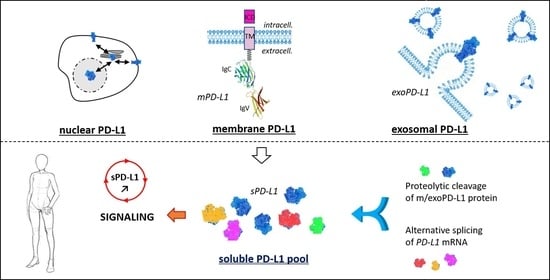
Soluble Programmed Death Ligand-1 (sPD-L1): A Pool of Circulating Proteins Implicated in Health and Diseases
Abstract
:Simple Summary
Abstract
1. The PD-1/PD-L1 Checkpoint
1.1. Membrane PD-L1
1.2. Exosomal PD-L1
1.3. Soluble PD-L1

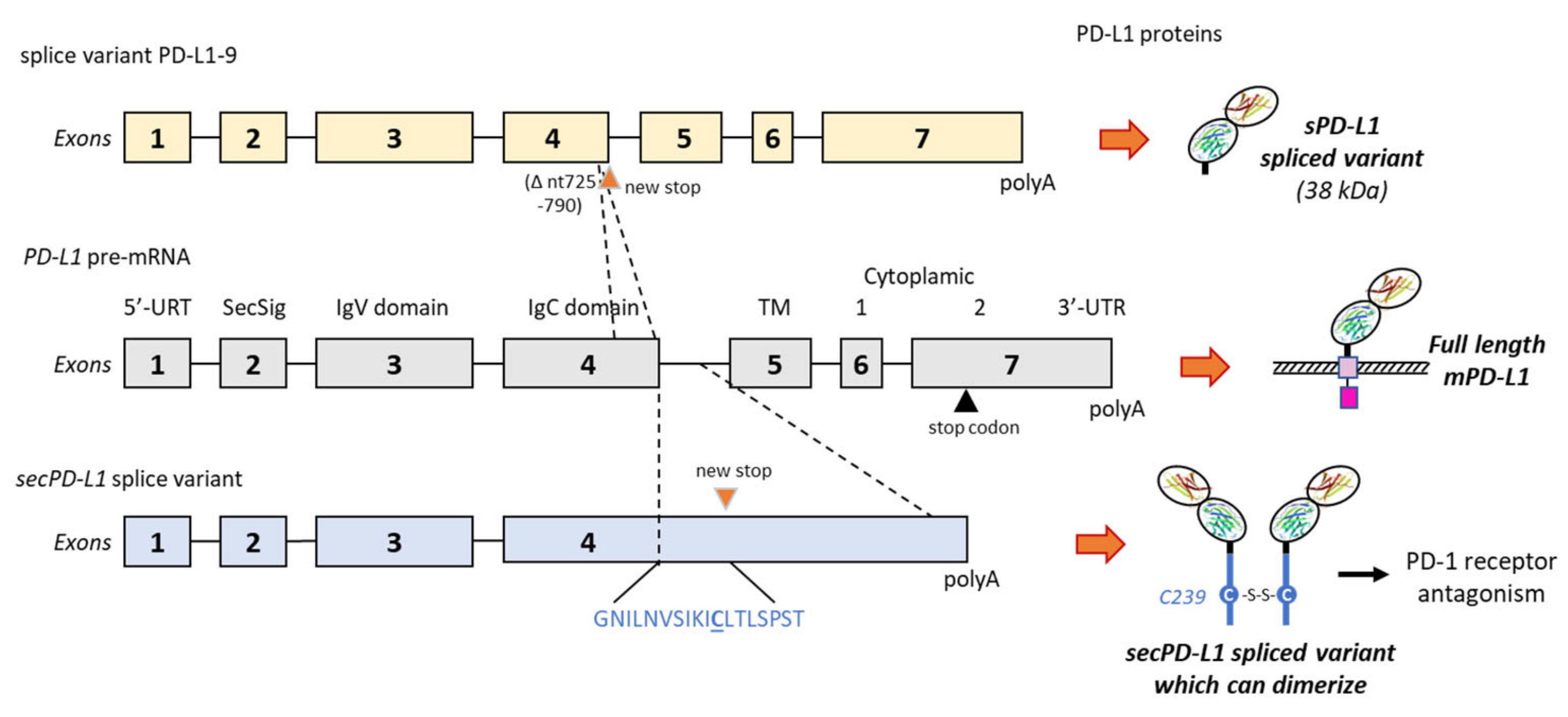
2. Generation of Soluble PD-L1: Proteolysis and Alternative Splicing
2.1. Structure and Domain Organization of mPD-L1/exPD-L1 Protein

2.2. Proteolytic Generation of sPD-L1
2.3. Alternative Splicing Generation of sPD-L1
3. Significance of Soluble PD-L1 in Cancer
3.1. sPD-L1 as a Cancer Biomarker
3.2. Functionality of sPD-L1 in Cancer
4. Soluble PD-L1 beyond Cancer
| Pathology or Condition | sPD-L1 Status and/or Function | References |
|---|---|---|
| Allergic rhinitis (AL) | Increased expression levels of both sPD-1 and sPD-L1 in peripheral blood of AR patients. | [134] |
| Acute liver failure (ALF) | sPD-L1 plasma levels increased in patients with ALF, notably in patients who developed sepsis or had a poor outcome. | [135] |
| Acute pancreatitis (AP) | Higher serum sPD-1 levels in AP patients with infection complications compared to patients without complication. Upregulation of sPD-L1 in patients with early AP and infectious complications. | [136,137] |
| HIV-1 infection (AIDS) | Much higher levels of sPD-L1 in HIV-infected patients compared to uninfected adults. | [138,139] |
| Acute respiratory distress syndrome (ARDS) | sPD-L1 upregulated in survivors of direct ARDS compared to non-survivors. sPD-L1 induces apoptosis of monocyte-derived macrophages in ARDS patients. | [140] |
| Community-acquired pneumonia (CAP) | Higher level of circulation sPD-L1 in patients with severe CAP compared to CAP group and healthy controls. Correlation between sPD-L1 level in CAP patients and survival prognosis. | [141] |
| Crohn’s disease | mPD-L1 cleaved from the cell surface by MMP-10 to generate a soluble form of PD-L1. | [28] |
| Chronic hepatitis C (CHC) | High level of serum sPD-L1 in CHC patients associated with disease progression. | [142] |
| Type 2 diabetes (T2DM) | Elevated amount of sPD-L1 (and IFN-g) in the sera of patients with T2DM compared to controls, notably in T2DM patients with an acute coronary syndrome. | [143] |
| Endometriosis | Elevated level of sPD-L1 in the serum and peritoneal fluid of patients with endometriosis vs. control. | [144] |
| Idiopathic pulmonary fibrosis (IPF) | Elevated concentrations of sPD-L1 in the serum of IPF patients compared to healthy population. | [27] |
| Immune thrombocytopenia (ITP) | Decreased levels of sPD-L1 in patients with newly diagnosed ITP compared to patients with chronic ITP. | [145] |
| Oral lichen planus (OLP) | Higher expression of sPD-1 and sPD-L1 in patients with OLP than in control group. Negative correlation between sPD-L1 expression level and CD4+ T lymphocytes. | [146] |
| Obstructive sleep apnea (OSA) | higher levels of sPD-L1 in severe OSA compared to mild OSA or non-OSA patients. | [147,148] |
| Recurrent aphthous ulcer (RAU) | Higher levels of both sPD-1 and sPD-L1 in RAU patients compared to control group. | [149] |
| Rheumatoid arthritis (RA) Psoriatic arthritis (PsA) | Increased concentrations of sPD-1 and sPD-L1 in knee synovial fluid and serum in the rabbits of the RA-model group compared to control. Increased levels of sPD-1 in RA and PsA. | [150,151] |
| Sepsis | High levels of circulating sPD-L1 in sepsis, positively correlated with the sepsis severity. | [135,152] |
| Systemic lupus erythematosus (SLE) | Higher levels of both sPD-1 and sPD-L1 in SLE patients compared to control group. | [153] |
| Cutaneous systemic sclerosis (SSc) | Elevated levels of sPD-L1 in patients with diffuse or limited cutaneous SSc. Possible marker of the severity of skin sclerosis. | [154] |
| Hantavirus-associated virus hemorrhagic fever (VHF) | High amounts of sPD-L1 and sPD-L2 in sera from hantavirus-infected patients. | [155] |
4.1. sPD-L1 in Pulmonary Diseases
4.2. sPD-L1 in Inflammatory and Autoimmune Diseases
4.3. sPD-L1 and Sepsis
4.4. sPD-L1 in Virus-Mediated Diseases
4.5. sPD-L1 in Pregnancy
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghosh, C.; Luong, G.; Sun, Y. A snapshot of the PD-1/PD-L1 pathway. J. Cancer 2021, 12, 2735–2746. [Google Scholar] [CrossRef]
- Zam, W.; Ali, L. Immune checkpoint inhibitors in the treatment of cancer. Curr. Clin. Pharmacol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Persico, P.; Lorenzi, E.; Dipasquale, A.; Pessina, F.; Navarria, P.; Politi, L.S.; Santoro, A.; Simonelli, M. Checkpoint inhibitors as high-grade gliomas treatment: State of the art and future perspectives. J. Clin. Med. 2021, 10, 1367. [Google Scholar] [CrossRef] [PubMed]
- Jimbu, L.; Mesaros, O.; Popescu, C.; Neaga, A.; Berceanu, I.; Dima, D.; Gaman, M.; Zdrenghea, M. Is there a place for PD-1-PD-L blockade in acute myeloid leukemia? Pharmaceuticals 2021, 14, 288. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Thuru, X.; Quesnel, B. Combined cytotoxic chemotherapy and immunotherapy of cancer: Modern times. NAR Cancer 2020, 2, zcaa002. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, C.; Zhu, S.; Liang, X.; Zhang, Q.; Luo, X.; Yuan, L.; Song, L. PD-1/PD-L1 immune checkpoint blockade-based combinational treatment: Immunotherapeutic amplification strategies against colorectal cancer. Int. Immunopharmacol. 2021, 96, 107607. [Google Scholar] [CrossRef]
- Sato, K.; Uehara, T.; Nakajima, T.; Iwaya, M.; Miyagawa, Y.; Watanabe, T.; Ota, H. Inverse correlation between PD-L1 expression and LGR5 expression in tumor budding of stage II/III colorectal cancer. Ann. Diagn. Pathol. 2021, 52, 151739. [Google Scholar] [CrossRef]
- Seliger, B. Basis of PD1/PD-L1 Therapies. J. Clin. Med. 2019, 8, 2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front. Immunol. 2019, 10, 2298. [Google Scholar] [CrossRef]
- Meggyes, M.; Nagy, D.U.; Szereday, L. Investigation of the PD-1 and PD-L1 immune checkpoint molecules throughout healthy human pregnancy and in nonpregnant women. J. Clin. Med. 2020, 9, 2536. [Google Scholar] [CrossRef]
- Dall’Olio, F.G.; Gelsomino, F.; Conci, N.; Marcolin, L.; De Giglio, A.; Grilli, G.; Sperandi, F.; Fontana, F.; Terracciano, M.; Fragomeno, B.; et al. PD-L1 expression in circulating tumor cells as a promising prognostic biomarker in advanced non-small-cell lung cancer treated with immune checkpoint inhibitors. Clin. Lung Cancer 2021. [Google Scholar] [CrossRef] [PubMed]
- Ntzifa, A.; Strati, A.; Kallergi, G.; Kotsakis, A.; Georgoulias, V.; Lianidou, E. Gene expression in circulating tumor cells reveals a dynamic role of EMT and PD-L1 during osimertinib treatment in NSCLC patients. Sci. Rep. 2021, 11, 2313. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Liu, Y.; Zhang, T.; Wang, Z.; Gu, M.; Li, Y.; Wang, D.D.; Li, W.; Lin, P.P. PD-L1+ aneuploid circulating tumor endothelial cells (CTECs) exhibit resistance to the checkpoint blockade immunotherapy in advanced NSCLC patients. Cancer Lett. 2020, 469, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, T. Expression and analysis of PD-L1 in peripheral blood circulating tumor cells of lung cancer. Future Oncol. 2021, 17, 1625–1635. [Google Scholar] [CrossRef]
- Kloten, V.; Lampignano, R.; Krahn, T.; Schlange, T. Circulating Tumor Cell PD-L1 expression as biomarker for therapeutic efficacy of immune checkpoint inhibition in NSCLC. Cells 2019, 8, 809. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yu, D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 455–468. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Tsai, H.I.; Xu, Z.; Yan, F.; Wu, Y.; Xiao, Y.; Liu, X.; Wu, Y.; Parvanian, S.; Zhu, W.; et al. Exosomal PD-L1 functions as an immunosuppressant to promote wound healing. J. Extracell Vesicles 2019, 9, 1709262. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Xu, M.; Lu, J.; Mao, L.; Wang, S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol. Cancer 2019, 18, 146. [Google Scholar] [CrossRef] [Green Version]
- Lawler, S.E.; Nowicki, M.O.; Ricklefs, F.L.; Chiocca, E.A. Immune escape mediated by exosomal PD-L1 in cancer. Adv. Biosyst. 2020, 4, e2000017. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, P.; Wang, Y.; Wang, J.; Su, M.; Wang, Y.; Zhou, L.; Zhou, J.; Xiong, W.; Zeng, Z.; et al. The biogenesis, biology, and clinical significance of exosomal PD-L1 in cancer. Front. Immunol. 2020, 11, 604. [Google Scholar] [CrossRef]
- Zhou, K.; Guo, S.; Li, F.; Sun, Q.; Liang, G. Exosomal PD-L1: New insights into tumor immune escape mechanisms and therapeutic strategies. Front. Cell. Dev. Biol. 2020, 8, 569219. [Google Scholar] [CrossRef]
- Morrissey, S.M.; Yan, J. Exosomal PD-L1: Roles in tumor progression and Immunotherapy. Trends Cancer 2020, 6, 550–558. [Google Scholar] [CrossRef]
- Liang, B.; Hu, X.; Ding, Y.; Liu, M. Tumor-derived exosomes in the PD-1/PD-L1 axis: Significant regulators as well as promising clinical targets. J. Cell Physiol. 2021, 236, 4138–4151. [Google Scholar] [CrossRef]
- Yin, Z.; Yu, M.; Ma, T.; Zhang, C.; Huang, S.; Karimzadeh, M.R.; Momtazi-Borojeni, A.A.; Chen, S. Mechanisms underlying low-clinical responses to PD-1/PD-L1 blocking antibodies in immunotherapy of cancer: A key role of exosomal PD-L1. J. Immunother. Cancer 2021, 9, e001698. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Zhang, C.; Meng, X.; Sun, B.; Zhang, G.; Fan, Y.; Kang, X. The clinical significance of soluble programmed cell death-ligand 1 (sPD-L1) in patients with gliomas. Front. Oncol. 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, D.; Roksandic Milenkovic, M.; Kotur Stevuljevic, J.; Markovic, J.; Ceriman, V.; Kontic, M.; Skodric Trifunovic, V. Membrane PD-L1 expression and soluble PD-L1 plasma levels in idiopathic pulmonary fibrosis-a pilot study. J. Thorac. Dis. 2018, 10, 6660–6669. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, J.E.; Beswick, E.J.; Grim, C.; Uribe, G.; Tafoya, M.; Chacon Palma, G.; Samedi, V.; McKee, R.; Villeger, R.; Fofanov, Y.; et al. Matrix metalloproteinases cleave membrane-bound PD-L1 on CD90+ (myo-)fibroblasts in Crohn’s disease and regulate Th1/Th17 cell responses. Int. Immunol. 2020, 32, 57–68. [Google Scholar] [CrossRef]
- Okuyama, M.; Mezawa, H.; Kawai, T.; Urashima, M. Elevated Soluble PD-L1 in pregnant women’s serum suppresses the immune reaction. Front. Immunol. 2019, 10, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.; Zhao, Z.; Arooj, S.; Fu, Y.; Liao, G. Soluble PD-1: Predictive, prognostic, and therapeutic value for cancer immunotherapy. Front. Immunol. 2020, 11, 587460. [Google Scholar] [CrossRef] [PubMed]
- Wuethrich, A.; Rajkumar, A.R.; Shanmugasundaram, K.B.; Reza, K.K.; Dey, S.; Howard, C.B.; Sina, A.A.; Trau, M. Single droplet detection of immune checkpoints on a multiplexed electrohydrodynamic biosensor. Analyst 2019, 144, 6914–6921. [Google Scholar] [CrossRef]
- Goto, M.; Chamoto, K.; Higuchi, K.; Yamashita, S.; Noda, K.; Iino, T.; Miura, M.; Yamasaki, T.; Ogawa, O.; Sonobe, M.; et al. Analytical performance of a new automated chemiluminescent magnetic immunoassays for soluble PD-1, PD-L1, and CTLA-4 in human plasma. Sci. Rep. 2019, 9, 10144. [Google Scholar] [CrossRef]
- Reza, K.K.; Sina, A.A.; Wuethrich, A.; Grewal, Y.S.; Howard, C.B.; Korbie, D.; Trau, M. A SERS microfluidic platform for targeting multiple soluble immune checkpoints. Biosens. Bioelectron. 2019, 126, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Luo, B.; Wang, Y.; Lu, H.; Wu, S.; Lu, Y.; Shi, S.; Li, L.; Jiang, S.; Zhao, M. Label-free and specific detection of soluble programmed death ligand-1 using a localized surface plasmon resonance biosensor based on excessively tilted fiber gratings. Biomed. Opt. Express. 2019, 10, 5136–5148. [Google Scholar] [CrossRef] [PubMed]
- Abu Hejleh, T.; Furqan, M.; Ballas, Z.; Clamon, G. The clinical significance of soluble PD-1 and PD-L1 in lung cancer. Crit. Rev. Oncol. Hematol. 2019, 143, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical significance of PD-L1(+) exosomes in plasma of head and neck cancer patients. Clin. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Chen, M.; Gu, J.; Niu, K.; Zhao, X.; Zheng, L.; Xu, Z.; Yu, Y.; Li, F.; Meng, L.; et al. Novel biomarkers of dynamic blood PD-L1 expression for immune checkpoint inhibitors in advanced non-small-cell lung cancer patients. Front. Immunol. 2021, 12, 665133. [Google Scholar] [CrossRef]
- Tu, X.; Qin, B.; Zhang, Y.; Zhang, C.; Kahila, M.; Nowsheen, S.; Yin, P.; Yuan, J.; Pei, H.; Li, H.; et al. PD-L1 (B7-H1) Competes with the RNA exosome to regulate the DNA damage response and can be targeted to sensitize to radiation or chemotherapy. Mol. Cell 2019, 74, 1215–1226. [Google Scholar] [CrossRef]
- Yu, J.; Qin, B.; Moyer, A.M.; Nowsheen, S.; Tu, X.; Dong, H.; Boughey, J.C.; Goetz, M.P.; Weinshilboum, R.; Lou, Z.; et al. Regulation of sister chromatid cohesion by nuclear PD-L1. Cell Res. 2020, 30, 590–601. [Google Scholar] [CrossRef]
- Du, W.; Zhu, J.; Zeng, Y.; Liu, T.; Zhang, Y.; Cai, T.; Fu, Y.; Zhang, W.; Zhang, R.; Liu, Z.; et al. KPNB1-mediated nuclear translocation of PD-L1 promotes non-small cell lung cancer cell proliferation via the Gas6/MerTK signaling pathway. Cell Death Differ. 2021, 28, 1284–1300. [Google Scholar] [CrossRef]
- Yoshida, J.; Ishikawa, T.; Doi, T.; Ota, T.; Yasuda, T.; Okayama, T.; Sakamoto, N.; Inoue, K.; Dohi, O.; Yoshida, N.; et al. Clinical significance of soluble forms of immune checkpoint molecules in advanced esophageal cancer. Med. Oncol. 2019, 36, 60. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lee, K.H.; Kim, J.W.; Suh, K.J.; Nam, A.R.; Bang, J.H.; Jin, M.H.; Oh, K.S.; Kim, J.M.; Kim, T.Y.; et al. The prognostic role of soluble transforming growth factor-beta and its correlation with soluble programmed death-ligand 1 in biliary tract cancer. Liver Int. 2021, 41, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Nam, A.R.; Bang, J.H.; Park, J.E.; Kim, T.Y.; Lee, K.H.; Han, S.W.; Im, S.A.; Kim, T.Y.; Bang, Y.J.; et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget 2016, 7, 76604–76612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mair, M.J.; Pajenda, S.; Ilhan-Mutlu, A.; Steindl, A.; Kiesel, B.; Widhalm, G.; Dieckmann, K.; Feldmann, K.; Hainfellner, J.; Marosi, C.; et al. Soluble PD-L1 is associated with local and systemic inflammation markers in primary and secondary brain tumours. ESMO Open 2020, 5, e000863. [Google Scholar] [CrossRef] [PubMed]
- Larrinaga, G.; Solano-Iturri, J.D.; Errarte, P.; Unda, M.; Loizaga-Iriarte, A.; Pérez-Fernández, A.; Echevarría, E.; Asumendi, A.; Manini, C.; Angulo, J.C.; et al. Soluble PD-L1 is an independent prognostic factor in clear cell renal cell carcinoma. Cancers 2021, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Montemagno, C.; Hagege, A.; Borchiellini, D.; Thamphya, B.; Rastoin, O.; Ambrosetti, D.; Iovanna, J.; Rioux-Leclercq, N.; Porta, C.; Negrier, S.; et al. Soluble forms of PD-L1 and PD-1 as prognostic and predictive markers of sunitinib efficacy in patients with metastatic clear cell renal cell carcinoma. Oncoimmunology 2020, 9, 1846901. [Google Scholar] [CrossRef] [PubMed]
- Omura, Y.; Toiyama, Y.; Okugawa, Y.; Yin, C.; Shigemori, T.; Kusunoki, K.; Kusunoki, Y.; Ide, S.; Shimura, T.; Fujikawa, H.; et al. Prognostic impacts of tumoral expression and serum levels of PD-L1 and CTLA-4 in colorectal cancer patients. Cancer Immunol. Immunother. 2020, 69, 2533–2546. [Google Scholar] [CrossRef]
- Zhou, J.; Mahoney, K.M.; Giobbie-Hurder, A.; Zhao, F.; Lee, S.; Liao, X.; Rodig, S.; Li, J.; Wu, X.; Butterfield, L.H.; et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol. Res. 2017, 5, 480–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, Y.; Yu, J.; Li, Y.; Li, L.; Zhou, S.; Zhang, T.; Li, L.; Qiu, L.; Meng, B.; Pan, Y.; et al. Plasma soluble PD-L1 and STAT3 predict the prognosis in diffuse large B cell lymphoma patients. J. Cancer 2020, 11, 7001–7008. [Google Scholar] [CrossRef]
- Cho, I.; Lee, H.; Yoon, S.E.; Ryu, K.J.; Ko, Y.H.; Kim, W.S.; Kim, S.J. Serum levels of soluble programmed death-ligand 1 (sPD-L1) in patients with primary central nervous system diffuse large B-cell lymphoma. BMC Cancer 2020, 20, 120. [Google Scholar] [CrossRef] [Green Version]
- Buderath, P.; Schwich, E.; Jensen, C.; Horn, P.A.; Kimmig, R.; Kasimir-Bauer, S.; Rebmann, V. Soluble programmed death receptor ligands sPD-L1 and sPD-L2 as liquid biopsy markers for prognosis and platinum response in epithelial ovarian cancer. Front. Oncol. 2019, 9, 1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koukourakis, M.I.; Kontomanolis, E.; Sotiropoulou, M.; Mitrakas, A.; Dafa, E.; Pouliliou, S.; Sivridis, E.; Giatromanolaki, A. Increased soluble PD-L1 levels in the plasma of patients with epithelial ovarian cancer correlate with plasma levels of miR34a and miR200. Anticancer Res. 2018, 38, 5739–5745. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Wei, P.; Guo, Y.; Shi, D.; Yu, B.H.; Su, Y.F.; Li, X.Q.; Zhou, X.Y. Clinical significance of circulating exosomal PD-L1 and soluble PD-L1 in extranodal NK/T-cell lymphoma, nasal-type. Am. J. Cancer Res. 2020, 10, 4498–4512. [Google Scholar]
- Feng, Y.; Jing, C.; Yu, X.; Cao, X.; Xu, C. Predicting treatment response of patients with extranodal natural killer/T-cell lymphoma based on levels of PD-L1 mRNA and soluble PD-L1. Hematol. Oncol. 2020, 38, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Mocan, T.; Ilies, M.; Nenu, I.; Craciun, R.; Horhat, A.; Susa, R.; Minciuna, I.; Rusu, I.; Mocan, L.P.; Seicean, A.; et al. Serum levels of soluble programmed death-ligand 1 (sPD-L1): A possible biomarker in predicting post-treatment outcomes in patients with early hepatocellular carcinoma. Int. Immunopharmacol. 2021, 94, 107467. [Google Scholar] [CrossRef]
- Li, X.S.; Li, J.W.; Li, H.; Jiang, T. Prognostic value of programmed cell death ligand 1 (PD-L1) for hepatocellular carcinoma: A meta-analysis. Biosci. Rep. 2020, 40, BSR20200459. [Google Scholar] [CrossRef] [Green Version]
- El-Gebaly, F.; Abou-Saif, S.; Elkadeem, M.; Helmy, A.; Abd-Elsalam, S.; Yousef, M.; Elkhouly, R.A.; Amer, I.F.; El-Demerdash, T. Study of serum soluble programmed death ligand 1 as a prognostic factor in hepatocellular carcinoma in Egyptian patients. Curr. Cancer Drug Targets. 2019, 19, 896–905. [Google Scholar] [CrossRef]
- Veldman, J.; Alsada, Z.N.D.; van den Berg, A.; Plattel, W.J.; Diepstra, A.; Visser, L. Soluble PD-L1 is a promising disease biomarker but does not reflect tissue expression in classic Hodgkin lymphoma. Br. J. Haematol. 2021, 193, 506–514. [Google Scholar] [CrossRef]
- Chiarucci, C.; Cannito, S.; Daffinà, M.G.; Amato, G.; Giacobini, G.; Cutaia, O.; Lofiego, M.F.; Fazio, C.; Giannarelli, D.; Danielli, R.; et al. Circulating levels of PD-L1 in mesothelioma patients from the NIBIT-MESO-1 study: Correlation with survival. Cancers 2020, 12, 361. [Google Scholar] [CrossRef] [Green Version]
- Carosio, R.; Fontana, V.; Mastracci, L.; Ferro, P.; Grillo, F.; Banelli, B.; Canessa, P.A.; Dessanti, P.; Vigani, A.; Morabito, A.; et al. Characterization of soluble PD-L1 in pleural effusions of mesothelioma patients: Potential implications in the immune response and prognosis. J. Cancer Res. Clin. Oncol. 2021, 147, 459–468. [Google Scholar] [CrossRef]
- Kase, K.; Kondo, S.; Wakisaka, N.; Dochi, H.; Mizokami, H.; Kobayashi, E.; Kano, M.; Komori, T.; Hirai, N.; Ueno, T.; et al. Epstein-barr virus LMP1 induces soluble PD-L1 in nasopharyngeal carcinoma. Microorganisms 2021, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hu, M.; Bai, X.; Ding, X.; Xie, L.; Ma, J.; Fan, B.; Yu, J. Plasma levels of soluble programmed death ligand 1 (sPD-L1) in WHO II/III nasopharyngeal carcinoma (NPC): A preliminary study. Medicine 2019, 98, e17231. [Google Scholar] [CrossRef] [PubMed]
- Kushlinskii, N.E.; Gershtein, E.S.; Chang, V.L.; Korotkova, E.A.; Alferov, A.A.; Kontorshchikov, M.M.; Sokolov, N.Y.; Karamysheva, E.I.; Ognerubov, N.A.; Stilidi, I.S.; et al. Prognostic significance of soluble forms of immune checkpoint PD-1/PDL1 receptor and ligand in blood plasma of gastric cancer patients. Klin. Lab. Diagn. 2021, 66, 139–146. [Google Scholar] [CrossRef]
- Ando, K.; Hamada, K.; Watanabe, M.; Ohkuma, R.; Shida, M.; Onoue, R.; Kubota, Y.; Matsui, H.; Ishiguro, T.; Hirasawa, Y.; et al. Plasma levels of soluble PD-L1 correlate with tumor regression in patients with lung and gastric cancer treated with immune checkpoint inhibitors. Anticancer Res. 2019, 39, 5195–5201. [Google Scholar] [CrossRef] [PubMed]
- Shigemori, T.; Toiyama, Y.; Okugawa, Y.; Yamamoto, A.; Yin, C.; Narumi, A.; Ichikawa, T.; Ide, S.; Shimura, T.; Fujikawa, H.; et al. Soluble PD-L1 expression in circulation as a predictive marker for recurrence and prognosis in gastric cancer: Direct comparison of the clinical burden between tissue and serum PD-L1 expression. Ann. Surg. Oncol. 2019, 26, 876–883. [Google Scholar] [CrossRef]
- Murakami, S.; Shibaki, R.; Matsumoto, Y.; Yoshida, T.; Goto, Y.; Kanda, S.; Horinouchi, H.; Fujiwara, Y.; Yamamoto, N.; Ohe, Y.; et al. Association between serum level soluble programmed cell death ligand 1 and prognosis in patients with non-small cell lung cancer treated with anti-PD-1 antibody. Thorac. Cancer 2020, 11, 3585–3595. [Google Scholar] [CrossRef]
- Jia, Y.; Li, X.; Zhao, C.; Ren, S.; Su, C.; Gao, G.; Li, W.; Zhou, F.; Li, J.; Zhou, C. Soluble PD-L1 as a Predictor of the Response to EGFR-TKIs in non-small cell lung cancer patients with EGFR mutations. Front. Oncol. 2020, 10, 1455. [Google Scholar] [CrossRef]
- Wu, W.; Xia, X.; Cheng, C.; Niu, L.; Wu, J.; Qian, Y. Serum soluble PD-L1, PD-L2, and B7-H5 as potential diagnostic biomarkers of human pancreatic cancer. Clin. Lab. 2021, 67, 6. [Google Scholar] [CrossRef]
- Asanuma, K.; Nakamura, T.; Hayashi, A.; Okamoto, T.; Iino, T.; Asanuma, Y.; Hagi, T.; Kita, K.; Nakamura, K.; Sudo, A. Soluble programmed death-ligand 1 rather than PD-L1 on tumor cells effectively predicts metastasis and prognosis in soft tissue sarcomas. Sci. Rep. 2020, 10, 9077. [Google Scholar] [CrossRef]
- Kushlinskii, N.E.; Alferov, A.A.; Boulytcheva, I.V.; Timofeev, Y.S.; Korotkova, E.A.; Khvan, O.T.; Kuzmin, Y.B.; Kuznetsov, I.N.; Bondarev, A.V.; Shchupak, M.Y.; et al. Comparative analysis of the levels of soluble forms of receptor and ligand of the immunity control point PD-1/PD-L1 in the blood serum of patients with typical bone osteosarcoma and chondrosarcoma. Klin. Lab. Diagn. 2020, 65, 669–675. [Google Scholar] [CrossRef]
- Aghajani, M.J.; Roberts, T.L.; Yang, T.; McCafferty, C.E.; Caixeiro, N.J.; DeSouza, P.; Niles, N. Elevated levels of soluble PD-L1 are associated with reduced recurrence in papillary thyroid cancer. Endocr. Connect. 2019, 8, 1040–1051. [Google Scholar] [CrossRef] [Green Version]
- Yazdanpanah, P.; Alavianmehr, A.; Ghaderi, A.; Monabati, A.; Montazer, M.; Tahmasbi, K.; Farjadian, S. PD-L1 expression in tumor lesions and soluble PD-L1 serum levels in patients with breast cancer: TNBC versus TPBC. Breast Dis. 2021, 40, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Price-Troska, T.; Paludo, J.; Villasboas, J.; Kim, H.J.; Yang, Z.Z.; Novak, A.J.; Ansell, S.M. Soluble PD-1 ligands regulate T-cell function in Waldenstrom macroglobulinemia. Blood Adv. 2018, 2, 1985–1997. [Google Scholar] [CrossRef]
- Janakiram, M.; Shah, U.A.; Liu, W.; Zhao, A.; Schoenberg, M.P.; Zang, X. The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol. Rev. 2017, 276, 26–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Gao, Y.; Wei, W. Post-Translational Regulations of PD-L1 and PD-1: Mechanisms and Opportunities for Combined Immunotherapy; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Chen, Y.; Liu, P.; Gao, F.; Cheng, H.; Qi, J.; Gao, G.F. A dimeric structure of PD-L1: Functional units or evolutionary relics? Protein Cell. 2010, 1, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butte, M.J.; Peña-Cruz, V.; Kim, M.J.; Freeman, G.J.; Sharpe, A.H. Interaction of human PD-L1 and B7-1. Mol. Immunol. 2008, 45, 3567–3572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhri, A.; Xiao, Y.; Klee, A.N.; Wang, X.; Zhu, B.; Freeman, G.J. PD-L1 Binds to B7-1 only in cis on the same cell surface. Cancer Immunol. Res. 2018, 6, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, C.D.; Pulanco, M.C.; Cui, W.; Lu, L.; Zang, X. PD-L1 and B7-1 cis-interaction: New mechanisms in immune checkpoints and immunotherapies. Trends Mol. Med. 2021, 27, 207–219. [Google Scholar] [CrossRef]
- Kang, J.H.; Jung, M.Y.; Choudhury, M.; Leof, E.B. Transforming growth factor beta induces fibroblasts to express and release the immunomodulatory protein PD-L1 into extracellular vesicles. FASEB J. 2020, 34, 2213–2226. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhou, J.; Zhang, J.; Li, S.; Wang, H.; Du, J. Cancer-associated fibroblasts promote PD-L1 expression in mice cancer cells via secreting CXCL5. Int. J. Cancer 2019, 145, 1946–1957. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Liu, X.; Liang, J.; Habiel, D.M.; Kulur, V.; Coelho, A.L.; Deng, N.; Xie, T.; Wang, Y.; Liu, N.; et al. PD-L1 on invasive fibroblasts drives fibrosis in a humanized model of idiopathic pulmonary fibrosis. JCI Insight 2019, 4, e125326. [Google Scholar] [CrossRef] [Green Version]
- Duitman, J.; van den Ende, T.; Spek, C.A. Immune checkpoints as promising targets for the treatment of idiopathic pulmonary fibrosis? J. Clin. Med. 2019, 8, 1547. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Bai, W.; Ma, H.; Li, F. Regulatory Effect of PD1/PD-Ligand 1 (PD-L1) on treg cells in patients with idiopathic pulmonary fibrosis. Med. Sci. Monit. 2021, 27, e927577. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J.; Lina, I.; Ding, D.; Engle, E.L.; Taube, J.; Gelbard, A.; Hillel, A.T. Increased Expression of PD-1 and PD-L1 in Patients With Laryngotracheal Stenosis. Laryngoscope 2021, 131, 967–974. [Google Scholar] [CrossRef]
- Yao, Q.; Fischer, K.P.; Tyrrell, D.L.; Gutfreund, K.S. The Pekin duck programmed death-ligand 1: cDNA cloning, genomic structure, molecular characterization and mRNA expression analysis. Int. J. Immunogenet. 2015, 42, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.R.A.P.; Mwangi, W.; Sadigh, Y.; Nair, V. In vitro interactions of chicken programmed cell death 1 (PD-1) and PD-1 Ligand-1 (PD-L1). Front. Cell Infect. Microbiol. 2019, 9, 436. [Google Scholar] [CrossRef] [Green Version]
- Dezutter-Dambuyant, C.; Durand, I.; Alberti, L.; Bendriss-Vermare, N.; Valladeau-Guilemond, J.; Duc, A.; Magron, A.; Morel, A.P.; Sisirak, V.; Rodriguez, C.; et al. A novel regulation of PD-1 ligands on mesenchymal stromal cells through MMP-mediated proteolytic cleavage. Oncoimmunology 2015, 5, e1091146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hira-Miyazawa, M.; Nakamura, H.; Hirai, M.; Kobayashi, Y.; Kitahara, H.; Bou-Gharios, G.; Kawashiri, S. Regulation of programmed-death ligand in the human head and neck squamous cell carcinoma microenvironment is mediated through matrix metalloproteinase-mediated proteolytic cleavage. Int. J. Oncol. 2018, 52, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Arras, D.; Rose-John, S. Regulation of Fibrotic Processes in the Liver by ADAM Proteases. Cells 2019, 8, 1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, N.; Rose-John, S.; Schmidt-Arras, D. ADAM-mediated signalling pathways in gastrointestinal cancer formation. Int. J. Mol. Sci. 2020, 21, 5133. [Google Scholar] [CrossRef] [PubMed]
- Heib, M.; Rose-John, S.; Adam, D. Necroptosis, ADAM proteases and intestinal (dys)function. Int. Rev. Cell. Mol. Biol. 2020, 353, 83–152. [Google Scholar] [PubMed]
- Romero, Y.; Wise, R.; Zolkiewska, A. Proteolytic processing of PD-L1 by ADAM proteases in breast cancer cells. Cancer Immunol. Immunother. 2020, 69, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Pichler, R.; Lindner, A.K.; Schäfer, G.; Tulchiner, G.; Staudacher, N.; Mayr, M.; Comperat, E.; Orme, J.J.; Schachtner, G.; Thurnher, M.; et al. Expression of ADAM proteases in bladder cancer patients with BCG Failure: A pilot study. J. Clin. Med. 2021, 10, 764. [Google Scholar] [CrossRef]
- Yunusova, N.V.; Patysheva, M.R.; Molchanov, S.V.; Zambalova, E.A.; Grigor’eva, A.E.; Kolomiets, L.A.; Ochirov, M.O.; Tamkovich, S.N.; Kondakova, I.V. Metalloproteinases at the surface of small extrcellular vesicles in advanced ovarian cancer: Relationships with ascites volume and peritoneal canceromatosis index. Clin. Chim. Acta 2019, 494, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M. Extracellular vesicle-associated MMPs: A modulator of the tissue microenvironment. Adv. Clin. Chem. 2019, 88, 35–66. [Google Scholar]
- Orme, J.J.; Jazieh, K.A.; Xie, T.; Harrington, S.; Liu, X.; Ball, M.; Madden, B.; Charlesworth, M.C.; Azam, T.U.; Lucien, F.; et al. ADAM10 and ADAM17 cleave PD-L1 to mediate PD-(L)1 inhibitor resistance. Oncoimmunology 2020, 9, 1744980. [Google Scholar] [CrossRef] [Green Version]
- Hansen, H.P.; Paes Leme, A.F.; Hallek, M. Role of ADAM10 as a CD30 sheddase in classical hodgkin lymphoma. Front. Immunol. 2020, 11, 398. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, S.F.; Lemberg, M.K.; Fluhrer, R. Proteolytic ectodomain shedding of membrane proteins in mammals-hardware, concepts, and recent developments. EMBO J. 2018, 37, e99456. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Zarrin, V.; Moghadam, E.R.; Zabolian, A.; Tavakol, S.; Samarghandian, S.; Najafi, M. PD-1/PD-L1 axis regulation in cancer therapy: The role of long non-coding RNAs and microRNAs. Life Sci. 2020, 256, 117899. [Google Scholar] [CrossRef]
- Qu, S.; Jiao, Z.; Lu, G.; Yao, B.; Wang, T.; Rong, W.; Xu, J.; Fan, T.; Sun, X.; Yang, R.; et al. PD-L1 lncRNA splice isoform promotes lung adenocarcinoma progression via enhancing c-Myc activity. Genome Biol. 2021, 22, 104. [Google Scholar] [CrossRef]
- He, X.H.; Xu, L.H.; Liu, Y. Identification of a novel splice variant of human PD-L1 mRNA encoding an isoform-lacking Igv-like domain. Acta Pharmacol. Sin. 2005, 26, 462–468. [Google Scholar] [CrossRef]
- Wang, C.; Weng, M.; Xia, S.; Zhang, M.; Chen, C.; Tang, J.; Huang, D.; Yu, H.; Sun, W.; Zhang, H.; et al. Distinct roles of programmed death ligand 1 alternative splicing isoforms in colorectal cancer. Cancer Sci. 2021, 112, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Attig, J.; Young, G.R.; Ottina, E.; Papamichos, S.I.; Kotsianidis, I.; Kassiotis, G. Soluble PD-L1 generated by endogenous retroelement exaptation is a receptor antagonist. Elife 2019, 8, e50256. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, K.M.; Shukla, S.A.; Patsoukis, N.; Chaudhri, A.; Browne, E.P.; Arazi, A.; Eisenhaure, T.M.; Pendergraft, W.F., 3rd; Hua, P.; Pham, H.C.; et al. A secreted PD-L1 splice variant that covalently dimerizes and mediates immunosuppression. Cancer Immunol. Immunother. 2019, 68, 421–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, B.; Kiyotani, K.; Sakata, S.; Nagano, S.; Kumehara, S.; Baba, S.; Besse, B.; Yanagitani, N.; Friboulet, L.; Nishio, M.; et al. Secreted PD-L1 variants mediate resistance to PD-L1 blockade therapy in non-small cell lung cancer. J. Exp. Med. 2019, 216, 982–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodská, B.; Otevřelová, P.; Kuželová, K. Correlation of PD-L1 surface expression on leukemia cells with the ratio of PD-L1 mRNA variants and with electrophoretic mobility. Cancer Immunol. Res. 2016, 4, 815–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassounah, N.B.; Malladi, V.S.; Huang, Y.; Freeman, S.S.; Beauchamp, E.M.; Koyama, S.; Souders, N.; Martin, S.; Dranoff, G.; Wong, K.K.; et al. Identification and characterization of an alternative cancer-derived PD-L1 splice variant. Cancer Immunol. Immunother. 2019, 68, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Dahal, L.N.; Schwarz, H.; Ward, F.J. Hiding in plain sight: Soluble immunomodulatory receptors. Trends Immunol. 2018, 39, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lang, J. Soluble PD-1 and PD-L1: Predictive and prognostic significance in cancer. Oncotarget 2017, 8, 97671–97682. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Xu, B.; Wang, Y.; Wu, C.; Jiang, J.; Wu, C. Prognostic significance of circulating soluble programmed death ligand-1 in patients with solid tumors: A meta-analysis. Medicine 2018, 97, e9617. [Google Scholar] [CrossRef]
- Ding, X.C.; Wang, L.L.; Zhu, Y.F.; Li, Y.D.; Nie, S.L.; Yang, J.; Liang, H.; Weichselbaum, R.R.; Yu, J.M.; Hu, M. The change of soluble programmed cell death-ligand 1 in glioma patients receiving radiotherapy and its impact on clinical outcomes. Front. Immunol. 2020, 11, 580335. [Google Scholar] [CrossRef]
- Atanackovic, D.; Luetkens, T. Biomarkers for checkpoint inhibition in hematologic malignancies. Semin. Cancer Biol. 2018, 52, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, J.B.; Monrad, I.; Enemark, M.B.; Ludvigsen, M.; Kamper, P.; Bjerre, M.; d’Amore, F. Soluble programmed cell death protein 1 (sPD-1) and the soluble programmed cell death ligands 1 and 2 (sPD-L1 and sPD-L2) in lymphoid malignancies. Eur. J. Haematol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, A.; Okuma, Y. Perspective on immune oncology with liquid biopsy, peripheral blood mononuclear cells, and microbiome with non-invasive biomarkers in cancer patients. Clin. Transl. Oncol. 2018, 20, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, Y.; Fujimura, T.; Hidaka, T.; Aiba, S. Biomarkers for predicting efficacies of anti-PD1 antibodies. Front. Med. 2019, 6, 174. [Google Scholar] [CrossRef] [Green Version]
- Costantini, A.; Takam Kamga, P.; Dumenil, C.; Chinet, T.; Emile, J.F.; Giroux Leprieur, E. Plasma biomarkers and immune checkpoint inhibitors in non-small cell lung cancer: New tools for better patient selection? Cancers 2019, 11, 1269. [Google Scholar] [CrossRef] [Green Version]
- Carretero-González, A.; Lora, D.; Martín Sobrino, I.; Sáez Sanz, I.; Bourlon, M.T.; Anido Herranz, U.; Martínez Chanzá, N.; Castellano, D.; de Velasco, G. The Value of PD-L1 expression as predictive biomarker in metastatic renal cell carcinoma patients: A meta-analysis of randomized clinical trials. Cancers 2020, 12, 1945. [Google Scholar] [CrossRef]
- Mildner, F.; Sopper, S.; Amann, A.; Pircher, A.; Pall, G.; Köck, S.; Naismith, E.; Wolf, D.; Gamerith, G. Systematic review: Soluble immunological biomarkers in advanced non-small-cell lung cancer (NSCLC). Crit. Rev. Oncol. Hematol. 2020, 153, 102948. [Google Scholar] [CrossRef] [PubMed]
- Duchemann, B.; Remon, J.; Naigeon, M.; Mezquita, L.; Ferrara, R.; Cassard, L.; Jouniaux, J.M.; Boselli, L.; Grivel, J.; Auclin, E.; et al. Integrating circulating biomarkers in the immune checkpoint inhibitor treatment in lung cancer. Cancers 2020, 12, 3625. [Google Scholar] [CrossRef]
- Cunha Pereira, T.; Rodrigues-Santos, P.; Almeida, J.S.; Rêgo Salgueiro, F.; Monteiro, A.R.; Macedo, F.; Soares, R.F.; Domingues, I.; Jacinto, P.; Sousa, G. Immunotherapy and predictive immunologic profile: The tip of the iceberg. Med. Oncol. 2021, 38, 51. [Google Scholar] [CrossRef]
- Sui, X.; Jiang, L.; Teng, H.; Mi, L.; Li, B.; Shi, A.; Yu, R.; Li, D.; Dong, X.; Yang, D.; et al. Prediction of clinical outcome in locally advanced non-small cell lung cancer patients treated with chemoradiotherapy by plasma markers. Front. Oncol. 2021, 10, 625911. [Google Scholar] [CrossRef] [PubMed]
- Castello, A.; Rossi, S.; Toschi, L.; Mansi, L.; Lopci, E. Soluble PD-L1 in NSCLC patients treated with checkpoint inhibitors and its correlation with metabolic parameters. Cancers 2020, 12, 1373. [Google Scholar] [CrossRef] [PubMed]
- Mazzaschi, G.; Minari, R.; Zecca, A.; Cavazzoni, A.; Ferri, V.; Mori, C.; Squadrilli, A.; Bordi, P.; Buti, S.; Bersanelli, M.; et al. Soluble PD-L1 and Circulating CD8+PD-1+ and NK cells enclose a prognostic and predictive immune effector score in immunotherapy treated NSCLC patients. Lung Cancer 2020, 148, 1–11. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, H.; Liu, H.; Sun, X.; Li, J.; Yu, H. Molecular mechanism of expression changes of immunological indexes of PD-1/sPD-L1 after radiotherapy in nonsmall cell lung cancer. Biomed. Res. Int. 2021, 2021, 8811751. [Google Scholar] [CrossRef]
- He, J.; Pan, Y.; Guo, Y.; Li, B.; Tang, Y. Study on the expression levels and clinical significance of PD-1 and PD-L1 in plasma of NSCLC Patients. J. Immunother. 2020, 43, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Doi, T.; Obayashi, K.; Hirai, A.; Yoneda, K.; Tanaka, F.; Iwai, Y. Soluble PD-L1 with PD-1-binding capacity exists in the plasma of patients with non-small cell lung cancer. Immunol. Lett. 2018, 196, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Tian, Y.; Cai, W.; Weng, Z.; Li, Y.; Zhang, H.; Bao, Y.; Li, Y. High-affinity human PD-L1 variants attenuate the suppression of T cell activation. Oncotarget 2017, 8, 88360–88375. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Dong, L.; Zhou, J.; Yang, Y.; Guo, J.; Xuan, Q.; Gao, K.; Xu, Z.; Lei, W.; Wang, J.; et al. The clinical implication of soluble PD-L1 (sPD-L1) in patients with breast cancer and its biological function in regulating the function of T lymphocyte. Cancer Immunol. Immunother. 2021. [Google Scholar] [CrossRef]
- Shi, B.; Du, X.; Wang, Q.; Chen, Y.; Zhang, X. Increased PD-1 on CD4(+)CD28(-) T cell and soluble PD-1 ligand-1 in patients with T2DM: Association with atherosclerotic macrovascular diseases. Metabolism 2013, 62, 778–785. [Google Scholar] [CrossRef]
- Frigola, X.; Inman, B.A.; Lohse, C.M.; Krco, C.J.; Cheville, J.C.; Thompson, R.H.; Leibovich, B.; Blute, M.L.; Dong, H.; Kwon, E.D.; et al. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin. Cancer Res. 2011, 17, 1915–1923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tannig, P.; Peter, A.S.; Lapuente, D.; Klessing, S.; Damm, D.; Tenbusch, M.; Überla, K.; Temchura, V. Modulation of vaccine-induced HIV-1-specific immune responses by co-electroporation of PD-L1 encoding DNA. Vaccines 2020, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Sun, Y.; Wang, S.; Du, J.; Gao, X.; Yuan, Y.; Zhao, L.; Yang, Y.; Xu, L.; Lei, Y.; et al. Secretion of human soluble programmed cell death protein 1 by chimeric antigen receptor-modified T cells enhances anti-tumor efficacy. Cytotherapy 2020, 22, 734–743. [Google Scholar] [CrossRef]
- Wen, S.L.; Li, F.; Zhao, F.; Zuo, J.J.; Deng, Y.Q.; Zhang, W.; Tao, Z.Z. Programmed cell death protein 1 and its ligands regulate immune balance in allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2020, 55, 384–390. [Google Scholar] [PubMed]
- Triantafyllou, E.; Gudd, C.L.; Mawhin, M.A.; Husbyn, H.C.; Trovato, F.M.; Siggins, M.K.; O’Connor, T.; Kudo, H.; Mukherjee, S.K.; Wendon, J.A.; et al. PD-1 blockade improves Kupffer cell bacterial clearance in acute liver injury. J. Clin. Investig. 2021, 131, e140196. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, M.; Liu, J.; Pan, T.; Zhou, T.; Liu, Z.; Tan, R.; Wang, X.; Tian, L.; Chen, E.; et al. sPD-L1 expression is associated with immunosuppression and infectious complications in patients with acute pancreatitis. Scand. J. Immunol. 2017, 86, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Pan, Y.; Fei, Q.; Lin, X.; Chen, Z.; Huang, H. Serum soluble PD-1 plays a role in predicting infection complications in patients with acute pancreatitis. Immun. Inflamm. Dis. 2021, 9, 310–318. [Google Scholar] [CrossRef]
- Avendaño-Ortiz, J.; Rubio-Garrido, M.; Lozano-Rodríguez, R.; Del Romero, J.; Rodríguez, C.; Moreno, S.; Aguirre, L.A.; Holguín, Á.; López-Collazo, E. Soluble PD-L1: A potential immune marker for HIV-1 infection and virological failure. Medicine 2020, 99, e20065. [Google Scholar] [CrossRef] [PubMed]
- León-Flores, A.; Del Río Estrada, P.M.; Álvarez-García, L.X.; Piten-Isidro, E.; Reyes-Terán, G. Increased levels of soluble co-stimulatory molecule PD-L1 (B7-H1) in the plasma of viraemic HIV-1(+) individuals. Immunol. Lett. 2018, 203, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, J.; Wang, X.; Tan, R.; Qi, X.; Liu, Z.; Qu, H.; Pan, T.; Zhan, Q.; Zuo, Y.; et al. Soluble PD-L1 improved direct ARDS by reducing monocyte-derived macrophages. Cell Death Dis. 2020, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- He, X.W.; Luo, Q.Z.; Zhao, L.L.; Shang, Y.; Gao, Z.C. The expression and clinical significance of serum soluble programmed cell death ligand-1 in adult patients with community-acquired pneumonia. Zhonghua Nei Ke Za Zhi 2021, 60, 243–246. [Google Scholar]
- Yamagiwa, S.; Ishikawa, T.; Waguri, N.; Sugitani, S.; Kamimura, K.; Tsuchiya, A.; Takamura, M.; Kawai, H.; Terai, S. Increase of soluble programmed cell death ligand 1 in patients with chronic hepatitis, C. Int. J. Med. Sci. 2017, 14, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.H.; Xing, Y.F.; Zhang, Z.L.; Huang, J.A.; Chen, Y.J. Effect of soluble PD-L1 released by lung cancer cells in regulating the function of T lymphocytes. Zhonghua Zhong Liu Za Zhi 2013, 35, 85–88. [Google Scholar]
- Santoso, B.; Sa’adi, A.; Dwiningsih, S.R.; Tunjungseto, A.; Widyanugraha, M.Y.A.; Mufid, A.F.; Rahmawati, N.Y.; Ahsan, F. Soluble immune checkpoints CTLA-4, HLA-G, PD-1, and PD-L1 are associated with endometriosis-related infertility. Am. J. Reprod. Immunol. 2020, 84, e13296. [Google Scholar] [CrossRef]
- Birtas Atesoglu, E.; Tarkun, P.; Demirsoy, E.T.; Geduk, A.; Mehtap, O.; Batman, A.; Kaya, F.; Cekmen, M.B.; Gulbas, Z.; Hacıhanefioglu, A. Soluble programmed death 1 (PD-1) is decreased in patients with immune thrombocytopenia (ITP): Potential involvement of PD-1 pathway in ITP immunopathogenesis. Clin. Appl. Thromb. Hemost. 2016, 22, 248–251. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Shu, M.; Li, S.; Cai, Y. Expression of soluble programmed death-1, soluble programmed death ligand 1 proteins and immune status in patients with oral lichen planus. Zhonghua Kou Qiang Yi Xue Za Zhi 2015, 50, 585–589. [Google Scholar] [PubMed]
- Cubillos-Zapata, C.; Balbás-García, C.; Avendaño-Ortiz, J.; Toledano, V.; Torres, M.; Almendros, I.; Casitas, R.; Zamarrón, E.; García-Sánchez, A.; Feliu, J.; et al. Age-dependent hypoxia-induced PD-L1 upregulation in patients with obstructive sleep apnoea. Respirology 2019, 24, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Zapata, C.; Martínez-García, M.Á.; Campos-Rodríguez, F.; Sánchez de la Torre, M.; Nagore, E.; Martorell-Calatayud, A.; Hernández Blasco, L.; Chiner Vives, E.; Abad-Capa, J.; Montserrat, J.M.; et al. Spanish sleep network. soluble PD-L1 is a potential biomarker of cutaneous melanoma aggressiveness and metastasis in obstructive sleep apnoea patients. Eur. Respir. J. 2019, 53, 1801298. [Google Scholar] [CrossRef]
- Nan, J.; Liang, L.; Li, L.; Xiaoqin, S.; Xing, J.; Yang, C. Soluble programmed death-1 and soluble programmed death ligand 1 protein expression and immune status in patients with recurrent aphthous ulcer. Hua Xi Kou Qiang Yi Xue Za Zhi 2017, 35, 286–290. [Google Scholar] [PubMed]
- Bommarito, D.; Hall, C.; Taams, L.S.; Corrigall, V.M. Inflammatory cytokines compromise programmed cell death-1 (PD-1)-mediated T cell suppression in inflammatory arthritis through up-regulation of soluble PD-1. Clin. Exp. Immunol. 2017, 188, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.M.; Wu, F.; Luo, X.C.; Chen, Y.; Ren, J.G.; Yang, X.; Ma, W.B.; Zhou, H.Y. Mechanism on moxibustion for rheumatoid arthritis based on PD-1/PD-L1 signaling pathway. Zhongguo Zhen Jiu 2020, 40, 976–982. [Google Scholar] [PubMed]
- Kawamoto, E.; Masui-Ito, A.; Eguchi, A.; Soe, Z.Y.; Prajuabjinda, O.; Darkwah, S.; Park, E.J.; Imai, H.; Shimaoka, M. Integrin and PD-1 ligand expression on circulating extracellular vesicles in systemic inflammatory response syndrome and sepsis. Shock 2019, 52, 13–22. [Google Scholar] [CrossRef]
- Du, Y.; Nie, L.; Xu, L.; Wu, X.; Zhang, S.; Xue, J. Serum levels of soluble programmed death-1 (sPD-1) and soluble programmed death ligand 1(sPD-L1) in systemic lupus erythematosus: Association with activity and severity. Scand. J. Immunol. 2020, 92, e12884. [Google Scholar] [CrossRef]
- Yanaba, K.; Hayashi, M.; Yoshihara, Y.; Nakagawa, H. Serum levels of soluble programmed death-1 and programmed death ligand-1 in systemic sclerosis: Association with extent of skin sclerosis. J. Dermatol. 2016, 43, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Raftery, M.J.; Abdelaziz, M.O.; Hofmann, J.; Schönrich, G. Hantavirus-driven PD-L1/PD-L2 upregulation: An imperfect viral immune evasion mechanism. Front. Immunol. 2018, 9, 2560. [Google Scholar] [CrossRef]
- Takahashi, N.; Iwasa, S.; Sasaki, Y.; Shoji, H.; Honma, Y.; Takashima, A.; Okita, N.T.; Kato, K.; Hamaguchi, T.; Yamada, Y. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Celada, L.J.; Kropski, J.A.; Herazo-Maya, J.D.; Luo, W.; Creecy, A.; Abad, A.T.; Chioma, O.S.; Lee, G.; Hassell, N.E.; Shaginurova, G.I.; et al. PD-1 up-regulation on CD4(+) T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-beta1 production. Sci. Transl. Med. 2018, 10, eaar8356. [Google Scholar] [CrossRef] [Green Version]
- Taz, T.A.; Ahmed, K.; Paul, B.K.; Kawsar, M.; Aktar, N.; Mahmud, S.M.H.; Moni, M.A. Network-based identification genetic effect of SARS-CoV-2 infections to Idiopathic pulmonary fibrosis (IPF) patients. Brief Bioinform. 2021, 22, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, Z.; Li, J.W.; Tan, K.; Yang, W.; Zhao, H.; Wang, G.Q. Discharge may not be the end of treatment: Pay attention to pulmonary fibrosis caused by severe COVID-19. J. Med. Virol. 2021, 93, 1378–1386. [Google Scholar] [CrossRef]
- Lin, F.; Ichim, T.E.; Pingle, S.; Jones, L.D.; Kesari, S.; Ashili, S. Mesenchymal stem cells as living anti-inflammatory therapy for COVID-19 related acute respiratory distress syndrome. World J. Stem Cells 2020, 12, 1067–1079. [Google Scholar] [CrossRef]
- Monaghan, S.F.; Chung, C.S.; Chen, Y.; Lomas-Neira, J.; Fairbrother, W.G.; Heffernan, D.S.; Cioffi, W.G.; Ayala, A. Soluble programmed cell death receptor-1 (sPD-1): A potential biomarker with anti-inflammatory properties in human and experimental acute respiratory distress syndrome (ARDS). J. Transl. Med. 2016, 14, 312. [Google Scholar] [CrossRef] [Green Version]
- Pan, T.; Zhou, T.; Li, L.; Liu, Z.; Chen, Y.; Mao, E.; Li, M.; Qu, H.; Liu, J. Monocyte programmed death ligand-1 expression is an early marker for predicting infectious complications in acute pancreatitis. Crit. Care 2017, 21, 186. [Google Scholar] [CrossRef] [Green Version]
- Kruger, S.; Legenstein, M.L.; Rösgen, V.; Haas, M.; Modest, D.P.; Westphalen, C.B.; Ormanns, S.; Kirchner, T.; Heinemann, V.; Holdenrieder, S.; et al. Serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death ligand 1 (sPD-L1) in advanced pancreatic cancer. Oncoimmunology 2017, 6, e1310358. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jiang, J.; Gao, L.; Wang, X.; Hu, X.; Wu, M.; Wu, J.; Xu, T.; Shi, Q.; Zhang, X. Soluble PD-1 aggravates progression of collagen-induced arthritis through Th1 and Th17 pathways. Arthritis Res. Ther. 2015, 17, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greisen, S.R.; Rasmussen, T.K.; Stengaard-Pedersen, K.; Hetland, M.L.; Hørslev-Petersen, K.; Hvid, M.; Deleuran, B. Increased soluble programmed death-1 (sPD-1) is associated with disease activity and radiographic progression in early rheumatoid arthritis. Scand. J. Rheumatol. 2014, 43, 101–108. [Google Scholar] [CrossRef]
- Wasén, C.; Erlandsson, M.C.; Bossios, A.; Ekerljung, L.; Malmhäll, C.; Töyrä Silfverswärd, S.; Pullerits, R.; Lundbäck, B.; Bokarewa, M.I. Smoking is associated with low levels of soluble PD-L1 in rheumatoid arthritis. Front. Immunol. 2018, 9, 1677. [Google Scholar] [CrossRef]
- Tong, M.; Fang, X.; Yang, J.; Wu, P.; Guo, Y.; Sun, J. Abnormal membrane-bound and soluble programmed death ligand 2 (PD-L2) expression in systemic lupus erythematosus is associated with disease activity. Immunol. Lett. 2020, 227, 96–101. [Google Scholar] [CrossRef]
- Her, M.; Kim, D.; Oh, M.; Jeong, H.; Choi, I. Increased expression of soluble inducible costimulator ligand (ICOSL) in patients with systemic lupus erythematosus. Lupus 2009, 18, 501–507. [Google Scholar] [CrossRef]
- Aarslev, K.; Dige, A.; Greisen, S.R.; Kreutzfeldt, M.; Jessen, N.; Vilstrup, H.; Deleuran, B.; Grønbæk, H. Soluble programmed death-1 levels are associated with disease activity and treatment response in patients with autoimmune hepatitis. Scand. J. Gastroenterol. 2017, 52, 93–99. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, N.; Wang, X.; Liu, Y.; Wang, X.; Wang, L.; Sun, M.; Yasen, H.; Zhao, F.; Fan, W.; et al. Percentages of PD-1(+)CD4(+)T cells and PD-L1(+)DCs are increased and sPD-1 level is elevated in patients with immune thrombocytopenia. Hum. Vaccin Immunother. 2018, 14, 832–838. [Google Scholar] [CrossRef]
- Costa, N.L.; Gonçalves, J.A.M.; de Lima, S.L.G.; de Arruda, J.A.A.; Miranda, A.C.C.; Mesquita, R.A.; da Silveira, É.J.D.; Batista, A.C. Evaluation of PD-L1, PD-L2, PD-1 and cytotoxic immune response in oral lichen planus. Oral Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jia, Y.; Li, C.; Shao, R.; Fang, Y. Predictive value of soluble programmed death-1 for severe sepsis and septic shock during the first week in an intensive care unit. Shock 2019, 51, 289–297. [Google Scholar] [CrossRef]
- Wilson, J.K.; Zhao, Y.; Singer, M.; Spencer, J.; Shankar-Hari, M. Lymphocyte subset expression and serum concentrations of PD-1/PD-L1 in sepsis-pilot study. Crit. Care 2018, 22, 95. [Google Scholar] [CrossRef]
- Shindo, Y.; McDonough, J.S.; Chang, K.C.; Ramachandra, M.; Sasikumar, P.G.; Hotchkiss, R.S. Anti-PD-L1 peptide improves survival in sepsis. J. Surg. Res. 2017, 208, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.Z.; Wang, X.L.; Xie, J.; Chen, L.P.; Li, Q.; Wang, X.X.; Wang, J.F.; Deng, X.M. Therapeutic effect of an anti-human programmed death-ligand 1 (PD-L1) nanobody on polymicrobial sepsis in humanized mice. Med. Sci. Monit. 2021, 27, e926820. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, R.; Huang, S.; Zu, B.; Zhang, S. Atezolizumab alleviates the immunosuppression induced by PD-L1-positive neutrophils and improves the survival of mice during sepsis. Mol. Med. Rep. 2021, 23, 144. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, S.; Chen, Y.; Shao, E.; Zhuang, T.; Lu, L.; Chen, X. Fatal adverse events associated with programmed cell death ligand 1 inhibitors: A systematic review and meta-analysis. Front. Pharmacol. 2020, 11, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Gu, Y.K.; Li, S.L.; Chen, H.; Chen, M.S.; Cai, Q.Q.; Deng, H.X.; Zuo, M.X.; Huang, J.H. Pre-treatment serum levels of soluble programmed cell death-ligand 1 predict prognosis in patients with hepatitis B-related hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2019, 145, 303–312. [Google Scholar] [CrossRef]
- Planès, R.; BenMohamed, L.; Leghmari, K.; Delobel, P.; Izopet, J.; Bahraoui, E. HIV-1 Tat protein induces PD-L1 (B7-H1) expression on dendritic cells through tumor necrosis factor alpha- and toll-like receptor 4-mediated mechanisms. J. Virol. 2014, 88, 6672–6689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Haij, N.; Planès, R.; Leghmari, K.; Serrero, M.; Delobel, P.; Izopet, J.; BenMohamed, L.; Bahraoui, E. HIV-1 Tat protein induces production of proinflammatory cytokines by human dendritic cells and monocytes/macrophages through engagement of TLR4-MD2-CD14 complex and activation of NF-kappaB pathway. PLoS ONE 2015, 10, e0129425. [Google Scholar] [CrossRef] [Green Version]
- Norton, T.D.; Zhen, A.; Tada, T.; Kim, J.; Kitchen, S.; Landau, N.R. Lentiviral vector-based dendritic cell vaccine suppresses HIV replication in humanized mice. Mol. Ther. 2019, 27, 960–973. [Google Scholar] [CrossRef] [Green Version]
- Tannig, P.; Peter, A.S.; Lapuente, D.; Klessing, S.; Schmidt, A.; Damm, D.; Tenbusch, M.; Überla, K.; Temchura, V. Genetic co-administration of soluble PD-1 ectodomains modifies immune responses against influenza a virus induced by DNA vaccination. Vaccines 2020, 8, 570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Aldo, P.; You, Y.; Ding, J.; Kaislasuo, J.; Petersen, J.F.; Lokkegaard, E.; Peng, G.; Paidas, M.J.; Simpson, S.; et al. Trophoblast-secreted soluble-PD-L1 modulates macrophage polarization and function. J. Leukoc. Biol. 2020, 108, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Enninga, E.A.L.; Harrington, S.M.; Creedon, D.J.; Ruano, R.; Markovic, S.N.; Dong, H.; Dronca, R.S. Immune checkpoint molecules soluble program death ligand 1 and galectin-9 are increased in pregnancy. Am. J. Reprod. Immunol. 2018, 79, e12795. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Morgan, J.; Lewis, D.F.; Cooper, D.B.; McCathran, C.E.; Wang, Y. Maternal soluble PD-1 levels are significantly increased in women with preeclampsia. Am. J. Reprod. Immunol. 2020, 83, e13193. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Zhou, S.; Zhao, K.; Song, Z.; Hu, G.; Zhang, T.; Li, Y.; Qiu, L.; Li, L.; et al. Plasma soluble programmed death ligand 1 levels predict clinical response in peripheral T-cell lymphomas. Hematol. Oncol. 2019, 37, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, C.; Zhi, C.; Liang, W.; Wang, X.; Chen, X.; Lv, T.; Shen, Q.; Song, Y.; Lin, D.; et al. Clinical significance of PD-L1 expression in serum-derived exosomes in NSCLC patients. J. Transl. Med. 2019, 17, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiako Meyo, M.; Jouinot, A.; Giroux-Leprieur, E.; Fabre, E.; Wislez, M.; Alifano, M.; Leroy, K.; Boudou-Rouquette, P.; Tlemsani, C.; Khoudour, N.; et al. Predictive Value of Soluble PD-1, PD-L1, VEGFA, CD40 ligand and CD44 for nivolumab therapy in advanced non-small cell lung cancer: A case-control study. Cancers 2020, 12, 473. [Google Scholar] [CrossRef] [Green Version]
- Gou, Q.; Dong, C.; Xu, H.; Khan, B.; Jin, J.; Liu, Q.; Shi, J.; Hou, Y. PD-L1 degradation pathway and immunotherapy for cancer. Cell Death Dis. 2020, 11, 955. [Google Scholar] [CrossRef]
- Xu, J.; Brosseau, J.P.; Shi, H. Targeted degradation of immune checkpoint proteins: Emerging strategies for cancer immunotherapy. Oncogene 2020, 39, 7106–7113. [Google Scholar] [CrossRef]
- Benicky, J.; Sanda, M.; Brnakova Kennedy, Z.; Grant, O.C.; Woods, R.J.; Zwart, A.; Goldman, R. PD-L1 Glycosylation and its impact on binding to clinical antibodies. J. Proteome Res. 2021, 20, 485–497. [Google Scholar] [CrossRef]
- Wadley, A.J.; Cullen, T.; Vautrinot, J.; Keane, G.; Bishop, N.C.; Coles, S.J. High intensity interval exercise increases the frequency of peripheral PD-1+ CD8(+) central memory T-cells and soluble PD-L1 in humans. Brain Behav. Immun. Health 2020, 3, 100049. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Xiao, Y.; Xue, M.; Cao, H.; Chen, J. Recent advances in the development of PD-L1 modulators: Degraders, downregulators, and covalent inhibitors. J. Med. Chem. 2020, 63, 15389–15398. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Vergoten, G. Protein homodimer sequestration with small molecules: Focus on PD-L1. Biochem. Pharmacol. 2020, 174, 113821. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Zhang, J.; Yang, Y.; Wang, L.; Zhou, J.; Zhang, H. Design, synthesis and biological evaluation of isoxazole-containing biphenyl derivatives as small-molecule inhibitors targeting the programmed cell death-1/ programmed cell death-ligand 1 immune checkpoint. Mol. Divers. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ye, W.; Wang, S.; He, Y.; Zhong, H.; Wang, Y.; Zhu, Y.; Han, J.; Bing, Z.; Ji, S.; et al. Discovery of a new inhibitor targeting PD-L1 for cancer immunotherapy. Neoplasia 2021, 23, 281–293. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.; Chen, H.; Feng, Z. Design, synthesis, evaluation, and SAR of 4-phenylindoline derivatives, a novel class of small-molecule inhibitors of the programmed cell death-1/ programmed cell death-ligand 1 (PD-1/PD-L1) interaction. Eur. J. Med. Chem. 2021, 211, 113001. [Google Scholar] [CrossRef]
- Orme, J.J.; Enninga, E.A.L.; Lucien-Matteoni, F.; Dale, H.; Burgstaler, E.; Harrington, S.M.; Ball, M.K.; Mansfield, A.S.; Park, S.S.; Block, M.S.; et al. Therapeutic plasma exchange clears circulating soluble PD-L1 and PD-L1-positive extracellular vesicles. J. Immunother. Cancer 2020, 8, e001113. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, C.; Thuru, X.; Quesnel, B. Soluble Programmed Death Ligand-1 (sPD-L1): A Pool of Circulating Proteins Implicated in Health and Diseases. Cancers 2021, 13, 3034. https://doi.org/10.3390/cancers13123034
Bailly C, Thuru X, Quesnel B. Soluble Programmed Death Ligand-1 (sPD-L1): A Pool of Circulating Proteins Implicated in Health and Diseases. Cancers. 2021; 13(12):3034. https://doi.org/10.3390/cancers13123034
Chicago/Turabian StyleBailly, Christian, Xavier Thuru, and Bruno Quesnel. 2021. "Soluble Programmed Death Ligand-1 (sPD-L1): A Pool of Circulating Proteins Implicated in Health and Diseases" Cancers 13, no. 12: 3034. https://doi.org/10.3390/cancers13123034
APA StyleBailly, C., Thuru, X., & Quesnel, B. (2021). Soluble Programmed Death Ligand-1 (sPD-L1): A Pool of Circulating Proteins Implicated in Health and Diseases. Cancers, 13(12), 3034. https://doi.org/10.3390/cancers13123034