Clinical Study of a Rare Tumour and Review of the Literature. Can. Urol. Assoc. J. 2015, 9, E589–E593. [Google Scholar] [CrossRef]
- Gupta, R.; Billis, A.; Shah, R.B.; Moch, H.; Osunkoya, A.O.; Jochum, W.; Hes, O.; Bacchi, C.E.; de Castro, M.G.; Hansel, D.E.; et al. Carcinoma of the Collecting Ducts of Bellini and Renal Medullary Carcinoma: Clinicopathologic Analysis of 52 Cases of Rare Aggressive Subtypes of Renal Cell Carcinoma With a Focus on Their Interrelationship. Am. J. Surg. Pathol. 2012, 36, 1265–1278. [Google Scholar] [CrossRef]
- Srigley, J.R.; Delahunt, B. Uncommon and Recently Described Renal Carcinomas. Mod. Pathol. 2009, 22, S2–S23. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Logothetis, C.J.; Markowitz, A.; Sella, A.; Amato, R.; Ro, J. Collecting Duct Carcinoma of the Kidney. Br. J. Urol. 1993, 71, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Levin, H.S.; Myles, J.L. The Pathology of Renal Neoplasms. In Renal Cell Carcinoma: Molecular Biology, Immunology, and Clinical Management; Bukowski, R.M., Novick, A.C., Eds.; Current Clinical Oncology; Humana Press: Totowa, NJ, USA, 2000; pp. 15–38. ISBN 978-1-59259-229-6. [Google Scholar]
- Oudard, S.; Banu, E.; Vieillefond, A.; Fournier, L.; Priou, F.; Medioni, J.; Banu, A.; Duclos, B.; Rolland, F.; Escudier, B.; et al. Prospective Multicenter Phase II Study of Gemcitabine Plus Platinum Salt for Metastatic Collecting Duct Carcinoma: Results of a GETUG (Groupe d’Etudes Des Tumeurs Uro-Génitales) Study. J. Urol. 2007, 177, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Horie, K.; Nagai, S.; Tsuchiya, T.; Saigo, C.; Kobayashi, K.; Miyazaki, T.; Deguchi, T. Response to Nivolumab in Metastatic Collecting Duct Carcinoma Expressing PD-L1: A Case Report. Mol. Clin. Oncol. 2017, 7, 988–990. [Google Scholar] [CrossRef]
- Yasuoka, S.; Hamasaki, T.; Kuribayashi, E.; Nagasawa, M.; Kawaguchi, T.; Nagashima, Y.; Kondo, Y. Nivolumab Therapy for Metastatic Collecting Duct Carcinoma after Nephrectomy: A Case Report. Medicine 2018, 97, e13173. [Google Scholar] [CrossRef]
- Rimar, K.J.; Meeks, J.J.; Kuzel, T.M. Anti-Programmed Death Receptor 1 Blockade Induces Clinical Response in a Patient with Metastatic Collecting Duct Carcinoma. Clin. Genitourin. Cancer 2016, 14, e431–e434. [Google Scholar] [CrossRef]
- Pal, S.K.; Choueiri, T.K.; Wang, K.; Khaira, D.; Karam, J.A.; Van Allen, E.; Palma, N.A.; Stein, M.N.; Johnson, A.; Squillace, R.; et al. Characterization of Clinical Cases of Collecting Duct Carcinoma of the Kidney Assessed by Comprehensive Genomic Profiling. Eur. Urol. 2016, 70, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Papanicolau-Sengos, A.; Chintala, S.; Wei, L.; Liu, B.; Hu, Q.; Miles, K.M.; Conroy, J.M.; Glenn, S.T.; Costantini, M.; et al. Collecting Duct Carcinoma of the Kidney Is Associated with CDKN2A Deletion and SLC Family Gene Up-Regulation. Oncotarget 2016, 7, 29901–29915. [Google Scholar] [CrossRef]
- Creighton, C.J.; Morgan, M.; Gunaratne, P.H.; Wheeler, D.A.; Gibbs, R.A.; Gordon Robertson, A.; Chu, A.; Beroukhim, R.; Cibulskis, K.; Signoretti, S.; et al. Comprehensive Molecular Characterization of Clear Cell Renal Cell Carcinoma. Nature 2013, 499, 43–49. [Google Scholar] [CrossRef]
- Wach, S.; Taubert, H.; Weigelt, K.; Hase, N.; Köhn, M.; Misiak, D.; Hüttelmaier, S.; Stöhr, C.G.; Kahlmeyer, A.; Haller, F.; et al. RNA Sequencing of Collecting Duct Renal Cell Carcinoma Suggests an Interaction between MiRNA and Target Genes and a Predominance of Deregulated Solute Carrier Genes. Cancers 2020, 12, 64. [Google Scholar] [CrossRef]
- Malouf, G.G.; Compérat, E.; Yao, H.; Mouawad, R.; Lindner, V.; Rioux-leclercq, N.; Verkarre, V.; Leroy, X.; Dainese, L.; Classe, M.; et al. Unique Transcriptomic Profile of Collecting Duct Carcinomas Relative to Upper Tract Urothelial Carcinomas and Other Kidney Carcinomas. Sci. Rep. 2016, 6, 30988. [Google Scholar] [CrossRef]
- Srigley, J.R.; Delahunt, B.; Eble, J.N.; Egevad, L.; Epstein, J.I.; Grignon, D.; Hes, O.; Moch, H.; Montironi, R.; Tickoo, S.K.; et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am. J. Surg. Pathol. 2013, 37, 1469–1489. [Google Scholar] [CrossRef]
- Yusenko, M.V.; Kuiper, R.P.; Boethe, T.; Ljungberg, B.; van Kessel, A.G.; Kovacs, G. High-Resolution DNA Copy Number and Gene Expression Analyses Distinguish Chromophobe Renal Cell Carcinomas and Renal Oncocytomas. BMC Cancer 2009, 9, 152. [Google Scholar] [CrossRef]
- Msaouel, P.; Malouf, G.G.; Su, X.; Yao, H.; Tripathi, D.N.; Soeung, M.; Gao, J.; Rao, P.; Coarfa, C.; Creighton, C.J.; et al. Comprehensive Molecular Characterization Identifies Distinct Genomic and Immune Hallmarks of Renal Medullary Carcinoma. Cancer Cell 2020, 37, 720–734.e13. [Google Scholar] [CrossRef]
- Colaprico, A.; Silva, T.C.; Olsen, C.; Garofano, L.; Cava, C.; Garolini, D.; Sabedot, T.S.; Malta, T.M.; Pagnotta, S.M.; Castiglioni, I.; et al. TCGAbiolinks: An R/Bioconductor Package for Integrative Analysis of TCGA Data. Nucleic Acids Res. 2016, 44, e71. [Google Scholar] [CrossRef]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416.e11. [Google Scholar] [CrossRef]
- Zhang, J.; Baran, J.; Cros, A.; Guberman, J.M.; Haider, S.; Hsu, J.; Liang, Y.; Rivkin, E.; Wang, J.; Whitty, B.; et al. International Cancer Genome Consortium Data Portal—A One-Stop Shop for Cancer Genomics Data. Database 2011, 2011. [Google Scholar] [CrossRef]
- Robinson, B.D.; Vlachostergios, P.J.; Bhinder, B.; Liu, W.; Li, K.; Moss, T.J.; Bareja, R.; Park, K.; Tavassoli, P.; Cyrta, J.; et al. Upper Tract Urothelial Carcinoma Has a Luminal-Papillary T-Cell Depleted Contexture and Activated FGFR3 Signaling. Nat. Commun. 2019, 10, 2977. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Disccv. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia Enables Predictive Modelling of Anticancer Drug Sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef]
- Seashore-Ludlow, B.; Rees, M.G.; Cheah, J.H.; Cokol, M.; Price, E.V.; Coletti, M.E.; Jones, V.; Bodycombe, N.E.; Soule, C.K.; Gould, J.; et al. Harnessing Connectivity in a Large-Scale Small-Molecule Sensitivity Dataset. Cancer Discov. 2015, 5, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Athar, A.; Füllgrabe, A.; George, N.; Iqbal, H.; Huerta, L.; Ali, A.; Snow, C.; Fonseca, N.A.; Petryszak, R.; Papatheodorou, I.; et al. ArrayExpress Update-from Bulk to Single-Cell Expression Data. Nucleic Acids Res. 2019, 47, D711–D715. [Google Scholar] [CrossRef]
- Iorio, F.; Knijnenburg, T.A.; Vis, D.J.; Bignell, G.R.; Menden, M.P.; Schubert, M.; Aben, N.; Gonçalves, E.; Barthorpe, S.; Lightfoot, H.; et al. A Landscape of Pharmacogenomic Interactions in Cancer. Cell 2016, 166, 740–754. [Google Scholar] [CrossRef]
- Genomics of Drug Sensitivity in Cancer. Available online: https://www.cancerrxgene.org/downloads/bulk_download. (accessed on 14 October 2020).
- McCall, M.N.; Bolstad, B.M.; Irizarry, R.A. Frozen Robust Multiarray Analysis (FRMA). Biostatistics 2010, 11, 242–253. [Google Scholar] [CrossRef]
- Huber, W.; Carey, V.J.; Gentleman, R.; Anders, S.; Carlson, M.; Carvalho, B.S.; Bravo, H.C.; Davis, S.; Gatto, L.; Girke, T.; et al. Orchestrating High-Throughput Genomic Analysis with Bioconductor. Nat. Methods 2015, 12, 115–121. [Google Scholar] [CrossRef]
- Irizarry, R.A.; Hobbs, B.; Collin, F.; Beazer-Barclay, Y.D.; Antonellis, K.J.; Scherf, U.; Speed, T.P. Exploration, Normalization, and Summaries of High Density Oligonucleotide Array Probe Level Data. Biostatistics 2003, 4, 249–264. [Google Scholar] [CrossRef]
- Carvalho, B.S.; Irizarry, R.A. A Framework for Oligonucleotide Microarray Preprocessing. Bioinformatics 2010, 26, 2363–2367. [Google Scholar] [CrossRef]
- Du, P.; Kibbe, W.A.; Lin, S.M. Lumi: A Pipeline for Processing Illumina Microarray. Bioinformatics 2008, 24, 1547–1548. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Cai, C.; Langfelder, P.; Geschwind, D.H.; Kurian, S.M.; Salomon, D.R.; Horvath, S. Strategies for Aggregating Gene Expression Data: The CollapseRows R Function. BMC Bioinform. 2011, 12, 322. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Oshlack, A. A Scaling Normalization Method for Differential Expression Analysis of RNA-Seq Data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Stuart, T.; Butler, A.; Hoffman, P.; Hafemeister, C.; Papalexi, E.; Mauck, W.M.; Hao, Y.; Stoeckius, M.; Smibert, P.; Satija, R. Comprehensive Integration of Single-Cell Data. Cell 2019, 177, 1888–1902.e21. [Google Scholar] [CrossRef]
- Korsunsky, I.; Millard, N.; Fan, J.; Slowikowski, K.; Zhang, F.; Wei, K.; Baglaenko, Y.; Brenner, M.; Loh, P.; Raychaudhuri, S. Fast, Sensitive and Accurate Integration of Single-Cell Data with Harmony. Nat. Methods 2019, 16, 1289–1296. [Google Scholar] [CrossRef]
- Liao, J.; Yu, Z.; Chen, Y.; Bao, M.; Zou, C.; Zhang, H.; Liu, D.; Li, T.; Zhang, Q.; Li, J.; et al. Single-Cell RNA Sequencing of Human Kidney. Sci. Data 2020, 7, 4. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. Voom: Precision Weights Unlock Linear Model Analysis Tools for RNA-Seq Read Counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Society Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A Web Server for Functional Enrichment Analysis and Conversions of Gene Lists (2019 Update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Collins, J.R.; Alvord, W.G.; Roayaei, J.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. The DAVID Gene Functional Classification Tool: A Novel Biological Module-Centric Algorithm to Functionally Analyze Large Gene Lists. Genome Biol. 2007, 8, R183. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Su, G.; Kuchinsky, A.; Morris, J.H.; States, D.J.; Meng, F. GLay: Community Structure Analysis of Biological Networks. Bioinformatics 2010, 26, 3135–3137. [Google Scholar] [CrossRef]
- HUGO Gene Nomenclature Committee. Available online: https://www.genenames.org. (accessed on 4 November 2020).
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular Signatures Database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A.; et al. Inferring Tumour Purity and Stromal and Immune Cell Admixture from Expression Data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef]
- Foroutan, M.; Bhuva, D.D.; Lyu, R.; Horan, K.; Cursons, J.; Davis, M.J. Single Sample Scoring of Molecular Phenotypes. BMC Bioinform. 2018, 19, 404. [Google Scholar] [CrossRef]
- Aibar, S.; González-Blas, C.B.; Moerman, T.; Huynh-Thu, V.A.; Imrichova, H.; Hulselmans, G.; Rambow, F.; Marine, J.-C.; Geurts, P.; Aerts, J.; et al. SCENIC: Single-Cell Regulatory Network Inference and Clustering. Nat. Methods 2017, 14, 1083–1086. [Google Scholar] [CrossRef]
- Hoshida, Y.; Brunet, J.-P.; Tamayo, P.; Golub, T.R.; Mesirov, J.P. Subclass Mapping: Identifying Common Subtypes in Independent Disease Data Sets. PLoS ONE 2007, 2, e1195. [Google Scholar] [CrossRef]
- Reich, M.; Liefeld, T.; Gould, J.; Lerner, J.; Tamayo, P.; Mesirov, J.P. GenePattern 2.0. Nat. Genet. 2006, 38, 500–501. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1505917 (accessed on 12 April 2021).
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017, 171, 1437–1452.e17. [Google Scholar] [CrossRef]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ–Related MRNA Profile Predicts Clinical Response to PD-1 Blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef]
- Danaher, P.; Warren, S.; Lu, R.; Samayoa, J.; Sullivan, A.; Pekker, I.; Wallden, B.; Marincola, F.M.; Cesano, A. Pan-Cancer Adaptive Immune Resistance as Defined by the Tumor Inflammation Signature (TIS): Results from the Cancer Genome Atlas (TCGA). J. ImmunoTherapy Cancer 2018, 6, 63. [Google Scholar] [CrossRef]
- Damotte, D.; Warren, S.; Arrondeau, J.; Boudou-Rouquette, P.; Mansuet-Lupo, A.; Biton, J.; Ouakrim, H.; Alifano, M.; Gervais, C.; Bellesoeur, A.; et al. The Tumor Inflammation Signature (TIS) Is Associated with Anti-PD-1 Treatment Benefit in the CERTIM Pan-Cancer Cohort. J. Transl. Med. 2019, 17, 357. [Google Scholar] [CrossRef]
- Ohe, C.; Smith, S.C.; Sirohi, D.; Divatia, M.; de Peralta-Venturina, M.; Paner, G.P.; Agaimy, A.; Amin, M.B.; Argani, P.; Chen, Y.-B.; et al. Reappraisal of Morphologic Differences between Renal Medullary Carcinoma, Collecting Duct Carcinoma, and Fumarate Hydratase–Deficient Renal Cell Carcinoma. Am. J. Surg. Pathol. 2018, 42, 279–292. [Google Scholar] [CrossRef]
- Pagani, F.; Colecchia, M.; Sepe, P.; Apollonio, G.; Claps, M.; Verzoni, E.; de Braud, F.; Procopio, G. Collecting Ducts Carcinoma: An Orphan Disease. Literature Overview and Future Perspectives. Cancer Treat. Rev. 2019, 79, 101891. [Google Scholar] [CrossRef]
- Ji, X.; Qian, J.; Rahman, S.M.J.; Siska, P.J.; Zou, Y.; Harris, B.K.; Hoeksema, M.D.; Trenary, I.A.; Heidi, C.; Eisenberg, R.; et al. XCT (SLC7A11)-Mediated Metabolic Reprogramming Promotes Non-Small Cell Lung Cancer Progression. Oncogene 2018, 37, 5007–5019. [Google Scholar] [CrossRef]
- Drayton, R.M.; Dudziec, E.; Peter, S.; Bertz, S.; Hartmann, A.; Bryant, H.E.; Catto, J.W. Reduced Expression of MiRNA-27a Modulates Cisplatin Resistance in Bladder Cancer by Targeting the Cystine/Glutamate Exchanger SLC7A11. Clin. Cancer Res. 2014, 20, 1990–2000. [Google Scholar] [CrossRef]
- Liu, C.; Gong, X.; Zhang, S.; Shi, W.; Xie, F.; Wang, A.; Zhao, Z.; Tan, M.; Zhang, P.; Du, P.; et al. PT321-Comprehensive Molecular Characterization of Clear Cell Renal Cell Carcinoma with Caval Tumour Thrombus. Eur. Urol. Suppl. 2019, 18, e2100. [Google Scholar] [CrossRef]
- Schaller, L.; Lauschke, V.M. The Genetic Landscape of the Human Solute Carrier (SLC) Transporter Superfamily. Hum. Genet. 2019, 138, 1359–1377. [Google Scholar] [CrossRef]
- Higuchi, K.; Sakamoto, S.; Ando, K.; Maimaiti, M.; Takeshita, N.; Okunushi, K.; Reien, Y.; Imamura, Y.; Sazuka, T.; Nakamura, K.; et al. Characterization of the Expression of LAT1 as a Prognostic Indicator and a Therapeutic Target in Renal Cell Carcinoma. Sci. Rep. 2019, 9, 16776. [Google Scholar] [CrossRef]
- He, L.; Vasiliou, K.; Nebert, D.W. Analysis and Update of the Human Solute Carrier (SLC) Gene Superfamily. Hum. Genom. 2009, 3, 195. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Babu, E.; Ramachandran, S.; Yang, S.; Thangaraju, M.; Ganapathy, V. SLC Transporters as a Novel Class of Tumour Suppressors: Identity, Function and Molecular Mechanisms. Biochem. J. 2016, 473, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Dizman, N.; Philip, E.J.; Pal, S.K. Genomic Profiling in Renal Cell Carcinoma. Nat. Rev. Nephrol. 2020, 16, 435–451. [Google Scholar] [CrossRef]
- Cheval, L.; Pierrat, F.; Rajerison, R.; Piquemal, D.; Doucet, A. Of Mice and Men: Divergence of Gene Expression Patterns in Kidney. PLoS ONE 2012, 7, e46876. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, Y. Approaches of Targeting Rho GTPases in Cancer Drug Discovery. Expert Opin. Drug Discov. 2015, 10, 991–1010. [Google Scholar] [CrossRef]
- Bai, L.; Yang, J.C.; Ok, J.; Mack, P.C.; Kung, H.-J.; Evans, C.P. Simultaneous Targeting of Src Kinase and Receptor Tyrosine Kinase Results in Synergistic Inhibition of Renal Cell Carcinoma Proliferation and Migration. Int. J. Cancer 2012, 130, 2693–2702. [Google Scholar] [CrossRef]
- Suwaki, N.; Vanhecke, E.; Atkins, K.M.; Graf, M.; Swabey, K.; Huang, P.; Schraml, P.; Moch, H.; Cassidy, A.M.; Brewer, D.; et al. A HIF-Regulated VHL-PTP1B-Src Signaling Axis Identifies a Therapeutic Target in Renal Cell Carcinoma. Sci. Transl. Med. 2011, 3, 85ra47. [Google Scholar] [CrossRef]
- Miyake, H.; Haraguchi, T.; Takenaka, A.; Fujisawa, M. Metastatic Collecting Duct Carcinoma of the Kidney Responded to Sunitinib. Int. J. Clin. Oncol. 2011, 16, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Mennitto, A.; Verzoni, E.; Peverelli, G.; Alessi, A.; Procopio, G. Management of Metastatic Collecting Duct Carcinoma: An Encouraging Result in a Patient Treated with Cabozantinib. Clin. Genitourin. Cancer 2018, 16, e521–e523. [Google Scholar] [CrossRef]
- Ansari, J.; Fatima, A.; Chaudhri, S.; Bhatt, R.I.; Wallace, M.; James, N.D. Sorafenib Induces Therapeutic Response in a Patient with Metastatic Collecting Duct Carcinoma of Kidney. Oncol. Res. Treat. 2009, 32, 44–46. [Google Scholar] [CrossRef]
- Bronchud, M.H.; Castillo, S.; de Romaní, S.E.; Mourelo, S.; Fernández, A.; Baena, C.; Murillo, J.; Julia, J.C.; Esquius, J.; Romero, R.; et al. HER2 Blockade in Metastatic Collecting Duct Carcinoma (CDC) of the Kidney: A Case Report. Oncol. Res. Treat. 2012, 35, 776–779. [Google Scholar] [CrossRef]
- Procopio, G.; Testa, I.; Iacovelli, R.; Grassi, P.; Verzoni, E.; Garanzini, E.; Colecchia, M.; Torelli, T.; Braud, F.D. Treatment of Collecting Duct Carcinoma: Current Status and Future Perspectives. Anticancer Res. 2014, 34, 1027–1030. [Google Scholar]
- Sheng, X.; Cao, D.; Yuan, J.; Zhou, F.; Wei, Q.; Xie, X.; Cui, C.; Chi, Z.; Si, L.; Li, S.; et al. Sorafenib in Combination with Gemcitabine plus Cisplatin Chemotherapy in Metastatic Renal Collecting Duct Carcinoma: A Prospective, Multicentre, Single-Arm, Phase 2 Study. Eur. J. Cancer 2018, 100, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tannir, N.M.; Plimack, E.; Ng, C.; Tamboli, P.; Bekele, N.B.; Xiao, L.; Smith, L.; Lim, Z.; Pagliaro, L.; Araujo, J.; et al. A Phase 2 Trial of Sunitinib in Patients with Advanced Non–Clear Cell Renal Cell Carcinoma. Eur. Urol. 2012, 62, 1013–1019. [Google Scholar] [CrossRef]
- Litchfield, K.; Reading, J.L.; Puttick, C.; Thakkar, K.; Abbosh, C.; Bentham, R.; Watkins, T.B.K.; Rosenthal, R.; Biswas, D.; Rowan, A.; et al. Meta-Analysis of Tumor- and T Cell-Intrinsic Mechanisms of Sensitization to Checkpoint Inhibition. Cell 2021, 184, 596–614.e14. [Google Scholar] [CrossRef]






| Patient ID | Age | Sex | Histology | Matched Normal | Nephrectomy | Tumor Specimen Site | Treatment-Naïve Specimen | Vital Status | Overall Survival (Months) * |
|---|---|---|---|---|---|---|---|---|---|
| PG-2 | 67 | F | CDC | No | Yes | Metastasis (soft tissue) | No | Deceased | 54.6 |
| PG-3 | 64 | F | CDC | Yes | Yes | Primary tumor | Yes | Deceased | 3.3 |
| PG-4 | 43 | M | CDC | No | Yes | Primary tumor | Yes | Deceased | 7.7 |
| PG-5 | 36 | F | ccRCC | No | Yes | Primary tumor | Yes | Alive | 228.1 |
| PG-6 | 43 | M | CDC | No | No | Primary tumor | Yes | Deceased | 7.9 |
| PG-7 | 57 | M | ccRCC | Yes | Yes | Primary tumor | Yes | Deceased | 7.4 |
| PG-8 | 71 | M | ccRCC | Yes | Yes | Primary tumor | Yes | Deceased | 47.2 |
| PG-9 | 33 | F | ccRCC | Yes | Yes | Primary tumor | Yes | Deceased | 58.3 |
| PG-14 | 71 | F | ccRCC | No | Yes | Primary tumor | Yes | Alive | 40.9 |
| PG-15 | 46 | F | CDC | No | Yes | Primary tumor | Yes | Deceased | 6.7 |
| PG-16 | 36 | F | CDC | No | Yes | Primary tumor | Yes | Deceased | 24.0 |
| Authors | Study Label | Reference | CDC (n) | ccRCC (n) | Chromophobe (n) | Oncocytoma (n) | Papillary (n) | Normal Kidney (n) |
|---|---|---|---|---|---|---|---|---|
| Gargiuli C et al. | INT-CDC | This paper | 6 | 5 | 0 | 0 | 0 | 4 |
| Wang J et al. | GSE89122 | [12] | 7 | 0 | 0 | 0 | 0 | 6 |
| Yusenko MV et al. | GSE11151 | [17] | 2 | 26 | 4 | 4 | 19 | 5 |
| Wach S et al. | WACH | [14] | 2 | 0 | 0 | 0 | 0 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gargiuli, C.; Sepe, P.; Tessari, A.; Sheetz, T.; Colecchia, M.; de Braud, F.G.M.; Procopio, G.; Sensi, M.; Verzoni, E.; Dugo, M. Integrative Transcriptomic Analysis Reveals Distinctive Molecular Traits and Novel Subtypes of Collecting Duct Carcinoma. Cancers 2021, 13, 2903. https://doi.org/10.3390/cancers13122903
Gargiuli C, Sepe P, Tessari A, Sheetz T, Colecchia M, de Braud FGM, Procopio G, Sensi M, Verzoni E, Dugo M. Integrative Transcriptomic Analysis Reveals Distinctive Molecular Traits and Novel Subtypes of Collecting Duct Carcinoma. Cancers. 2021; 13(12):2903. https://doi.org/10.3390/cancers13122903
Chicago/Turabian StyleGargiuli, Chiara, Pierangela Sepe, Anna Tessari, Tyler Sheetz, Maurizio Colecchia, Filippo Guglielmo Maria de Braud, Giuseppe Procopio, Marialuisa Sensi, Elena Verzoni, and Matteo Dugo. 2021. "Integrative Transcriptomic Analysis Reveals Distinctive Molecular Traits and Novel Subtypes of Collecting Duct Carcinoma" Cancers 13, no. 12: 2903. https://doi.org/10.3390/cancers13122903
APA StyleGargiuli, C., Sepe, P., Tessari, A., Sheetz, T., Colecchia, M., de Braud, F. G. M., Procopio, G., Sensi, M., Verzoni, E., & Dugo, M. (2021). Integrative Transcriptomic Analysis Reveals Distinctive Molecular Traits and Novel Subtypes of Collecting Duct Carcinoma. Cancers, 13(12), 2903. https://doi.org/10.3390/cancers13122903






