Pulmonary Toxicity after Total Body Irradiation—An Underrated Complication? Estimation of Risk via Normal Tissue Complication Probability Calculations and Correlation with Clinical Data
Abstract
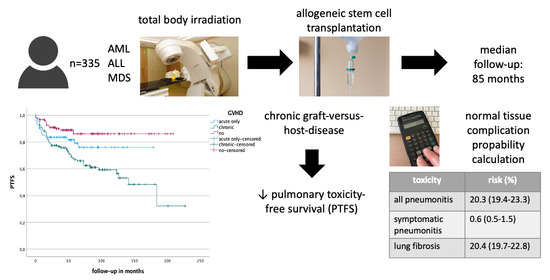
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Data
2.2. Statistical Analysis
2.3. Planning
2.4. Radiation Technique
2.5. NTCP Calculation and Replanning
3. Results
NTCP Calculation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, J.Y.C.; Filippi, A.R.; Dabaja, B.S.; Yahalom, J.; Specht, L. Total Body Irradiation: Guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.Y.C.; Filippi, A.R.; Scorsetti, M.; Hui, S.; Muren, L.P.; Mancosu, P. Total Marrow and Total Lymphoid Irradiation in Bone Marrow Transplantation for Acute Leukaemia. Lancet Oncol. 2020, 21, e477–e487. [Google Scholar] [CrossRef]
- Hoeller, U.; Borgmann, K.; Oertel, M.; Haverkamp, U.; Budach, V.; Eich, H.T. Late Sequelae of Radiotherapy—The Effect of Technical and Conceptual Innovations in Radiation Oncology. Dtsch. Aerzteblatt Online 2021, 118, 205–212. [Google Scholar] [CrossRef]
- Türkkan, G.; Willems, Y.; Hendriks, L.E.L.; Mostard, R.; Conemans, L.; Gietema, H.A.; Mitea, C.; Peeters, S.; De Ruysscher, D. Idiopathic Pulmonary Fibrosis: Current Knowledge, Future Perspectives and Its Importance in Radiation Oncology. Radiother. Oncol. 2021, 155, 269–277. [Google Scholar] [CrossRef]
- Hanania, A.N.; Mainwaring, W.; Ghebre, Y.T.; Hanania, N.A.; Ludwig, M. Radiation-Induced Lung Injury: Assessment and Management. Chest 2019, 156, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.B.; Bentzen, S.M.; Deasy, J.O.; Kong, F.-M.S.; Bradley, J.D.; Vogelius, I.S.; El Naqa, I.; Hubbs, J.L.; Lebesque, J.V.; Timmerman, R.D.; et al. Radiation Dose–Volume Effects in the Lung. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S70–S76. [Google Scholar] [CrossRef] [Green Version]
- Barrett, A.; Depledge, M.H.; Powles, R.L. Interstitial Pneumonitis Following Bone Marrow Transplantation after Low Dose Rate Total Body Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1983, 9, 1029–1033. [Google Scholar] [CrossRef]
- Cosset, J.M.; Baume, D.; Pico, J.L.; Shank, B.; Girinski, T.; Benhamou, E.; Briot, E.; Malaise, E.; Hayat, M.; Dutreix, J. Single Dose versus Hyperfractionated Total Body Irradiation before Allogeneic Bone Marrow Transplantation: A Non-Randomized Comparative Study of 54 Patients at the Institut Gustave-Roussy. Radiother. Oncol. 1989, 15, 151–160. [Google Scholar] [CrossRef]
- Latini, P.; Aristei, C.; Aversa, F.; Checcaglini, F.; Maranzano, E.; Panizza, B.M.; Perrucci, E.; Carotti, A.; Martelli, M.F. Interstitial Pneumonitis after Hyperfractionated Total Body Irradiation in HLA-Matched T-Depleted Bone Marrow Transplantation. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 401–405. [Google Scholar] [CrossRef]
- Latini, P.; Aristei, C.; Aversa, F.; Checcaglini, F.; Maranzano, E.; Raymondi, C.; Panizza, B.M.; Perrucci, E.; Martelli, M.F. Lung Damage Following Bone Marrow Transplantation after Hyperfractionated Total Body Irradiation. Radiother. Oncol. 1991, 22, 127–132. [Google Scholar] [CrossRef]
- Socie, G.; Devergie, A.; Girinsky, T.; Reiffers, J.; Vernant, J.P.; Le Bourgeois, J.P.; Herve, P.; Guyotat, D.; Maraninchi, D.; Rio, B. Influence of the Fractionation of Total Body Irradiation on Complications and Relapse Rate for Chronic Myelogenous Leukemia. The Groupe d’Etude Des Greffes de Moelle Osseuse (GEGMO). Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 397–404. [Google Scholar] [CrossRef]
- Chiang, Y.; Tsai, C.-H.; Kuo, S.-H.; Liu, C.-Y.; Yao, M.; Li, C.-C.; Huang, S.-Y.; Ko, B.-S.; Lin, C.-T.; Hou, H.-A.; et al. Reduced Incidence of Interstitial Pneumonitis after Allogeneic Hematopoietic Stem Cell Transplantation Using a Modified Technique of Total Body Irradiation. Sci. Rep. 2016, 6, 36730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oya, N.; Sasai, K.; Tachiiri, S.; Sakamoto, T.; Nagata, Y.; Okada, T.; Yano, S.; Ishikawa, T.; Uchiyama, T.; Hiraoka, M. Influence of Radiation Dose Rate and Lung Dose on Interstitial Pneumonitis after Fractionated Total Body Irradiation: Acute Parotitis May Predict Interstitial Pneumonitis. Int. J. Hematol. 2006, 83, 86–91. [Google Scholar] [CrossRef]
- Carruthers, S.A.; Wallington, M.M. Total Body Irradiation and Pneumonitis Risk: A Review of Outcomes. Br. J. Cancer 2004, 90, 2080–2084. [Google Scholar] [CrossRef] [PubMed]
- Gogna, N.K.; Morgan, G.; Downs, K.; Atkinson, K.; Biggs, J. Lung Dose Rate and Interstitial Pneumonitis in Total Body Irradiation for Bone Marrow Transplantation. Australas. Radiol. 1992, 36, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Pepper, N.B.; Oertel, M.; Kittel, C.; Kröger, K.J.; Elsayad, K.; Haverkamp, U.; Eich, H.T. Impact of Radiation Techniques on Lung Toxicity in Patients with Mediastinal Hodgkin’s Lymphoma. Strahlenther. Onkol. 2020. [Google Scholar] [CrossRef]
- Reinartz, G.; Baehr, A.; Kittel, C.; Oertel, M.; Haverkamp, U.; Eich, H.T. Biophysical Analysis of Acute and Late Toxicity of Radiotherapy in Gastric Marginal Zone Lymphoma—Impact of Radiation Dose and Planning Target Volume. Cancers 2021, 13, 1390. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 25 April 2021).
- Lyman, J.T. Complication Probability as Assessed from Dose-Volume Histograms. Radiat. Res. Suppl. 1985, 8, S13–S19. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, V. Dose–Volume Analysis of Lung Complications in the Radiation Treatment of Malignant Thymoma: A Retrospective Review. Radiother. Oncol. 2003, 67, 265–274. [Google Scholar] [CrossRef]
- Lawton, C.A.; Barber-Derus, S.; Murray, K.J.; Casper, J.T.; Ash, R.C.; Gillin, M.T.; Frank Wilson, J. Technical Modifications in Hyperfractionated Total Body Irradiation for T-Lymphocyte Deplete Bone Marrow Transplant. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 319–322. [Google Scholar] [CrossRef]
- Byun, H.K.; Yoon, H.I.; Cho, J.; Kim, H.J.; Min, Y.H.; Lyu, C.J.; Cheong, J.-W.; Kim, J.S.; Kim, H.S.; Kim, S.-J.; et al. Factors Associated with Pulmonary Toxicity after Myeloablative Conditioning Using Fractionated Total Body Irradiation. Radiat. Oncol. J 2017, 35, 257–267. [Google Scholar] [CrossRef]
- Abugideiri, M.; Nanda, R.H.; Butker, C.; Zhang, C.; Kim, S.; Chiang, K.-Y.; Butker, E.; Khan, M.K.; Haight, A.E.; Chen, Z.; et al. Factors Influencing Pulmonary Toxicity in Children Undergoing Allogeneic Hematopoietic Stem Cell Transplantation in the Setting of Total Body Irradiation-Based Myeloablative Conditioning. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 349–359. [Google Scholar] [CrossRef]
- Kelsey, C.R.; Horwitz, M.E.; Chino, J.P.; Craciunescu, O.; Steffey, B.; Folz, R.J.; Chao, N.J.; Rizzieri, D.A.; Marks, L.B. Severe Pulmonary Toxicity After Myeloablative Conditioning Using Total Body Irradiation: An Assessment of Risk Factors. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 812–818. [Google Scholar] [CrossRef]
- Weshler, Z.; Breuer, R.; Or, R.; Naparstek, E.; Pfeffer, M.R.; Lowental, E.; Slavin, S. Interstitial Pneumonitis after Total Body Irradiation: Effect of Partial Lung Shielding. Br. J. Haematol. 1990, 74, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Durie, E.; Nicholson, E.; Anthias, C.; Dunne, E.M.; Potter, M.; Ethell, M.; Messiou, C.; Brennan, J.; Eagle, S.; Talbot, J.; et al. Determining the Incidence of Interstitial Pneumonitis and Chronic Kidney Disease Following Full Intensity Haemopoetic Stem Cell Transplant Conditioned Using a Forward-Planned Intensity Modulated Total Body Irradiation Technique. Radiother. Oncol. 2021, 158, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.W.; Weisdorf, D.J.; DeFor, T.E.; Ehler, E.; Dusenbery, K.E. Influence of Total Body Irradiation Dose Rate on Idiopathic Pneumonia Syndrome in Acute Leukemia Patients Undergoing Allogeneic Hematopoietic Cell Transplantation. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 180–189. [Google Scholar] [CrossRef]
- Sampath, S.; Schultheiss, T.E.; Wong, J. Dose Response and Factors Related to Interstitial Pneumonitis after Bone Marrow Transplant. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Weiner, R.S.; Bortin, M.M.; Gale, R.P.; Gluckman, E.; Kay, H.E.; Kolb, H.J.; Hartz, A.J.; Rimm, A.A. Interstitial Pneumonitis after Bone Marrow Transplantation. Assessment of Risk Factors. Ann. Intern. Med. 1986, 104, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Shank, B.; O’Reilly, R.J.; Cunningham, I.; Kernan, N.; Yaholom, J.; Brochstein, J.; Castro-Malaspina, H.; Kutcher, G.J.; Mohan, R.; Bonfiglio, P. Total Body Irradiation for Bone Marrow Transplantation: The Memorial Sloan-Kettering Cancer Center Experience. Radiother. Oncol. 1990, 18, 68–81. [Google Scholar] [CrossRef]
- Shinde, A.; Yang, D.; Frankel, P.; Liu, A.; Han, C.; Del Vecchio, B.; Schultheiss, T.; Cheng, J.; Li, R.; Kim, D.; et al. Radiation-Related Toxicities Using Organ Sparing Total Marrow Irradiation Transplant Conditioning Regimens. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1025–1033. [Google Scholar] [CrossRef]
- Zuro, D.; Vagge, S.; Broggi, S.; Agostinelli, S.; Takahashi, Y.; Brooks, J.; Leszcynska, P.; Liu, A.; Zucchetti, C.; Saldi, S.; et al. Multi-Institutional Evaluation of MVCT Guided Patient Registration and Dosimetric Precision in Total Marrow Irradiation: A Global Health Initiative by the International Consortium of Total Marrow Irradiation. Radiother. Oncol. 2019, 141, 275–282. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | n (% or Range) |
|---|---|
| Number of patients | 335 |
| Median age at transplantation | 48 (18–74) |
| Sex | |
| Male | 192 (57.3) |
| Female | 143 (42.7) |
| Diseases | |
| AML | 219 (65.4) |
| ALL | 98 (29.3) |
| T-cell ALL | 22 (22.4 of ALL) |
| B-cell ALL | 76 (77.6 of ALL) |
| MDS | 15 (4.5) |
| Biphenotypic leukemia | 2 (0.6) |
| T-PLL | 1 (0.3) |
| Chemotherapy regimen | |
| Fludarabine | 147 (43.9) |
| Melphalan-fludarabine | 96 (28.7) |
| Cyclophosphamide | 74 (22.1) |
| Etoposide | 11 (3.3) |
| Other | 7 (2.1) |
| Graft-versus-host-disease | |
| No | 124 (37.0) |
| Acute only | 75 (22.4) |
| Chronic | 131 (39.1) |
| No information | 5 (1.5) |
| TBI dose | |
| 8 Gy | 244 (72.8) |
| 12 Gy | 86 (25.7) |
| <8 Gy | 5 (1.5) |
| Pulmonary Toxicity | n (% or Range) |
|---|---|
| Type of toxicity | |
| Overall | 83 (24.8) |
| Pneumonia | 45 (13.4) |
| Bronchial obstruction | 20 (6.0) |
| Dyspnea | 9 (2.7) |
| Pleural effusion | 7 (2.1) |
| ARDS | 4 (1.2) |
| Other | 4 (1.2) |
| Maximum grade of toxicity | |
| Grade 1–2 | 51 (61.4) |
| Grade 3–5 | 32 (38.6) |
| Type of Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total |
|---|---|---|---|---|---|---|
| ARDS | 0 (0.0%) | 0 (0.0%) | 2 (50.0%) | 0 (0.0%) | 2 (50.0%) | 4 (100.0%) |
| Bronchial obstruction | 1 (5.0%) | 15 (75.0%) | 3 (15.0%) | 0 (0.0%) | 1 (5.0%) | 20 (100.0%) |
| Dyspnea | 5 (55.6%) | 4 (44.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 9 (100.0%) |
| Lung edema | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (100.0%) |
| Pleural effusion | 0 (0.0%) | 2 (28.6%) | 4 (57.1%) | 0 (0.0%) | 1 (14.3%) | 7 (100.0%) |
| Pneumonia | 0 (0.0%) | 27 (60.0%) | 7 (15.6%) | 5 (11.1%) | 6 (13.3%) | 45 (100.0%) |
| Pneumothorax | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (100.0%) |
| Pulmonary hypertension | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (100.0%) |
| Vital capacity decrease | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (100.0%) |
| Total | 6 (6.7%) | 52 (58.4%) | 16 (18.0%) | 5 (5.6%) | 10 (11.2%) | 89 (100%) |
| Variable | Comparison | Univariate Analysis | Multivariate Analysis (Step 1) | Multivariate Analysis (Step 2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RR | Range | p | RR | Range | p | RR | Range | p | ||
| GVHD | acute vs. none | 1.97 | 0.96–4.04 | 0.065 | 1.92 | 0.93–3.95 | 0.076 | 1.90 | 0.92–3.91 | 0.081 |
| chronic vs. none | 3.34 | 1.88–5.95 | ≤0.001 | 3.33 | 1.85–5.99 | ≤0.001 | 3.31 | 1.84–5.95 | ≤0.001 | |
| Conditioning chemotherapy | cyc vs. flu | 0.51 | 0.28–0.94 | 0.030 | 0.59 | 0.29–1.16 | 0.126 | 0.52 | 0.28–0.96 | 0.036 |
| flu and mel vs. flu | 0.75 | 0.45–1.27 | 0.288 | |||||||
| Disease | ALL vs. AML | 0.97 | 0.60–1.57 | 0.895 | ||||||
| MDS vs. AML | 0.75 | 0.23–2.38 | 0.621 | |||||||
| Sex | male vs. female | 1.26 | 0.81–1.96 | 0.316 | ||||||
| RT dose | 8 Gy vs. 12 Gy | 1.34 | 0.80–2.26 | 0.271 | ||||||
| Age at SCT | 1.01 | 1.00–1.03 | 0.120 | 1.01 | 0.99–1.03 | 0.433 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oertel, M.; Kittel, C.; Martel, J.; Mikesch, J.-H.; Glashoerster, M.; Stelljes, M.; Eich, H.T. Pulmonary Toxicity after Total Body Irradiation—An Underrated Complication? Estimation of Risk via Normal Tissue Complication Probability Calculations and Correlation with Clinical Data. Cancers 2021, 13, 2946. https://doi.org/10.3390/cancers13122946
Oertel M, Kittel C, Martel J, Mikesch J-H, Glashoerster M, Stelljes M, Eich HT. Pulmonary Toxicity after Total Body Irradiation—An Underrated Complication? Estimation of Risk via Normal Tissue Complication Probability Calculations and Correlation with Clinical Data. Cancers. 2021; 13(12):2946. https://doi.org/10.3390/cancers13122946
Chicago/Turabian StyleOertel, Michael, Christopher Kittel, Jonas Martel, Jan-Henrik Mikesch, Marco Glashoerster, Matthias Stelljes, and Hans Theodor Eich. 2021. "Pulmonary Toxicity after Total Body Irradiation—An Underrated Complication? Estimation of Risk via Normal Tissue Complication Probability Calculations and Correlation with Clinical Data" Cancers 13, no. 12: 2946. https://doi.org/10.3390/cancers13122946
APA StyleOertel, M., Kittel, C., Martel, J., Mikesch, J.-H., Glashoerster, M., Stelljes, M., & Eich, H. T. (2021). Pulmonary Toxicity after Total Body Irradiation—An Underrated Complication? Estimation of Risk via Normal Tissue Complication Probability Calculations and Correlation with Clinical Data. Cancers, 13(12), 2946. https://doi.org/10.3390/cancers13122946