Epithelial–Mesenchymal Transition Associated with Head and Neck Squamous Cell Carcinomas: A Review
Abstract
Simple Summary
Abstract
1. Introductory Comments Related to HNSCC and EMT Phenomena
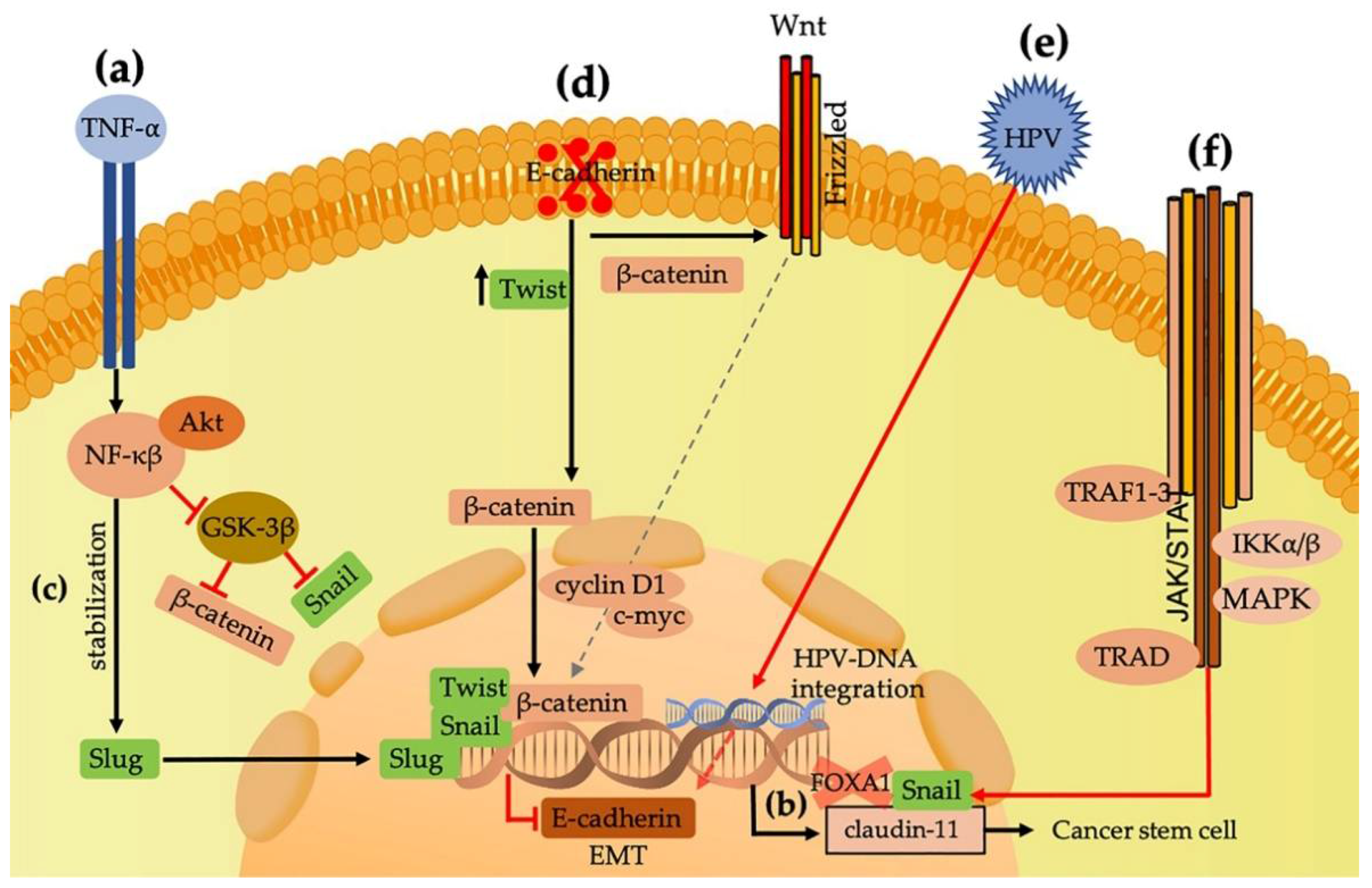
2. Snail, Slug, Twist, and ZEB Are Transcription Factors Related to EMT Induction
3. Complex E-cadherin/Beta-Catenin Associations with EMT
4. Vimentin and N-Cadherin (Mesenchymal Markers) Related to the Promotion of EMT
5. Plasticity Is an Important Phenomenon Related to EMT and Metastasis
6. Human Papillomaviruses and Their Influence on EMT
7. Epstein–Barr Virus Induces EMT in Nasopharyngeal Carcinomas through Latent Membrane Protein-1
8. DNA Methyltransferases, G9a, and N-Glycosylation Are Related to EMT
9. Hypoxia as an Important Factor Related to EMT
10. Tumor Microenvironment and Inflammation Are Related to HNSCC Progression
11. Overexpression of Matrix Metalloproteinases Are Related to Tumor Progression and EMT Induction
12. Inhibition of EMT Is Important in the Treatment of HNSCC
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Cardin, G.B.; Bernard, M.; Bahig, H.; Nguyen-Tan, P.F.; Ballivy, O.; Filion, E.; Soulieres, D.; Philouze, P.; Ayad, T.; Guertin, L.; et al. Single nucleotide polymorphism rs6942067 is a risk factor in young and in non-smoking patients with HPV negative head and neck squamous cell carcinoma. Cancers 2019, 12, 55. [Google Scholar] [CrossRef]
- Domingo-Vidal, M.; Whitaker-Menezes, D.; Martos-Rus, C.; Tassone, P.; Snyder, C.M.; Tuluc, M.; Philp, N.; Curry, J.; Martinez-Outschoorn, U. Cigarette smoke induces metabolic reprogramming of the tumor stroma in head and neck squamous cell carcinoma. Mol. Cancer Res. 2019, 17, 1893. [Google Scholar] [CrossRef]
- Pastushenko, I.; Blanpain, C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef]
- Umbreit, C.; Flanjak, J.; Weiss, C.; Erben, P.; Aderhold, C.; Faber, A.; Stern-Straeter, J.; Hoermann, K.; Schultz, J.D. Incomplete epithelial-mesenchymal transition in p16-positive squamous cell carcinoma cells correlates with β-catenin expression. Anticancer Res. 2014, 34, 7061–7069. [Google Scholar] [PubMed]
- Bommi, P.V.; Ravindran, S.; Raychaudhuri, P.; Bagchi, S. DDB2 regulates Epithelial-to-Mesenchymal Transition (EMT) in Oral/head and neck squamous cell carcinoma. Oncotarget 2018, 9, 34708–34718. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin switch in epithelial-to-mesenchymal transition: Signaling, therapeutic implications, and challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Chang, J.W.; Gwak, S.Y.; Shim, G.A.; Liu, L.; Lim, Y.C.; Kim, J.M.; Jung, M.G.; Koo, B.S. EZH2 is associated with poor prognosis in head-and-neck squamous cell carcinoma via regulating the epithelial-to-mesenchymal transition and chemosensitivity. Oral Oncol. 2016, 52, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ota, I.; Masui, T.; Kurihara, M.; Yook, J.-I.; Mikami, S.; Kimura, T.; Shimada, K.; Konishi, N.; Yane, K.; Yamanaka, T.; et al. Snail-induced EMT promotes cancer stem cell-like properties in head and neck cancer cells. Oncol. Rep. 2015, 35. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.N.; Burton, L.J.; Henderson, V.; Randle, D.D.; Morton, D.J.; Smith, B.A.; Taliaferro-Smith, L.; Nagappan, P.; Yates, C.; Zayzafoon, M.; et al. Snail promotes epithelial mesenchymal transition in breast cancer cells in part via activation of nuclear ERK2. PLoS ONE 2014, 9, e104987. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, R.; Mallets, E.; Gomez-Cambronero, J. The transcription factors Slug (SNAI2) and Snail (SNAI1) regulate phospholipase D (PLD) promoter in opposite ways towards cancer cell invasion. Mol. Oncol. 2016, 10, 663–676. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. Snail. Cell Adhes. Migr. 2010, 4, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.; Wang, G.; Luo, J.; Lin, Y.; Dohadwala, M.; Abemayor, E.; Elashoff, D.A.; Sharma, S.; Dubinett, S.M.; St John, M.A. Snail controls the mesenchymal phenotype and drives erlotinib resistance in oral epithelial and head and neck squamous cell carcinoma cells. Otolaryngol. Head Neck Surg. 2012, 147, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Li, C.F.; Chen, J.Y.; Ho, Y.H.; Hsu, W.H.; Wu, L.C.; Lan, H.Y.; Hsu, D.S.; Tai, S.K.; Chang, Y.C.; Yang, M.H. Snail-induced claudin-11 prompts collective migration for tumour progression. Nat. Cell Biol. 2019, 21, 251–262. [Google Scholar] [CrossRef]
- Barrallo-Gimeno, A.; Nieto, M.A. The Snail genes as inducers of cell movement and survival: Implications in development and cancer. Development 2005, 132, 3151–3161. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, Q.; Zhou, Y.; Wang, Y.; Chen, X. Slug is a key mediator of hypoxia induced cadherin switch in HNSCC: Correlations with poor prognosis. Oral Oncol. 2013, 49, 1043–1050. [Google Scholar] [CrossRef]
- Katafiasz, D.; Smith, L.M.; Wahl, J.K., 3rd. Slug (SNAI2) expression in oral SCC cells results in altered cell-cell adhesion and increased motility. Cell Adh. Migr. 2011, 5, 315–322. [Google Scholar] [CrossRef]
- Lo, J.F.; Yu, C.C.; Chiou, S.H.; Huang, C.Y.; Jan, C.I.; Lin, S.C.; Liu, C.J.; Hu, W.Y.; Yu, Y.H. The epithelial-mesenchymal transition mediator S100A4 maintains cancer-initiating cells in head and neck cancers. Cancer Res. 2011, 71, 1912–1923. [Google Scholar] [CrossRef]
- Boye, K.; Maelandsmo, G.M. S100A4 and metastasis: A small actor playing many roles. Am. J. Pathol. 2010, 176, 528–535. [Google Scholar] [CrossRef]
- Wetting, H.L.; Hadler-Olsen, E.; Magnussen, S.; Rikardsen, O.; Steigen, S.E.; Sundkvist, E.; Loennechen, T.; Kanapathippillai, P.; Kildalsen, H.; Winberg, J.O.; et al. S100A4 expression in xenograft tumors of human carcinoma cell lines is induced by the tumor microenvironment. Am. J. Pathol. 2011, 178, 2389–2396. [Google Scholar] [CrossRef]
- Candido, J.; Hagemann, T. Cancer-related inflammation. J. Clin. Immunol 2013, 33, S79–S84. [Google Scholar] [CrossRef]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef]
- López-Lago, M.A.; Posner, S.; Thodima, V.J.; Molina, A.M.; Motzer, R.J.; Chaganti, R.S. Neutrophil chemokines secreted by tumor cells mount a lung antimetastatic response during renal cell carcinoma progression. Oncogene 2013, 32, 1752–1760. [Google Scholar] [CrossRef]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef]
- Liu, S.; Shi, L.; Wang, Y.; Ye, D.; Ju, H.; Ma, H.; Yang, W.; Hu, J.; Deng, J.; Zhang, Z. Stabilization of slug by NF-κB is essential for TNF-α-induced migration and epithelial-mesenchymal transition in head and neck squamous cell carcinoma cells. Cell Physiol. Biochem. 2018, 47, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Ou, D.L.; Chien, H.F.; Chen, C.L.; Lin, T.C.; Lin, L.I. Role of Twist in head and neck carcinoma with lymph node metastasis. Anticancer Res. 2008, 28, 1355–1359. [Google Scholar]
- Yang, M.H.; Wu, M.Z.; Chiou, S.H.; Chen, P.M.; Chang, S.Y.; Liu, C.J.; Teng, S.C.; Wu, K.J. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat. Cell Biol. 2008, 10, 295–305. [Google Scholar] [CrossRef] [PubMed]
- May, C.D.; Sphyris, N.; Evans, K.W.; Werden, S.J.; Guo, W.; Mani, S.A. Epithelial-mesenchymal transition and cancer stem cells: A dangerously dynamic duo in breast cancer progression. Breast Cancer Res. 2011, 13, 202. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, W.; Wang, P. Smoking and gender modify the effect of TWIST on patient survival in head and neck squamous carcinoma. Oncotarget 2017, 8, 85816–85827. [Google Scholar] [CrossRef] [PubMed]
- Min, A.; Xiong, H.; Wang, W.; Hu, X.; Wang, C.; Mao, T.; Yang, L.; Huang, D.; Xia, K.; Su, T. CD147 promotes proliferation and migration of oral cancer cells by inhibiting junctions between E-cadherin and β-catenin. J. Oral Pathol. Med. 2020, 49, 1019–1029. [Google Scholar] [CrossRef]
- Vergara, D.; Simeone, P.; Latorre, D.; Cascione, F.; Leporatti, S.; Trerotola, M.; Giudetti, A.M.; Capobianco, L.; Lunetti, P.; Rizzello, A.; et al. Proteomics analysis of E-cadherin knockdown in epithelial breast cancer cells. J. Biotechnol. 2015, 202, 3–11. [Google Scholar] [CrossRef]
- Wong, S.H.M.; Fang, C.M.; Chuah, L.-H.; Leong, C.O.; Ngai, S.C. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit. Rev. Oncol. Hematol. 2018, 121, 11–22. [Google Scholar] [CrossRef]
- Cao, Q.; Yu, J.; Dhanasekaran, S.M.; Kim, J.H.; Mani, R.S.; Tomlins, S.A.; Mehra, R.; Laxman, B.; Cao, X.; Kleer, C.G.; et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 2008, 27, 7274–7284. [Google Scholar] [CrossRef]
- Tan, E.J.; Kahata, K.; Idås, O.; Thuault, S.; Heldin, C.H.; Moustakas, A. The high mobility group A2 protein epigenetically silences the Cdh1 gene during epithelial-to-mesenchymal transition. Nucleic Acids Res. 2015, 43, 162–178. [Google Scholar] [CrossRef] [PubMed]
- Cercelaru, L.; Stepan, A.E.; Mărgăritescu, C.; Osman, A.; Popa, I.C.; Florescu, M.M.; Simionescu, C.E.; Mărgăritescu, O.C. E-cadherin, β-catenin and Snail immunoexpression in laryngeal squamous cell carcinoma. Rom. J. Morphol. Embryol. 2017, 58, 761–766. [Google Scholar] [PubMed]
- Steinbichler, T.B.; Dudas, J.; Ingruber, J.; Glueckert, R.; Sprung, S.; Fleischer, F.; Cidlinsky, N.; Dejaco, D.; Kofler, B.; Giotakis, A.I.; et al. Slug is a surrogate marker of epithelial to mesenchymal transition (EMT) in head and neck cancer. J. Clin. Med. 2020, 9, 2061. [Google Scholar] [CrossRef] [PubMed]
- Von Zeidler, S.V.; de Souza Botelho, T.; Mendonça, E.F.; Batista, A.C. E-cadherin as a potential biomarker of malignant transformation in oral leukoplakia: A retrospective cohort study. BMC Cancer 2014, 14, 972. [Google Scholar] [CrossRef] [PubMed]
- De Morais, E.F.; Santos, H.B.P.; Cavalcante, I.L.; Rabenhorst, S.H.B.; dos Santos, J.N.; Galvão, H.C.; Freitas, R.A. Twist and E-cadherin deregulation might predict poor prognosis in lower lip squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 318–329. [Google Scholar] [CrossRef]
- Miyashita, H.; Mori, S.; Motegi, K.; Fukumoto, M.; Uchida, T. Pin1 is overexpressed in oral squamous cell carcinoma and its levels correlate with cyclin D1 overexpression. Oncol. Rep. 2003, 10, 455–461. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Z.; Niu, B.; Zhang, J.; Tan, T.K.; Lee, S.R.; Zhao, Y.; Harris, D.C.H.; Zheng, G. E-cadherin/β-catenin complex and the epithelial barrier. J. Biomed. Biotechnol. 2011, 2011, 567305. [Google Scholar] [CrossRef]
- Shang, S.; Hua, F.; Hu, Z.W. The regulation of β-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 2017, 8, 33972–33989. [Google Scholar] [CrossRef] [PubMed]
- Dal Vechio, A.M.; Giudice, F.S.; Sperandio, F.F.; Mantesso, A.; dos Santos Pinto, D., Jr. Vimentin expression and the influence of Matrigel in cell lines of head and neck squamous cell carcinoma. Braz. Oral Res. 2011, 25, 235–240. [Google Scholar] [CrossRef]
- Liu, P.F.; Kang, B.H.; Wu, Y.M.; Sun, J.H.; Yen, L.M.; Fu, T.Y.; Lin, Y.C.; Liou, H.H.; Lin, Y.S.; Sie, H.C.; et al. Vimentin is a potential prognostic factor for tongue squamous cell carcinoma among five epithelial-mesenchymal transition-related proteins. PLoS ONE 2017, 12, e0178581. [Google Scholar] [CrossRef]
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the move: New developments in cell migration. F1000Res 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Eriksson, J.E. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef]
- Wangmo, C.; Charoen, N.; Jantharapattana, K.; Dechaphunkul, A.; Thongsuksai, P. Epithelial-mesenchymal transition predicts survival in oral squamous cell carcinoma. Pathol. Oncol. Res. 2020, 26, 1511–1518. [Google Scholar] [CrossRef]
- Luo, W.R.; Wu, A.B.; Fang, W.Y.; Li, S.Y.; Yao, K.T. Nuclear expression of N-cadherin correlates with poor prognosis of nasopharyngeal carcinoma. Histopathology 2012, 61, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Blaschuk, O.W. N-cadherin antagonists as oncology therapeutics. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015, 370, 20140039. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Nguyen, D.; Chea, C.; Miyauchi, M.; Fujii, M.; Takata, T. Interaction between N-cadherin and decoy receptor-2 regulates apoptosis in head and neck cancer. Oncotarget 2018, 9, 31516–31530. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.Q.; Wang, Z.; Leng, P. Aberrant N-cadherin expression in cancer. Biomed. Pharmacother. 2019, 118, 109320. [Google Scholar] [CrossRef]
- Gupta, P.B.; Pastushenko, I.; Skibinski, A.; Blanpain, C.; Kuperwasser, C. Phenotypic plasticity: Driver of cancer initiation, progression, and therapy resistance. Cell Stem Cell 2019, 24, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Brown, J.; Kanarek, A.; Rajagopal, J.; Melton, D.A. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008, 455, 627–632. [Google Scholar] [CrossRef]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Südhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041. [Google Scholar] [CrossRef]
- Szabo, E.; Rampalli, S.; Risueño, R.M.; Schnerch, A.; Mitchell, R.; Fiebig-Comyn, A.; Levadoux-Martin, M.; Bhatia, M. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 2010, 468, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Ieda, M.; Fu, J.D.; Delgado-Olguin, P.; Vedantham, V.; Hayashi, Y.; Bruneau, B.G.; Srivastava, D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010, 142, 375–386. [Google Scholar] [CrossRef]
- Efe, J.A.; Hilcove, S.; Kim, J.; Zhou, H.; Ouyang, K.; Wang, G.; Chen, J.; Ding, S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat. Cell Biol. 2011, 13, 215–222. [Google Scholar] [CrossRef]
- Kim, J.; Efe, J.A.; Zhu, S.; Talantova, M.; Yuan, X.; Wang, S.; Lipton, S.A.; Zhang, K.; Ding, S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc. Natl. Acad. Sci. USA 2011, 108, 7838–7843. [Google Scholar] [CrossRef]
- Tanabe, K.; Ang, C.E.; Chanda, S.; Olmos, V.H.; Haag, D.; Levinson, D.F.; Südhof, T.C.; Wernig, M. Transdifferentiation of human adult peripheral blood T cells into neurons. Proc. Natl. Acad. Sci. USA 2018, 115, 6470. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.-P.; Lièvre, M.; Thomas, C.; Hinkal, G.; Ansieau, S.; Puisieux, A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE 2008, 3, e2888. [Google Scholar] [CrossRef]
- Zhang, Y.; Weinberg, R.A. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Puré, E.; Hingorani, S.R. Mesenchymal cell plasticity and perfidy in epithelial malignancy. Trends Cancer 2018, 4, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zheng, M.; Zhang, M.; Yang, X.; Li, L.; Wang, S.S.; Wu, J.S.; Yu, X.H.; Wu, J.B.; Pang, X.; et al. PRRX1 Regulates cellular phenotype plasticity and dormancy of head and neck squamous cell carcinoma through miR-642b-3p. Neoplasia 2019, 21, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Biddle, A.; Liang, X.; Gammon, L.; Fazil, B.; Harper, L.J.; Emich, H.; Costea, D.E.; Mackenzie, I.C. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Res. 2011, 71, 5317–5326. [Google Scholar] [CrossRef]
- Shigeishi, H.; Biddle, A.; Gammon, L.; Emich, H.; Rodini, C.O.; Gemenetzidis, E.; Fazil, B.; Sugiyama, M.; Kamata, N.; Mackenzie, I.C. Maintenance of stem cell self-renewal in head and neck cancers requires actions of GSK3β influenced by CD44 and RHAMM. Stem Cells 2013, 31, 2073–2083. [Google Scholar] [CrossRef]
- Brabletz, T. EMT and MET in metastasis: Where are the cancer stem cells? Cancer Cell 2012, 22, 699–701. [Google Scholar] [CrossRef]
- Thierauf, J.; Veit, J.A.; Hess, J. Epithelial-to-mesenchymal transition in the pathogenesis and therapy of head and neck cancer. Cancers 2017, 9, 76. [Google Scholar] [CrossRef]
- Van Dijk, D.; Sharma, R.; Nainys, J.; Yim, K.; Kathail, P.; Carr, A.J.; Burdziak, C.; Moon, K.R.; Chaffer, C.L.; Pattabiraman, D.; et al. Recovering gene interactions from single-cell data using data diffusion. Cell 2018, 174, 716–729. [Google Scholar] [CrossRef]
- Pastushenko, I.; Brisebarre, A.; Sifrim, A.; Fioramonti, M.; Revenco, T.; Boumahdi, S.; van Keymeulen, A.; Brown, D.; Moers, V.; Lemaire, S.; et al. Identification of the tumour transition states occurring during EMT. Nature 2018, 556, 463–468. [Google Scholar] [CrossRef]
- Kisoda, S.; Shao, W.; Fujiwara, N.; Mouri, Y.; Tsunematsu, T.; Jin, S.; Arakaki, R.; Ishimaru, N.; Kudo, Y. Prognostic value of partial EMT-related genes in head and neck squamous cell carcinoma by a bioinformatic analysis. Oral Dis. 2020, 26, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Stasikowska-Kanicka, O.; Wągrowska-Danilewicz, M.; Danilewicz, M. Immunohistochemical study EMT-related proteins in HPV-, and EBV-negative patients with sinonasal tumours. Pathol. Oncol. Res. 2016, 22, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Mizumachi, T.; Sakashita, T.; Kano, S.; Homma, A.; Fukuda, S. Epithelial-mesenchymal transition in human papillomavirus-positive and -negative oropharyngeal squamous cell carcinoma. Oncol. Rep. 2014, 32, 2673–2679. [Google Scholar] [CrossRef][Green Version]
- Cho, Y.A.; Kim, E.K.; Cho, B.C.; Koh, Y.W.; Yoon, S.O. Twist and snail/slug expression in oropharyngeal squamous cell carcinoma in correlation with lymph node metastasis. Anticancer Res. 2019, 39, 6307–6316. [Google Scholar] [CrossRef]
- Wushou, A.; Pan, H.Y.; Liu, W.; Tian, Z.; Wang, L.Z.; Shali, S.; Zhang, Z.Y. Correlation of increased twist with lymph node metastasis in patients with oral squamous cell carcinoma. J. Oral Maxillofac. Surg. 2012, 70, 1473–1479. [Google Scholar] [CrossRef]
- Mroz, E.A.; Tward, A.D.; Pickering, C.R.; Myers, J.N.; Ferris, R.L.; Rocco, J.W. High intratumor genetic heterogeneity is related to worse outcome in patients with head and neck squamous cell carcinoma. Cancer 2013, 119, 3034–3042. [Google Scholar] [CrossRef] [PubMed]
- Mroz, E.A.; Rocco, J.W. MATH, a novel measure of intratumor genetic heterogeneity, is high in poor-outcome classes of head and neck squamous cell carcinoma. Oral Oncol. 2013, 49, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Rocco, J.W. Mutant allele tumor heterogeneity (MATH) and head and neck squamous cell carcinoma. Head Neck Pathol. 2015, 9, 1–5. [Google Scholar] [CrossRef]
- Kagohara, L.T.; Zamuner, F.; Davis-Marcisak, E.F.; Sharma, G.; Considine, M.; Allen, J.; Yegnasubramanian, S.; Gaykalova, D.A.; Fertig, E.J. Integrated single-cell and bulk gene expression and ATAC-seq reveals heterogeneity and early changes in pathways associated with resistance to cetuximab in HNSCC-sensitive cell lines. Br. J. Cancer 2020, 123, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, T.; Yoshizaki, T.; Kondo, S.; Furukawa, M.; Kaizaki, Y.; Pagano, J.S. Epstein-Barr Virus latent membrane protein 1 induces snail and epithelial-mesenchymal transition in metastatic nasopharyngeal carcinoma. Br. J. Cancer 2011, 104, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Kieser, A.; Sterz, K.R. The latent membrane protein 1 (LMP1). Curr. Top. Microbiol. Immunol. 2015, 391, 119–149. [Google Scholar] [CrossRef]
- Ye, D.; Zhu, J.; Zhao, Q.; Ma, W.; Xiao, Y.; Xu, G.; Zhang, Z. LMP1 Up-regulates calreticulin to induce epithelial-mesenchymal transition via TGF-β/Smad3/NRP1 Pathway in nasopharyngeal carcinoma cells. J. Cancer 2020, 11, 1257–1269. [Google Scholar] [CrossRef]
- Ammous-Boukhris, N.; Ayadi, W.; Derbel, M.; Allaya-Jaafar, N.; Charfi, S.; Daoud, J.; Sellami-Boudawara, T.; Mokdad-Gargouri, R. FOXA1 Expression in nasopharyngeal carcinoma: Association with clinicopathological characteristics and EMT markers. BioMed Res. Int. 2020, 2020, 4234632. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, L.; Li, J.; Wang, W.; Cai, J.; Ban, Y.; Zhou, Y.; Hu, M.; Mei, Y.; Zeng, Z.; et al. FOXA1 Suppresses the growth, migration, and invasion of nasopharyngeal carcinoma cells through repressing miR-100-5p and miR-125b-5p. J. Cancer 2020, 11, 2485–2495. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Cedar, H.; Bergman, Y. Linking DNA methylation and histone modification: Patterns and paradigms. Nat. Rev. Genet. 2009, 10, 295–304. [Google Scholar] [CrossRef]
- Lin, Y.; Dong, C.; Zhou, B.P. Epigenetic regulation of EMT: The Snail story. Curr. Pharm. Des. 2014, 20, 1698–1705. [Google Scholar] [CrossRef]
- Fukagawa, A.; Ishii, H.; Miyazawa, K.; Saitoh, M. δEF1 associates with DNMT1 and maintains DNA methylation of the E-cadherin promoter in breast cancer cells. Cancer Med. 2015, 4, 125–135. [Google Scholar] [CrossRef]
- Subramaniam, D.; Thombre, R.; Dhar, A.; Anant, S. DNA methyltransferases: A novel target for prevention and therapy. Front. Oncol. 2014, 4, 80. [Google Scholar] [CrossRef]
- Lyko, F. The DNA methyltransferase family: A versatile toolkit for epigenetic regulation. Nat. Rev. Genet. 2018, 19, 81–92. [Google Scholar] [CrossRef]
- Luczak, M.W.; Jagodziński, P.P. The role of DNA methylation in cancer development. Folia Histochem. Cytobiol. 2006, 44, 143–154. [Google Scholar]
- Bestor, T.H. The DNA methyltransferases of mammals. Hum. Mol. Genet. 2000, 9, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Chen, L.H.; Hsu, W.L.; Tseng, Y.J.; Liu, D.W.; Weng, C.F. Involvement of DNMT 3B promotes epithelial-mesenchymal transition and gene expression profile of invasive head and neck squamous cell carcinomas cell lines. BMC Cancer 2016, 16, 431. [Google Scholar] [CrossRef]
- Cardenas, H.; Vieth, E.; Lee, J.; Segar, M.; Liu, Y.; Nephew, K.P.; Matei, D. TGF-β induces global changes in DNA methylation during the epithelial-to-mesenchymal transition in ovarian cancer cells. Epigenetics 2014, 9, 1461–1472. [Google Scholar] [CrossRef]
- Shiah, S.G.; Hsiao, J.R.; Chang, H.J.; Hsu, Y.M.; Wu, G.H.; Peng, H.Y.; Chou, S.T.; Kuo, C.C.; Chang, J.Y. MiR-30a and miR-379 modulate retinoic acid pathway by targeting DNA methyltransferase 3B in oral cancer. J. Biomed. Sci. 2020, 27, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Du, S.; Zhang, J.; Wang, Y.; Wu, Q.; Ni, J. Polymorphism of DNA methyltransferase 3B-149C/T and cancer risk: A meta-analysis. Med. Oncol. 2015, 32, 399. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Sugimoto, K.; Nozaki, M.; Ueda, J.; Ohta, T.; Ohki, M.; Fukuda, M.; Takeda, N.; Niida, H.; Kato, H.; et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002, 16, 1779–1791. [Google Scholar] [CrossRef]
- Lehnertz, B.; Pabst, C.; Su, L.; Miller, M.; Liu, F.; Yi, L.; Zhang, R.; Krosl, J.; Yung, E.; Kirschner, J.; et al. The methyltransferase G9a regulates HoxA9-dependent transcription in AML. Genes Dev. 2014, 28, 317–327. [Google Scholar] [CrossRef]
- Dong, C.; Wu, Y.; Yao, J.; Wang, Y.; Yu, Y.; Rychahou, P.G.; Evers, B.M.; Zhou, B.P. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J. Clin. Investig. 2012, 122, 1469–1486. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Shen, L.; Ahmed, S.; Boumber, Y.; Sekido, Y.; Haddad, B.R.; Issa, J.-P.J. Downregulation of histone H3 lysine 9 methyltransferase G9a induces centrosome disruption and chromosome instability in cancer cells. PLoS ONE 2008, 3, e2037. [Google Scholar] [CrossRef]
- Chen, H.; Yan, Y.; Davidson, T.L.; Shinkai, Y.; Costa, M. Hypoxic stress induces dimethylated histone H3 lysine 9 through histone methyltransferase G9a in mammalian cells. Cancer Res. 2006, 66, 9009–9016. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, T.; Wang, X.; Zhao, E.; Choi, J.H.; Yang, L.; Zha, Y.; Dong, Z.; Huang, S.; Asara, J.M.; et al. The histone H3 methyltransferase G9A epigenetically activates the serine-glycine synthesis pathway to sustain cancer cell survival and proliferation. Cell Metab. 2013, 18, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ye, D.; Guo, W.; Yu, W.; He, Y.; Hu, J.; Wang, Y.; Zhang, L.; Liao, Y.; Song, H.; et al. G9a is essential for EMT-mediated metastasis and maintenance of cancer stem cell-like characters in head and neck squamous cell carcinoma. Oncotarget 2015, 6, 6887–6901. [Google Scholar] [CrossRef]
- Pinho, S.S.; Osório, H.; Nita-Lazar, M.; Gomes, J.; Lopes, C.; Gärtner, F.; Reis, C.A. Role of E-cadherin N-glycosylation profile in a mammary tumor model. Biochem. Biophys. Res. Commun. 2009, 379, 1091–1096. [Google Scholar] [CrossRef]
- Vargas, D.A.; Sun, M.; Sadykov, K.; Kukuruzinska, M.A.; Zaman, M.H. The integrated role of Wnt/β-catenin, N-glycosylation, and E-cadherin-mediated adhesion in network dynamics. PLoS Comput. Biol. 2016, 12, e1005007. [Google Scholar] [CrossRef] [PubMed]
- Varelas, X.; Bouchie, M.P.; Kukuruzinska, M.A. Protein N-glycosylation in oral cancer: Dysregulated cellular networks among DPAGT1, E-cadherin adhesion and canonical Wnt signaling. Glycobiology 2014, 24, 579–591. [Google Scholar] [CrossRef]
- Liu, G.; Sengupta, P.K.; Jamal, B.; Yang, H.Y.; Bouchie, M.P.; Lindner, V.; Varelas, X.; Kukuruzinska, M.A. N-glycosylation induces the CTHRC1 protein and drives oral cancer cell migration. J. Biol. Chem. 2013, 288, 20217–20227. [Google Scholar] [CrossRef]
- Sengupta, P.K.; Bouchie, M.P.; Nita-Lazar, M.; Yang, H.Y.; Kukuruzinska, M.A. Coordinate regulation of N-glycosylation gene DPAGT1, canonical Wnt signaling and E-cadherin adhesion. J. Cell Sci. 2013, 126, 484–496. [Google Scholar] [CrossRef]
- Jamal, B.; Sengupta, P.K.; Gao, Z.N.; Nita-Lazar, M.; Amin, B.; Jalisi, S.; Bouchie, M.P.; Kukuruzinska, M.A. Aberrant amplification of the crosstalk between canonical Wnt signaling and N-glycosylation gene DPAGT1 promotes oral cancer. Oral Oncol. 2012, 48, 523–529. [Google Scholar] [CrossRef]
- Joseph, J.P.; Harishankar, M.K.; Pillai, A.A.; Devi, A. Hypoxia induced EMT: A review on the mechanism of tumor progression and metastasis in OSCC. Oral Oncol. 2018, 80, 23–32. [Google Scholar] [CrossRef]
- Möglich, A.; Ayers, R.A.; Moffat, K. Structure and signaling mechanism of Per-ARNT-Sim domains. Structure 2009, 17, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Brahimi-Horn, M.C.; Pouysségur, J. Hypoxia in cancer cell metabolism and pH regulation. Essays Biochem. 2007, 43, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.H.; Semenza, G.L.; Bauer, C.; Marti, H.H. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am. J. Physiol. 1996, 271, C1172–C1180. [Google Scholar] [CrossRef] [PubMed]
- Dunwoodie, S.L. The role of hypoxia in development of the mammalian embryo. Dev. Cell 2009, 17, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ning, X.; Zhang, Y.; Lu, Y.; Nie, Y.; Han, S.; Liu, L.; Du, R.; Xia, L.; He, L.; et al. Hypoxia-inducible factor-1alpha induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transition. Kidney Int. 2009, 75, 1278–1287. [Google Scholar] [CrossRef]
- Domingos, P.L.B.; Souza, M.G.; Guimarães, T.A.; Santos, E.S.; Farias, L.C.; de Carvalho Fraga, C.A.; Jones, K.M.; Santos, S.H.S.; de Paula, A.M.B.; Guimarães, A.L.S. Hypoxia reduces the E-cadherin expression and increases OSCC cell migration regardless of the E-cadherin methylation profile. Pathol. Res. Pract 2017, 213, 496–501. [Google Scholar] [CrossRef]
- Duan, Y.; He, Q.; Yue, K.; Si, H.; Wang, J.; Zhou, X.; Wang, X. Hypoxia induced Bcl-2/Twist1 complex promotes tumor cell invasion in oral squamous cell carcinoma. Oncotarget 2017, 8, 7729–7739. [Google Scholar] [CrossRef] [PubMed]
- Ramamonjisoa, N.; Ackerstaff, E. Characterization of the tumor microenvironment and tumor-stroma interaction by non-invasive preclinical imaging. Front. Oncol. 2017, 7, 3. [Google Scholar] [CrossRef]
- Curry, J.M.; Sprandio, J.; Cognetti, D.; Luginbuhl, A.; Bar-ad, V.; Pribitkin, E.; Tuluc, M. Tumor microenvironment in head and neck squamous cell carcinoma. Semin. Oncol. 2014, 41, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Peltanova, B.; Raudenska, M.; Masarik, M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: A systematic review. Mol. Cancer 2019, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Dobrenis, K.; Gauthier, L.R.; Barroca, V.; Magnon, C. Granulocyte colony-stimulating factor off-target effect on nerve outgrowth promotes prostate cancer development. Int. J. Cancer 2015, 136, 982–988. [Google Scholar] [CrossRef]
- Hu, P.; Wang, G.; Shen, M.; Zhang, P.; Zhang, J.; Du, J.; Liu, Q. Intratumoral polymorphonuclear granulocyte is associated with poor prognosis in squamous esophageal cancer by promoting epithelial-mesenchymal transition. Future Oncol. 2015, 11, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Oft, M.; Heider, K.H.; Beug, H. TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr. Biol. 1998, 8, 1243–1252. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, Z.; Zhou, W.; Wu, Q.; Donnola, S.; Liu, J.K.; Fang, X.; Sloan, A.E.; Mao, Y.; Lathia, J.D.; et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 2013, 153, 139–152. [Google Scholar] [CrossRef]
- Quezada, S.A.; Peggs, K.S.; Simpson, T.R.; Allison, J.P. Shifting the equilibrium in cancer immunoediting: From tumor tolerance to eradication. Immunol. Rev. 2011, 241, 104–118. [Google Scholar] [CrossRef]
- Bremnes, R.M.; Dønnem, T.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Sirera, R.; Camps, C.; Marinez, I.; Busund, L.T. The role of tumor stroma in cancer progression and prognosis: Emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 209–217. [Google Scholar] [CrossRef]
- Cirri, P.; Chiarugi, P. Cancer associated fibroblasts: The dark side of the coin. Am. J. Cancer Res. 2011, 1, 482–497. [Google Scholar]
- Alcolea, S.; Antón, R.; Camacho, M.; Soler, M.; Alfranca, A.; Avilés-Jurado, F.-X.; Redondo, J.-M.; Quer, M.; León, X.; Vila, L. Interaction between head and neck squamous cell carcinoma cells and fibroblasts in the biosynthesis of PGE2. J. Lipid Res. 2012, 53, 630–642. [Google Scholar] [CrossRef]
- Mironska, A.; Łukaszewicz-Zajac, M.; Mroczko, B. Clinical significance of selected chemokines in thyroid cancer. Anticancer Res. 2019, 39, 2715–2720. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zafar, M.S.; Khan, R.S.; Najeeb, S.; Slowey, P.D.; Rehman, I.U. Role of salivary biomarkers in oral cancer detection. Adv. Clin. Chem. 2018, 86, 23–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, Z.-M.; Huang, H.-B.; Sun, L.-S.; Sun, A.-Q.; Li, K. Association of IL-8 gene promoter-251 A/T and IL-18 gene promoter-137 G/C polymorphisms with head and neck cancer risk: A comprehensive meta-analysis. Cancer Manag. Res. 2018, 10, 2589–2604. [Google Scholar] [CrossRef]
- Riley, P.; Glenny, A.M.; Worthington, H.V.; Littlewood, A.; Fernandez Mauleffinch, L.M.; Clarkson, J.E.; McCabe, M.G. Interventions for preventing oral mucositis in patients with cancer receiving treatment: Cytokines and growth factors. Cochrane Database Syst. Rev. 2017, 11, Cd011990. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Nassri, A.; Kindt, N.; Brown, D.N.; Journe, F.; Saussez, S. Role of macrophage migration inhibitory factor in head and neck cancer and novel therapeutic targets: A systematic review. Head Neck 2017, 39, 2573–2584. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Chen, S.-W.; Liu, L.-L.; Li, L.; Gao, F.; Zhuang, S.-M.; Wang, L.-P.; Li, Y.; Song, M. Cancer-associated fibroblasts provide a suitable microenvironment for tumor development and progression in oral tongue squamous cancer. J. Transl. Med. 2015, 13, 198. [Google Scholar] [CrossRef]
- Leef, G.; Thomas, S.M. Molecular communication between tumor-associated fibroblasts and head and neck squamous cell carcinoma. Oral Oncol. 2013, 49, 381–386. [Google Scholar] [CrossRef]
- Bocci, F.; Tripathi, S.C.; Vilchez Mercedes, S.A.; George, J.T.; Casabar, J.P.; Wong, P.K.; Hanash, S.M.; Levine, H.; Onuchic, J.N.; Jolly, M.K. NRF2 activates a partial epithelial-mesenchymal transition and is maximally present in a hybrid epithelial/mesenchymal phenotype. Integr. Biol. 2019, 11, 251–263. [Google Scholar] [CrossRef]
- Lin, D.T.; Subbaramaiah, K.; Shah, J.P.; Dannenberg, A.J.; Boyle, J.O. Cyclooxygenase-2: A novel molecular target for the prevention and treatment of head and neck cancer. Head Neck 2002, 24, 792–799. [Google Scholar] [CrossRef]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef]
- Chow, V.; Yuen, A.P.; Lam, K.Y.; Tsao, G.S.; Ho, W.K.; Wei, W.I. A comparative study of the clinicopathological significance of E-cadherin and catenins (alpha, beta, gamma) expression in the surgical management of oral tongue carcinoma. J. Cancer Res. Clin. Oncol. 2001, 127, 59–63. [Google Scholar] [CrossRef]
- Fontemaggi, G.; Gurtner, A.; Damalas, A.; Costanzo, A.; Higashi, Y.; Sacchi, A.; Strano, S.; Piaggio, G.; Blandino, G. deltaEF1 repressor controls selectively p53 family members during differentiation. Oncogene 2005, 24, 7273–7280. [Google Scholar] [CrossRef]
- St John, M.A.; Dohadwala, M.; Luo, J.; Wang, G.; Lee, G.; Shih, H.; Heinrich, E.; Krysan, K.; Walser, T.; Hazra, S.; et al. Proinflammatory mediators upregulate snail in head and neck squamous cell carcinoma. Clin. Cancer Res. 2009, 15, 6018–6027. [Google Scholar] [CrossRef] [PubMed]
- St John, M.A. Inflammatory mediators drive metastasis and drug resistance in head and neck squamous cell carcinoma. Laryngoscope 2015, 125, S1–S11. [Google Scholar] [CrossRef] [PubMed]
- Karpathiou, G.; Vieville, M.; Gavid, M.; Camy, F.; Dumollard, J.M.; Magné, N.; Froudarakis, M.; Prades, J.M.; Peoc’h, M. Prognostic significance of tumor budding, tumor-stroma ratio, cell nests size, and stroma type in laryngeal and pharyngeal squamous cell carcinomas. Head Neck 2019, 41, 1918–1927. [Google Scholar] [CrossRef]
- Libra, M.; Scalisi, A.; Vella, N.; Clementi, S.; Sorio, R.; Stivala, F.; Spandidos, D.A.; Mazzarino, C. Uterine cervical carcinoma: Role of matrix metalloproteinases (review). Int. J. Oncol. 2009, 34, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.H.; Zhu, W.; Li, M.Y.; Li, X.H.; Yi, H.; Zeng, G.Q.; Wan, X.X.; He, Q.Y.; Li, J.H.; Qu, J.Q.; et al. Activation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT-like phenotype change and MMP-9-mediated degradation of E-cadherin. J. Cell Biochem. 2011, 112, 2508–2517. [Google Scholar] [CrossRef]
- Aseervatham, J.; Ogbureke, K.U.E. Effects of DSPP and MMP20 silencing on adhesion, metastasis, angiogenesis, and epithelial-mesenchymal transition proteins in oral squamous cell carcinoma cells. Int. J. Mol. Sci. 2020, 21, 4734. [Google Scholar] [CrossRef]
- Ardalan Khales, S.; Abbaszadegan, M.R.; Majd, A.; Forghanifard, M.M. TWIST1 upregulates matrix metalloproteinase (MMP) genes family in esophageal squamous carcinoma cells. Gene Expr. Patterns 2020, 37, 119127. [Google Scholar] [CrossRef]
- Pietruszewska, W.; Bojanowska-Poźniak, K.; Kobos, J. Matrix metalloproteinases MMP1, MMP2, MMP9 and their tissue inhibitors TIMP1, TIMP2, TIMP3 in head and neck cancer: An immunohistochemical study. Otolaryngol. Pol. 2016, 70, 32–43. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, A.C.; Kowalski, L.P.; Campos, A.H.; Soares, F.A.; Carvalho, A.L.; Vettore, A.L. Clinical significance of molecular alterations in histologically negative surgical margins of head and neck cancer patients. Oral Oncol. 2012, 48, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Wang, C.W. S100A4 promotes squamous cell laryngeal cancer Hep-2 cell invasion via NF-kB/MMP-9 signal. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1361–1367. [Google Scholar]
- Yan, X.; Cao, N.; Chen, Y.; Lan, H.Y.; Cha, J.H.; Yang, W.H.; Yang, M.H. MT4-MMP promotes invadopodia formation and cell motility in FaDu head and neck cancer cells. Biochem. Biophys. Res. Commun. 2020, 522, 1009–1014. [Google Scholar] [CrossRef]
- Huang, C.-H.; Yang, W.-H.; Chang, S.-Y.; Tai, S.-K.; Tzeng, C.-H.; Kao, J.-Y.; Wu, K.-J.; Yang, M.-H. Regulation of membrane-type 4 matrix metalloproteinase by SLUG contributes to hypoxia-mediated metastasis. Neoplasia 2009, 11, 1371–1382. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Iorio, M.V.; Croce, C.M. MicroRNAs in cancer: Small molecules with a huge impact. J. Clin. Oncol. 2009, 27, 5848–5856. [Google Scholar] [CrossRef]
- Cho, E.S.; Kang, H.E.; Kim, N.H.; Yook, J.I. Therapeutic implications of cancer epithelial-mesenchymal transition (EMT). Arch. Pharmacal Res. 2019, 42, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Yin, J.J.; Abou-Kheir, W.; Hynes, P.G.; Casey, O.M.; Fang, L.; Yi, M.; Stephens, R.M.; Seng, V.; Sheppard-Tillman, H.; et al. MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via Slug-independent mechanisms. Oncogene 2013, 32, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, J.S.; Lee, S.; Lee, H.; Yoon, J.S.; Hong, J.H.; Chun, S.H.; Sun, S.; Won, H.S.; Hong, S.A.; et al. QKI, a miR-200 target gene, suppresses epithelial-to-mesenchymal transition and tumor growth. Int. J. Cancer 2019, 145, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhu, H.; Lei, Q.; Chen, L.; Yang, D.; Sui, W. MicroRNA-149-3p inhibits cell proliferation by targeting AKT2 in oral squamous cell carcinoma. Mol. Med. Rep. 2021, 23. [Google Scholar] [CrossRef]
- Dou, C.; Liu, Z.; Xu, M.; Jia, Y.; Wang, Y.; Li, Q.; Yang, W.; Zheng, X.; Tu, K.; Liu, Q. miR-187-3p inhibits the metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting S100A4. Cancer Lett. 2016, 381, 380–390. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, D.; Zhu, L.; Zhong, S.; Zhao, J.; Tang, J. miR-149 in human cancer: A systemic review. J. Cancer 2018, 9, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tao, C.; Dai, L.; Cui, C.; Chen, C.; Wu, H.; Wei, Q.; Zhou, X. MicroRNA-625 inhibits cell invasion and epithelial-mesenchymal transition by targeting SOX4 in laryngeal squamous cell carcinoma. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Tiwari, N.; Tiwari, V.K.; Waldmeier, L.; Balwierz, P.J.; Arnold, P.; Pachkov, M.; Meyer-Schaller, N.; Schübeler, D.; van Nimwegen, E.; Christofori, G. Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell 2013, 23, 768–783. [Google Scholar] [CrossRef]
- Vervoort, S.J.; van Boxtel, R.; Coffer, P.J. The role of SRY-related HMG box transcription factor 4 (SOX4) in tumorigenesis and metastasis: Friend or foe? Oncogene 2013, 32, 3397–3409. [Google Scholar] [CrossRef]
- Noda, T.; Nagano, H.; Takemasa, I.; Yoshioka, S.; Murakami, M.; Wada, H.; Kobayashi, S.; Marubashi, S.; Takeda, Y.; Dono, K.; et al. Activation of Wnt/beta-catenin signalling pathway induces chemoresistance to interferon-alpha/5-fluorouracil combination therapy for hepatocellular carcinoma. Br. J. Cancer 2009, 100, 1647–1658. [Google Scholar] [CrossRef]
- Flahaut, M.; Meier, R.; Coulon, A.; Nardou, K.A.; Niggli, F.K.; Martinet, D.; Beckmann, J.S.; Joseph, J.M.; Mühlethaler-Mottet, A.; Gross, N. The Wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the Wnt/beta-catenin pathway. Oncogene 2009, 28, 2245–2256. [Google Scholar] [CrossRef]
- Guo, K.; Wolf, V.; Dharmarajan, A.M.; Feng, Z.; Bielke, W.; Saurer, S.; Friis, R. Apoptosis-associated gene expression in the corpus luteum of the rat. Biol. Reprod. 1998, 58, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Lacher, M.D.; Siegenthaler, A.; Jäger, R.; Yan, X.; Hett, S.; Xuan, L.; Saurer, S.; Lareu, R.R.; Dharmarajan, A.M.; Friis, R. Role of DDC-4/sFRP-4, a secreted frizzled-related protein, at the onset of apoptosis in mammary involution. Cell Death Differ. 2003, 10, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.F.; Zhao, W.; Bentel, J.; Shearwood, A.M.; Zeps, N.; Joseph, D.; Iacopetta, B.; Dharmarajan, A. Expression of sFRP-4 and beta-catenin in human colorectal carcinoma. Cancer Lett. 2006, 231, 129–137. [Google Scholar] [CrossRef]
- Hewitt, D.P.; Mark, P.J.; Dharmarajan, A.M.; Waddell, B.J. Placental expression of secreted frizzled related protein-4 in the rat and the impact of glucocorticoid-induced fetal and placental growth restriction. Biol. Reprod. 2006, 75, 75–81. [Google Scholar] [CrossRef]
- Warrier, S.; Bhuvanalakshmi, G.; Arfuso, F.; Rajan, G.; Millward, M.; Dharmarajan, A. Cancer stem-like cells from head and neck cancers are chemosensitized by the Wnt antagonist, sFRP4, by inducing apoptosis, decreasing stemness, drug resistance and epithelial to mesenchymal transition. Cancer Gene Ther. 2014, 21, 381–388. [Google Scholar] [CrossRef]
- Palazzi, M.; Maluta, S.; Dall’Oglio, S.; Romano, M. The role of hyperthermia in the battle against cancer. Tumori 2010, 96, 902–910. [Google Scholar] [CrossRef]
- Yu, J.; Liang, P.; Yu, X.; Wang, Y.; Gao, Y. Ultrasound-guided percutaneous microwave ablation of splenic metastasis: Report of four cases and literature review. Int. J. Hyperth. 2011, 27, 517–522. [Google Scholar] [CrossRef]
- Lui, P.C.; Fan, Y.S.; Xu, G.; Ngai, C.Y.; Fung, K.P.; Tse, G.M.; Yu, A.M.; Li, J.Y. Apoptotic and necrotic effects of tumour necrosis factor-alpha potentiated with hyperthermia on L929 and tumour necrosis factor-alpha-resistant L929. Int. J. Hyperth. 2010, 26, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Alcala, M.A., Jr.; Park, K.; Yoo, J.; Lee, D.H.; Park, B.H.; Lee, B.C.; Bartlett, D.L.; Lee, Y.J. Effect of hyperthermia in combination with TRAIL on the JNK-Bim signal transduction pathway and growth of xenograft tumors. J. Cell Biochem. 2010, 110, 1073–1081. [Google Scholar] [CrossRef]
- Tang, Y.L.; Jiang, J.; Liu, J.; Zheng, M.; He, Y.W.; Chen, W.; Fan, Y.L.; Chen, Q.M.; Liao, C.H.; Liang, X.H. Hyperthermia inhibited the migration of tongue squamous cell carcinoma through TWIST2. J. Oral Pathol. Med. 2015, 44, 337–344. [Google Scholar] [CrossRef]
- Fowke, J.H. Head and neck cancer: A case for inhibition by isothiocyanates and indoles from cruciferous vegetables. Eur J. Cancer Prev. 2007, 16, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, Y.; Han, R.; Wang, S. Benzyl isothiocyanate inhibits invasion and induces apoptosis via reducing S100A4 expression and increases PUMA expression in oral squamous cell carcinoma cells. Braz. J. Med. Biol. Res. 2019, 52, e8409. [Google Scholar] [CrossRef]
- Guo, X.G.; Wang, S.; Xu, Y.B.; Zhuang, J. Propofol suppresses invasion, angiogenesis and survival of EC-1 cells in vitro by regulation of S100A4 expression. Eur Rev. Med. Pharmacol. Sci. 2015, 19, 4858–4865. [Google Scholar]
- Li, C.; Xia, M.; Wang, H.; Li, W.; Peng, J.; Jiang, H. Propofol facilitates migration and invasion of oral squamous cell carcinoma cells by upregulating SNAI1 expression. Life Sci. 2020, 241, 117143. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-González, R.; Ortiz-Sarabia, G.; Molina-Frechero, N.; Salas-Pacheco, J.M.; Salas-Pacheco, S.M.; Lavalle-Carrasco, J.; López-Verdín, S.; Tremillo-Maldonado, O.; Bologna-Molina, R. Epithelial–Mesenchymal Transition Associated with Head and Neck Squamous Cell Carcinomas: A Review. Cancers 2021, 13, 3027. https://doi.org/10.3390/cancers13123027
González-González R, Ortiz-Sarabia G, Molina-Frechero N, Salas-Pacheco JM, Salas-Pacheco SM, Lavalle-Carrasco J, López-Verdín S, Tremillo-Maldonado O, Bologna-Molina R. Epithelial–Mesenchymal Transition Associated with Head and Neck Squamous Cell Carcinomas: A Review. Cancers. 2021; 13(12):3027. https://doi.org/10.3390/cancers13123027
Chicago/Turabian StyleGonzález-González, Rogelio, Gamaliel Ortiz-Sarabia, Nelly Molina-Frechero, José Manuel Salas-Pacheco, Sergio Manuel Salas-Pacheco, Jesús Lavalle-Carrasco, Sandra López-Verdín, Omar Tremillo-Maldonado, and Ronell Bologna-Molina. 2021. "Epithelial–Mesenchymal Transition Associated with Head and Neck Squamous Cell Carcinomas: A Review" Cancers 13, no. 12: 3027. https://doi.org/10.3390/cancers13123027
APA StyleGonzález-González, R., Ortiz-Sarabia, G., Molina-Frechero, N., Salas-Pacheco, J. M., Salas-Pacheco, S. M., Lavalle-Carrasco, J., López-Verdín, S., Tremillo-Maldonado, O., & Bologna-Molina, R. (2021). Epithelial–Mesenchymal Transition Associated with Head and Neck Squamous Cell Carcinomas: A Review. Cancers, 13(12), 3027. https://doi.org/10.3390/cancers13123027









