The Characteristics of 206 Long-Term Survivors with Peritoneal Metastases from Colorectal Cancer Treated with Curative Intent Surgery: A Multi-Center Cohort from PSOGI
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
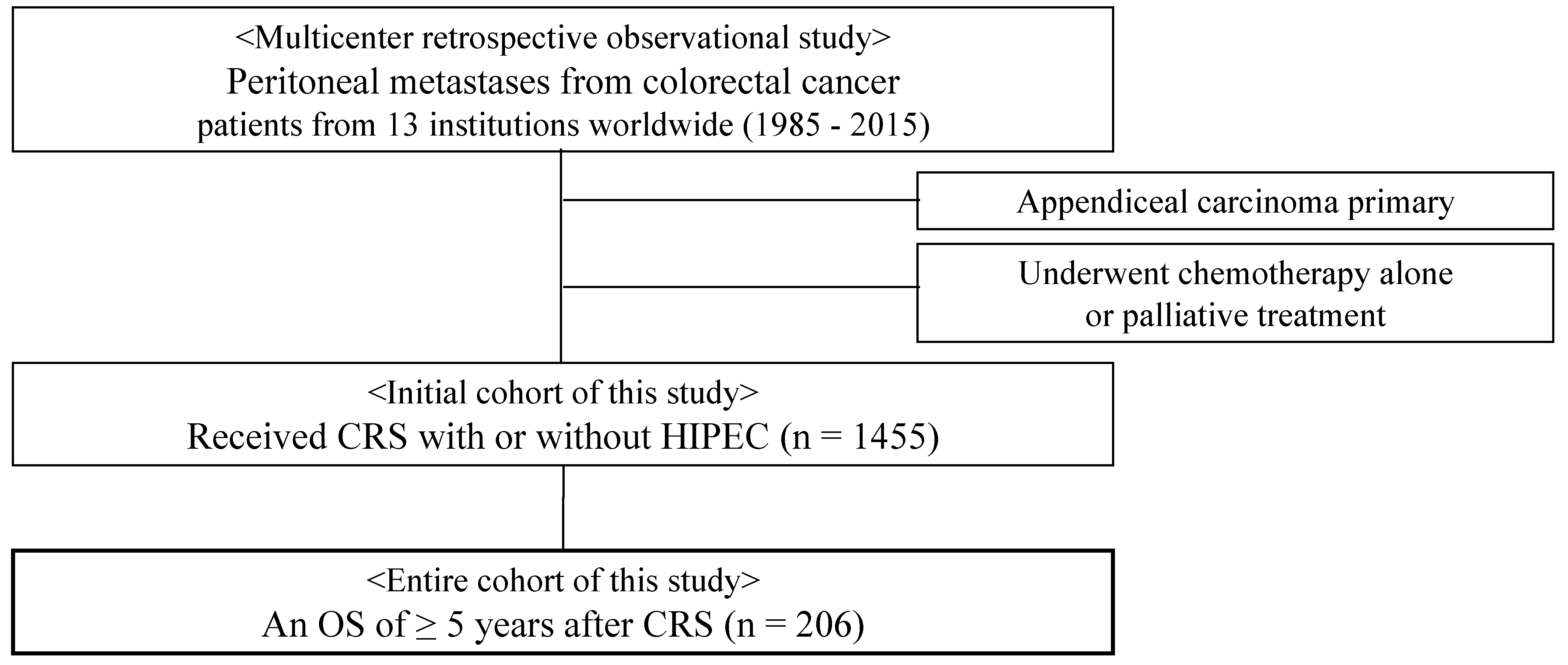
2.1. Patient Characteristics
2.2. Treatment Factors
2.3. Long-Term Outcomes
3. Discussion
4. Materials and Methods
4.1. Study Protocol
4.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations:
| CRS | cytoreductive surgery |
| HIPEC | hyperthermic intraperitoneal chemotherapy |
| OS | overall survival |
References
- Lemmens, V.E.; Klaver, Y.L.; Verwaal, V.J.; Rutten, H.J.; Coebergh, J.W.W.; de Hingh, I.H. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: A population-based study. Int. J. Cancer 2011, 128, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.G.; Fook, S.; Loi, C.; Seow-Choen, F. Peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2002, 89, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, B.; Arvieux, C.; Glehen, O.; Beaujard, A.C.; Rivoire, M.; Baulieux, J.; Fontaumard, E.; Brachet, A.; Caillot, J.L.; Faure, J.L.; et al. Peritoneal carcinomatosis from non-gynecologic malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer 2000, 88, 358–363. [Google Scholar] [CrossRef]
- Klaver, Y.L.B.; Simkens, L.H.J.; Lemmens, V.E.P.P.; Koopman, M.; Teerenstra, S.; Bleichrodt, R.P.; de Hingh, I.H.J.T.; Punt, C.J.A. Outcomes of colorectal cancer patients with peritoneal carcinomatosis treated with chemotherapy with and without targeted therapy. Eur. J. Surg. Oncol. 2012, 38, 617–623. [Google Scholar] [CrossRef]
- van Oudheusden, T.R.; Razenberg, L.G.; van Gestel, Y.R.; Creemers, G.J.; Lemmens, V.E.; de Hingh, I.H. Systemic treatment of patients with metachronous peritoneal carcinomatosis of colorectal origin. Sci. Rep. 2015, 5, 18632. [Google Scholar] [CrossRef]
- Franko, J.; Shi, Q.; Goldman, C.D.; Pockaj, B.A.; Nelson, G.D.; Goldberg, R.M.; Pitot, H.C.; Grothey, A.; Alberts, S.R.; Sargent, D.J. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: A pooled analysis of North Central Cancer Treatment Group Phase III Trials N9741 and N9841. J. Clin. Oncol 2012, 30, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Lefevre, J.H.; Chevalier, J.; Brouquet, A.; Marchal, F.; Classe, J.-M.; Ferron, G.; Guilloit, J.-M.; Meeus, P.; Goéré, D.; et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J. Clin. Oncol. 2009, 27, 681–685. [Google Scholar] [CrossRef]
- Elias, D.; Gilly, F.; Boutitie, F.; Quenet, F.; Bereder, J.-M.; Mansvelt, B.; Lorimier, G.; Dubè, P.; Glehen, O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric french study. J. Clin. Oncol. 2010, 28, 63–68. [Google Scholar] [CrossRef]
- Franko, J.; Ibrahim, Z.; Gusani, N.J.; Holtzman, M.P.; Bartlett, D.L.; Zeh, H.J. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer 2010, 116, 3756–3762. [Google Scholar] [CrossRef]
- Hompes, D.; D’Hoore, A.; Van Cutsem, E.; Fieuws, S.; Ceelen, W.; Peeters, M.; Van der Speeten, K.; Bertrand, C.; Legendre, H.; Kerger, J. The treatment of peritoneal carcinomatosis of colorectal cancer with complete cytoreductive surgery and Hyperthermic Intraperitoneal Peroperative Chemotherapy (HIPEC) with oxaliplatin: A Belgian Multicentre Prospective Phase II Clinical Study. Ann. Surg. Oncol. 2012, 19, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Quenet, F.; Goéré, D.; Mehta, S.S.; Roca, L.; Dumont, F.; Hessissen, M.; Saint-Aubert, B.; Elias, D. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann. Surg. 2011, 254, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Kwiatkowski, F.; Sugarbaker, P.H.; Elias, D.; Levine, E.A.; De Simone, M.; Barone, R.; Yonemura, Y.; Cavaliere, F.; Quenet, F.; et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J. Clin. Oncol. 2004, 22, 3284–3292. [Google Scholar] [CrossRef]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Sloothen, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A.N. Randomized Trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef]
- Goéré, D.; Malka, D.; Tzanis, D.; Gava, V.; Boige, V.; Eveno, C.; Maggiori, L.; Dumont, F.; Ducreux, M.; Elias, D. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann. Surg. 2013, 257, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Kyang, L.S.; Alzahrani, N.A.; Valle, S.J.; Rahman, M.K.; Arrowaili, A.; Liauw, W.; Morris, D.L. Long-term survival outcomes of cytoreductive surgery and perioperative intraperitoneal chemotherapy: Single-institutional experience with 1225 cases. J. Surg. Oncol. 2019, 120, 794–802. [Google Scholar] [CrossRef]
- Elias, D.; Goere, D.; Blot, F.; Billard, V.; Pocard, M.; Kohneh-Shahri, N.; Raynard, B. Optimization of hyperthermic intraperitoneal chemotherapy with oxaliplatin plus irinotecan at 43 degrees C after compete cytoreductive surgery: Mortality and morbidity in 106 consecutive patients. Ann. Surg. Oncol. 2007, 14, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Kusamura, S.; Younan, R.; Baratti, D.; Costanzo, P.; Favaro, M.; Gavazzi, C.; Deraco, M. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: Analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer 2006, 106, 1144–1153. [Google Scholar] [CrossRef]
- Ceelen, W.P.; Peeters, M.; Houtmeyers, P.; Breusegem, C.; De Somer, F.; Pattyn, P. Safety and efficacy of hyperthermic intraperitoneal chemoperfusion with high-dose oxaliplatin in patients with peritoneal carcinomatosis. Ann. Surg. Oncol. 2008, 15, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Hallam, S.; Tyler, R.; Price, M.; Beggs, A.; Youssef, H. Meta-analysis of prognostic factors for patients with colorectal peritoneal metastasis undergoing cytoreductive surgery and heated intraperitoneal chemotherapy. BJS Open 2019, 3, 585–594. [Google Scholar] [CrossRef]
- Kwakman, R.; Schrama, A.M.; van Olmen, J.P.; Otten, R.H.; de Lange-de Klerk, E.S.; de Cuba, E.M.; Kazemier, G.; Te Velde, E.A. Clinicopathological parameters in patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer metastases: A meta-analysis. Ann. Surg. 2016, 263, 1102–1111. [Google Scholar] [CrossRef]
- Cavaliere, F.; De Simone, M.; Virzì, S.; Deraco, M.; Rossi, C.R.; Garofalo, A.; Di Filippo, F.; Giannarelli, D.; Vaira, M.; Valle, M.; et al. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur. J. Surg. Oncol. 2011, 37, 148–154. [Google Scholar] [CrossRef]
- Yonemura, Y.; Canbay, E.; Ishibashi, H. Prognostic factors of peritoneal metastases from colorectal cancer following cytoreductive surgery and perioperative chemotherapy. Sci. World J. 2013, 2013, 978394. [Google Scholar] [CrossRef] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Yan, T.D.; Black, D.; Savady, R.; Sugarbaker, P.H. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J. Clin. Oncol. 2006, 24, 4011–4019. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.M.; Sleightholm, R.; Patel, A.; Shostrom, V.; Hall, B.; Neilsen, B.; Bartlett, D.; Smith, L. Morbidity and mortality rates following cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy compared with other high-risk surgical oncology procedures. JAMA Netw. Open 2019, 2, e186847. [Google Scholar] [CrossRef] [PubMed]
- Choudry, M.H.A.; Shuai, Y.; Jones, H.L.; Pai, R.K.; Pingpank, J.F.; Ahrendt, S.S.; Holtzman, M.P.; Zeh, H.J.; Bartlett, D.L. Postoperative complications independently predict cancer-related survival in peritoneal malignancies. Ann. Surg. Oncol. 2018, 25, 3950–3959. [Google Scholar] [CrossRef]
- Goéré, D.; Sourrouille, I.; Gelli, M.; Benhaim, L.; Faron, M.; Honoré, C. Peritoneal metastases from colorectal cancer: Treatment principles and perspectives. Surg. Oncol. Clin. North Am. 2018, 27, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, J.; Lowy, A.M.; Markman, M.; Chua, T.; Pelz, J.; Baratti, D.; Baumgartner, J.M.; Berri, R.; Bretcha-Boix, P.; Deraco, M.; et al. The American society of peritoneal surface malignancies (ASPSM) multiinstitution evaluation of the peritoneal surface disease severity score (PSDSS) in 1,013 patients with colorectal cancer with peritoneal carcinomatosis. Ann. Surg. Oncol. 2014, 21, 4195–4201. [Google Scholar] [CrossRef]
- Glehen, O.; Gilly, F.N.; Boutitie, F.; Bereder, J.M.; Quenet, F.; Sideris, L.; Mansvelt, B.; Lorimier, G.; Msika, S.; Elias, D. French Surgical Association. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: A multi-institutional study of 1,290 patients. Cancer 2010, 116, 5608–5618. [Google Scholar] [CrossRef]
- NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines): NCCN, Colon Cancer. Version 2. 2021. Available online: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 21 January 2021).
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Kamada, Y.; Hida, K.; Ishibashi, H.; Sako, S.; Mizumoto, A.; Ichinose, M.; Padmanabhan, N.; Yoshida, S.; Yonemura, Y. Thirty-three long-term survivors after cytoreductive surgery in patients with peritoneal metastases from colorectal cancer: A retrospective descriptive study. World J. Surg. Oncol. 2021, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Faron, M.; Macovei, R.; Goéré, D.; Honoré, C.; Benhaim, L.; Elias, D. Linear Relationship of Peritoneal Cancer Index and Survival in Patients with Peritoneal Metastases from Colorectal Cancer. Ann. Surg. Oncol. 2016, 23, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Morris, D.L. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for isolated colorectal peritoneal carcinomatosis: Experimental therapy or standard of care? Ann. Surg. 2008, 248, 829–835. [Google Scholar] [CrossRef]
- Goéré, D.; Souadka, A.; Faron, M.; Cloutier, A.S.; Viana, B.; Honoré, C.; Dumont, F.; Elias, D. Extent of Colorectal peritoneal carcinomatosis: Attempt to define a threshold above which HIPEC Does not offer survival benefit: A comparative study. Ann. Surg. Oncol. 2015, 22, 2958–2964. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Benizri, E.; Vernerey, D.; Eldweny, H.; Dipietrantonio, D.; Pocard, M. Preoperative criteria of incomplete resectability of peritoneal carcinomatosis from non-appendiceal colorectal carcinoma. Gastroenterol. Clin. Biol. 2005, 29, 1010–1013. [Google Scholar] [CrossRef]
- Benizri, E.I.; Bernard, J.-L.; Rahili, A.; Benchimol, D.; Bereder, J.-M. Small bowel involvement is a prognostic factor in colorectal carcinomatosis treated with complete cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. World J. Surg. Oncol. 2012, 10, 56. [Google Scholar] [CrossRef]
- Spiliotis, J.; Kalles, V.; Kyriazanos, I.; Terra, A.; Prodromidou, A.; Raptis, A.; Kopanakis, N.; Christopoulou, A. CRS and HIPEC in patients with peritoneal metastasis secondary to colorectal cancer: The small-bowel PCI score as a predictor of survival. Pleura Peritoneum 2019, 4, 20190018. [Google Scholar] [CrossRef]
- Thomassen, I.; van Gestel, Y.R.; Lemmens, V.E.; de Hingh, I.H. Incidence, prognosis, and treatment options for patients with synchronous peritoneal carcinomatosis and liver metastases from colorectal origin. Dis. Colon Rectum 2013, 56, 1373–1380. [Google Scholar] [CrossRef]
- van Oudheusden, T.R.; Braam, H.J.; Nienhuijs, S.W.; Wiezer, M.J.; van Ramshorst, B.; Luyer, P.; de Hingh, I.H. Poor outcome after cytoreductive surgery and HIPEC for colorectal peritoneal carcinomatosis with signet ring cell histology. J. Surg. Oncol. 2015, 111, 237–242. [Google Scholar] [CrossRef]
- Winer, J.; Zenati, M.; Ramalingam, L.; Jones, H.; Zureikat, A.; Holtzman, M.; Lee, K.; Ahrendt, S.; Pingpank, J.; Zeh, H.J.; et al. Impact of aggressive histology and location of primary tumor on the efficacy of surgical therapy for peritoneal carcinomatosis of colorectal origin. Ann. Surg. Oncol. 2014, 21, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Pelz, J.O.W.; Chua, T.C.; Esquivel, J.; Stojadinovic, A.; Doerfer, J.; Morris, D.L.; Maeder, U.; Germer, C.-T.; Kerscher, A.G. Evaluation of best supportive care and systemic chemotherapy as treatment stratified according to the retrospective peritoneal surface disease severity score (PSDSS) for peritoneal carcinomatosis of colorectal origin. BMC Cancer 2010, 10, 689. [Google Scholar] [CrossRef]
- Van Sweringen, H.L.; Hanseman, D.J.; Ahmad, S.A.; Edwards, M.J.; Sussman, J.J. Predictors of survival in patients with high-grade peritoneal metastases undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Surgery 2012, 152, 617–624. [Google Scholar] [CrossRef]
- Tonello, M.; Ortega-Perez, G.; Alonso-Casado, O.; Torres-Mesa, P.; Guiñez, G.; Gonzalez-Moreno, S. Peritoneal carcinomatosis arising from rectal or colonic adenocarcinoma treated with cytoreductive surgery (CRS) hyperthermic intraperitoneal chemotherapy (HIPEC): Two different diseases. Clin. Transl. Oncol. 2018, 20, 1268–1273. [Google Scholar] [CrossRef]
- da Silva, R.G.; Cabanas, J.; Sugarbaker, P.H. Limited survival in the treatment of carcinomatosis from rectal cancer. Dis. Colon Rectum 2005, 48, 2258–2263. [Google Scholar] [CrossRef]
- Verwaal, V.J.; van Tinteren, H.; van Ruth, S.; Zoetmulder, F.A.N. Predicting the survival of patients with peritoneal carcinomatosis of colorectal origin treated by aggressive cytoreduction and hyperthermic intraperitoneal chemotherapy. Br. J. Surg. 2004, 91, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Ihemelandu, C.; Sugarbaker, P.H. Management for peritoneal metastasis of colonic origin: Role of Cytoreductive surgery and perioperative intraperitoneal chemotherapy: A single institution’s experience during two decades. Ann. Surg. Oncol. 2017, 24, 898–905. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Peritoneal Metastases from Gastrointestinal Cancer. Curr. Oncol. Rep. 2018, 20, 62. [Google Scholar] [CrossRef]
- Braam, H.J.; van Oudheusden, T.R.; de Hingh, I.H.J.T.; Nienhuijs, S.W.; Boerma, D.; Wiezer, M.J.; van Ramshorst, B. Patterns of recurrence following complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. J. Surg. Oncol. 2014, 109, 841–847. [Google Scholar] [CrossRef]
- Chua, T.C.; Yan, T.D.; Ng, K.M.; Zhao, J.; Morris, D.L. Significance of lymph node metastasis in patients with colorectal cancer peritoneal carcinomatosis. World J. Surg. 2009, 33, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.M.; Tobin, L.; Heavey, S.F.; Kelly, K.J.; Roeland, E.J.; Lowy, A.M. Predictors of Progression in high-grade appendiceal or colorectal peritoneal carcinomatosis after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2015, 22, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Simkens, G.A.; van Oudheusden, T.R.; Nieboer, D.; Steyerberg, E.W.; Rutten, H.J.; Luyer, M.D.; Nienhuijs, S.W.; de Hingh, I.H. Development of a Prognostic nomogram for patients with peritoneally metastasized colorectal cancer treated with cytoreductive surgery and HIPEC. Ann. Surg. Oncol 2016, 23, 4214–4221. [Google Scholar] [CrossRef] [PubMed]
- Shida, D.; Tsukamoto, S.; Ochiai, H.; Kanemitsu, Y. Long-term outcomes after R0 resection of synchronous peritoneal metastasis from colorectal cancer without cytoreductive surgery or hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2018, 25, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
| Variable | Long-Term Survivors | Cured Patients |
|---|---|---|
| (n = 206) | (n = 84) | |
| Age, y, median (IQR) | 58 (49–66) | 55 (44–64) |
| Gender | ||
| Male | 101 (49.0%) | 40 (47.6%) |
| Female | 105 (51.0%) | 44 (52.4%) |
| ASA grade | ||
| I | 97 (47.1%) | 46 (54.8%) |
| II | 84 (40.1%) | 30 (35.7%) |
| III | 6 (2.9%) | 1 (1.2%) |
| Missing | 19 (9.2%) | 7 (8.3%) |
| Date of CRS | ||
| Before 2001 | 16 (7.8%) | 5 (6.0%) |
| Between 2001 and 2010 | 76 (36.9%) | 37 (44.0%) |
| 2011 or later | 114 (55.3%) | 42 (50.0%) |
| Onset | ||
| Synchronous | 96 (46.6%) | 42 (50.0%) |
| Metachronous | 96 (46.6%) | 35 (41.7%) |
| Missing | 14 (6.8%) | 7 (8.3%) |
| Location of primary tumor | ||
| Right colon | 90 (43.7%) | 38 (45.2%) |
| Left colon | 101 (49.0%) | 42 (50.0%) |
| Rectum | 14 (6.8%) | 4 (4.8%) |
| Missing | 1 (0.5%) | 0 (0%) |
| Histology | ||
| Well to moderately | 149 (72.3%) | 67 (79.8%) |
| Mucinous | 50 (24.3%) | 15 (17.9%) |
| Poorly or signet ring cell | 6 (2.9%) | 2 (2.4%) |
| Missing | 1 (0.5%) | 0 (0%) |
| pT category | ||
| pT ≤ 3 | 89 (43.2%) | 33 (39.3%) |
| pT4 | 100 (48.5%) | 45 (53.6%) |
| Missing | 17 (8.3%) | 6 (7.1%) |
| pN category | ||
| N0 | 64 (31.1%) | 25 (29.8%) |
| N1/2 | 123 (59.7%) | 51 (60.7%) |
| Missing | 19 (9.2%) | 8 (9.5%) |
| Extraperitoneal metastases | ||
| None | 177 (85.9%) | 78 (92.9%) |
| Liver metastases | 27 (13.1%) | 5 (6.0%) |
| Lung metastases | 2 (1.0% | 1 (1.2%) |
| PCI, median (IQR) | 4 (2–7) | 3 (2–5) |
| 0–5 | 129 (62.6%) | 66 (78.6%) |
| 6–10 | 40 (19.4%) | 14 (16.7%) |
| 11–15 | 15 (7.3%) | 2 (2.4%) |
| 16–20 | 8 (3.9%) | 1 (1.2%) |
| ≥21 | 4 (1.9%) | 0 (0%) |
| Missing | 10 (4.9%) | 1 (1.2%) |
| SB-PCI, median (IQR) | 0 (0–2) | 0 (0–1) |
| 0 | 130 (63.1%) | 60 (71.4%) |
| 1–4 | 50 (24.3%) | 15 (17.9%) |
| ≥5 | 9 (4.4%) | 2 (2.4%) |
| Missing | 16 (7.8%) | 7 (8.3%) |
| Variable | Long-Term Survivors | Cured Patients |
|---|---|---|
| (n = 206) | (n = 84) | |
| Preoperative chemotherapy | ||
| Not performed | 72 (35.0%) | 31 (36.9%) |
| 5-FU | 127 (61.7%) | 51 (60.7%) |
| Oxaliplatin | 65 (31.6%) | 30 (35.7%) |
| Irinotecan | 58 (28.2%) | 18 (21.4%) |
| Antiangiogenic | 54 (26.2%) | 25 (29.8%) |
| Anti-EGFR | 12 (46.2%) | 4 (4.8%) |
| Others | 5 (2.4%) | 5 (6.0%) |
| Chemotherapy cycles | ||
| >6 cycles | 29 (14.1%) | 8 (9.5%) |
| ≤6 cycles | 73 (35.4%) | 29 (34.5%) |
| Missing | 32 (15.5%) | 16 (19.0%) |
| Completeness of cytoreductive score | ||
| CC-0 | 180 (87.4%) | 77 (91.7%) |
| CC-1 | 22 (10.7%) | 7 (8.4%) |
| CC-2 | 2 (1.0%) | 0 (0%) |
| Missing | 2 (1.0%) | 0 (0%) |
| Number of organs resected, median (IQR) | 2 (1–3) | 2 (1–3) |
| Number of peritoneal sectors resected, median (IQR) | 3 (1–6) | 3 (1–4) |
| HIPEC | ||
| Done | 151 (73.3%) | 62 (73.8%) |
| Not performed | 55 (26.7%) | 22 (26.2%) |
| HIPEC agent | ||
| Mitomycin-based | 85 (56.3%) | 42 (67.7%) |
| Oxaliplatin-based | 63 (41.7%) | 19 (30.6%) |
| Others | 3 (2.0%) | 1 (1.6%) |
| Major complication (Clavien-Dindo III–IV) | ||
| None | 166 (80.6%) | 72 (85.7%) |
| Intra-abdominal | 31 (15.0%) | 9 (10.7%) |
| Extra-abdominal | 7 (3.4%) | 2 (2.4%) |
| Missing | 2 (1.0%) | 1 (1.2%) |
| Length of stay, median (IQR) | 19 (15–31) | 18 (14–27) |
| Postoperative chemotherapy | ||
| Systemic chemotherapy | 148 (71.8%) | 58 (69.1%) |
| Intraperitoneal chemotherapy | 7 (3.4%) | 2 (2.4%) |
| Systemic chemotherapy + Intraperitoneal chemotherapy | 1 (0.5%) | 1 (1.2%) |
| Not performed | 48 (23.3%) | 21 (25.0%) |
| Missing | 2 (1.0%) | 2 (2.4%) |
| Status | ||
| Alive | 134 (65.0%) | 78 (92.9%) |
| Dead | 72 (35.0%) | 6 (7.1%) |
| Variable | Total Number |
|---|---|
| (n = 122) | |
| Site of recurrence | |
| Isolated | |
| Peritoneum | 43 (35.3%) |
| Liver | 12 (9.8%) |
| Abdominal wall | 11 (9.0%) |
| Lung | 9 (7.4%) |
| Lymph nodes | 5 (4.1%) |
| Bone | 1 (0.8%) |
| Multiple | |
| Peritoneum + other site(s) | 36 (29.5%) |
| Others | 5 (4.1%) |
| Treatment for recurrence | |
| Reoperation ± chemotherapy | 70 (57.4%) |
| Chemotherapy | 21 (17.2%) |
| Palliative therapy | 5 (4.1%) |
| Unknown | 26 (21.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamada, Y.; Hida, K.; Yonemura, Y.; Sugarbaker, P.H.; Ghabra, S.; Ishihara, S.; Nagata, H.; Murono, K.; Goi, T.; Katayama, K.; et al. The Characteristics of 206 Long-Term Survivors with Peritoneal Metastases from Colorectal Cancer Treated with Curative Intent Surgery: A Multi-Center Cohort from PSOGI. Cancers 2021, 13, 2964. https://doi.org/10.3390/cancers13122964
Kamada Y, Hida K, Yonemura Y, Sugarbaker PH, Ghabra S, Ishihara S, Nagata H, Murono K, Goi T, Katayama K, et al. The Characteristics of 206 Long-Term Survivors with Peritoneal Metastases from Colorectal Cancer Treated with Curative Intent Surgery: A Multi-Center Cohort from PSOGI. Cancers. 2021; 13(12):2964. https://doi.org/10.3390/cancers13122964
Chicago/Turabian StyleKamada, Yasuyuki, Koya Hida, Yutaka Yonemura, Paul H. Sugarbaker, Shadin Ghabra, Soichiro Ishihara, Hiroshi Nagata, Koji Murono, Takanori Goi, Kanji Katayama, and et al. 2021. "The Characteristics of 206 Long-Term Survivors with Peritoneal Metastases from Colorectal Cancer Treated with Curative Intent Surgery: A Multi-Center Cohort from PSOGI" Cancers 13, no. 12: 2964. https://doi.org/10.3390/cancers13122964
APA StyleKamada, Y., Hida, K., Yonemura, Y., Sugarbaker, P. H., Ghabra, S., Ishihara, S., Nagata, H., Murono, K., Goi, T., Katayama, K., Morikawa, M., Rau, B., Piso, P., Acs, M., Coccolini, F., Canbay, E., Hsieh, M.-C., Bhatt, A., Bonnot, P.-E., & Glehen, O. (2021). The Characteristics of 206 Long-Term Survivors with Peritoneal Metastases from Colorectal Cancer Treated with Curative Intent Surgery: A Multi-Center Cohort from PSOGI. Cancers, 13(12), 2964. https://doi.org/10.3390/cancers13122964








