Gender-Specific Efficacy Revealed by Head-to-Head Comparison of Pasireotide and Octreotide in a Representative In Vivo Model of Nonfunctioning Pituitary Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Efficacy of Octreotide LAR vs. Pasireotide LAR against Endogenous NFPTs In Vivo
2.2. Proliferation Rates of NFPTs in the Three Treatment Groups
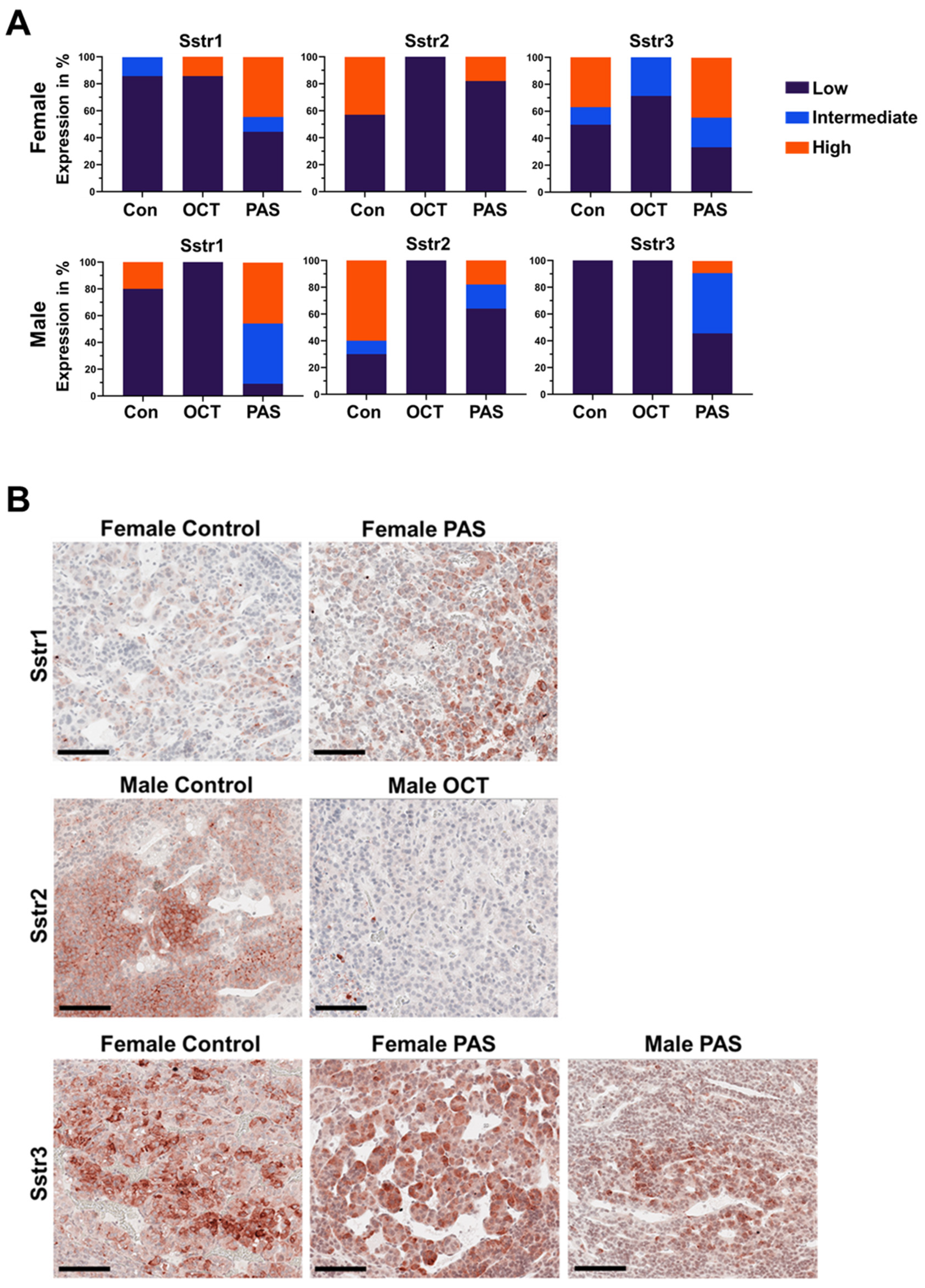
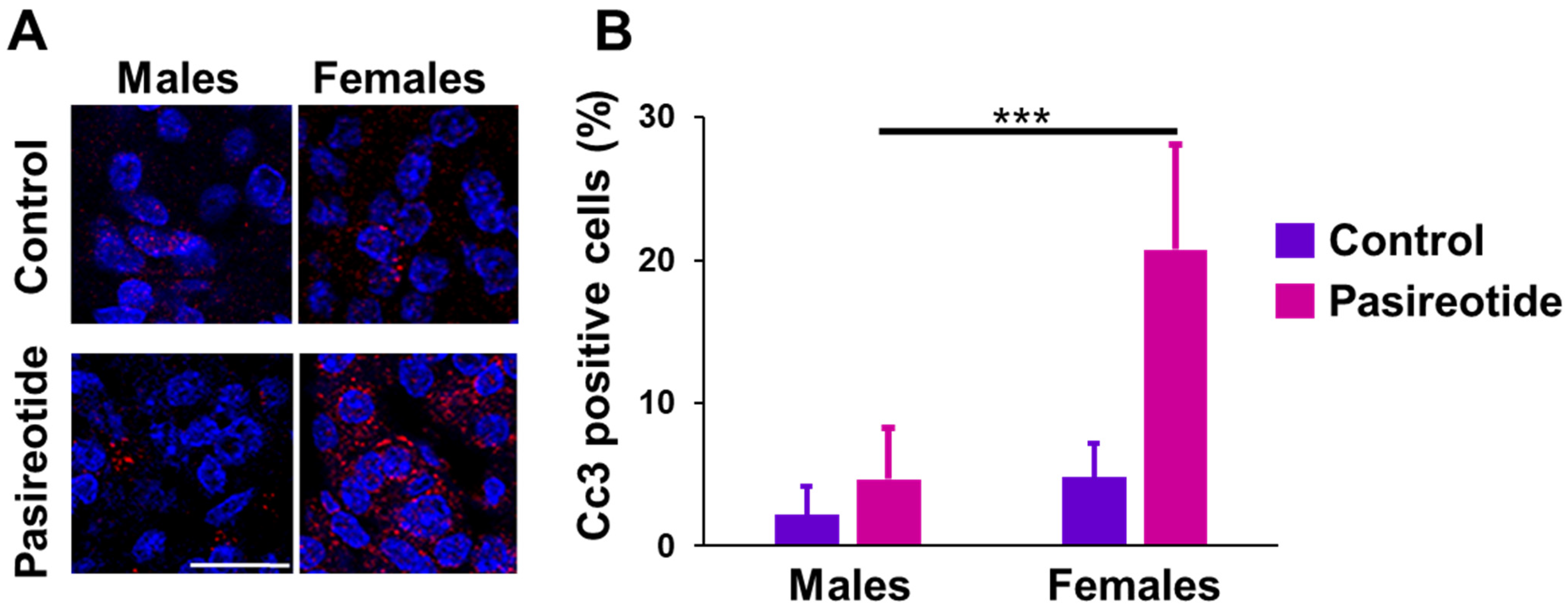
2.3. Expression of Sstrs and Apoptosis Markers in Rat NFPTs
2.4. SSTR3 Expression in Human NFPTs
3. Discussion
4. Materials and Methods
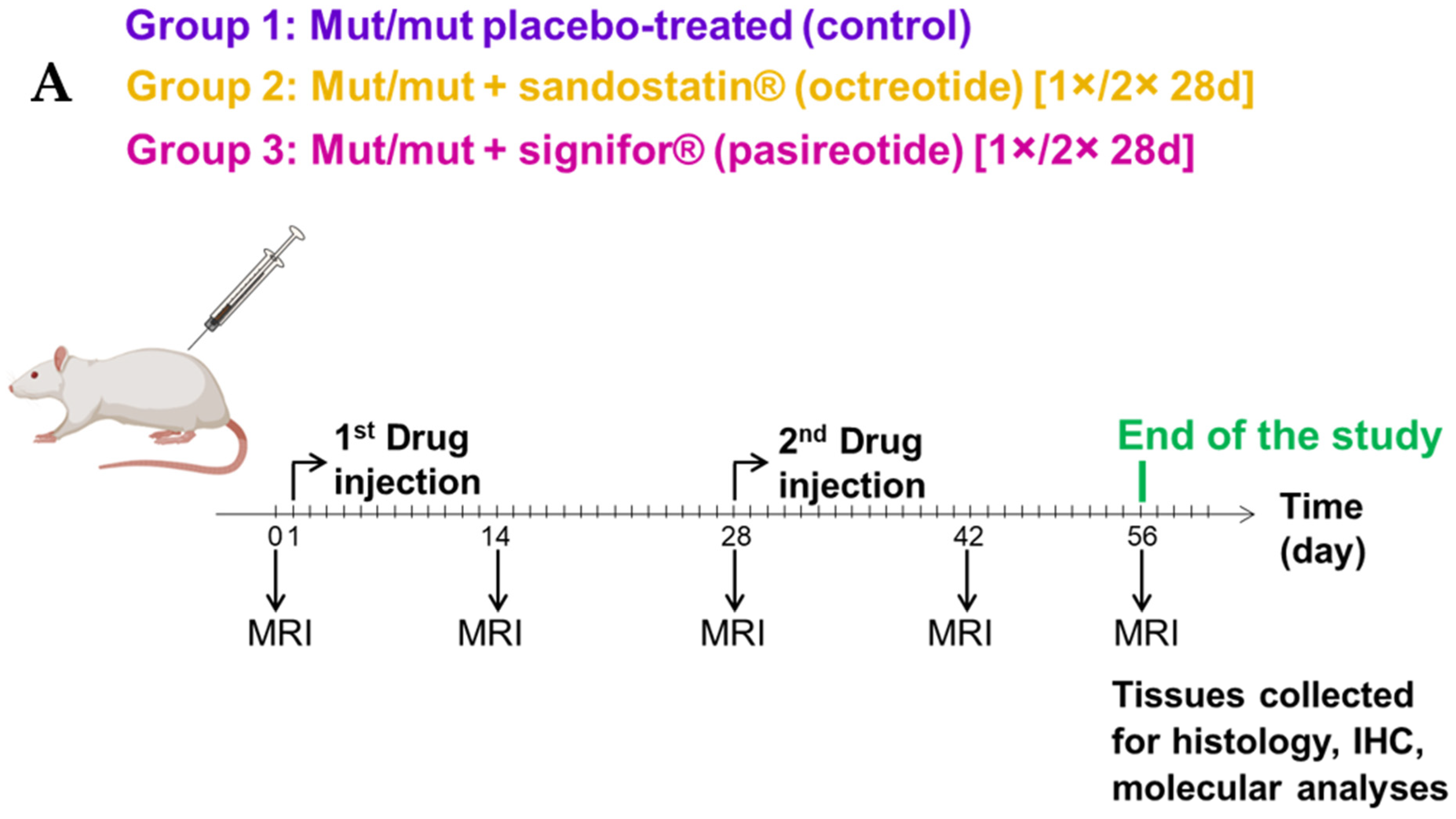
4.1. Rats and Treatment
4.2. Human Tissue Samples
4.3. Magnetic Resonance Imaging (MRI)
4.4. RNA Extraction and Quantitative RT-PCR
4.5. Immunohistochemistry (IHC)
4.6. Evaluation of Immunostains for SSTRs
4.7. Immunofluorescence (IF) and Quantification
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asa, S.L.; Casar-Borota, O.; Chanson, P.; Delgrange, E.; Earls, P.; Ezzat, S.; Grossman, A.; Ikeda, H.; Inoshita, N.; Karavitaki, N.; et al. From pituitary adenoma to pituitary neuroendocrine tumor (PT): An International Pituitary Pathology Club proposal. Endocr. Relat. Cancer 2017, 24, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Lobatto, D.J.; Steffens, A.N.V.; Najafabadi, A.H.Z.; Andela, C.D.; Pereira, A.M.; Hout, W.B.V.D.; Peul, W.C.; Vlieland, T.P.M.V.; Biermasz, N.R.; Van Furth, W.R. Work disability and its determinants in patients with pituitary tumor-related disease. Pituitary 2018, 21, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampourlou, M.; Fountas, A.; Ntali, G.; Karavitaki, N. Mortality in patients with non-functioning pituitary adenoma. Pituitary 2018, 21, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Drummond, J.; Roncaroli, F.; Grossman, A.B.; Korbonits, M. Clinical and Pathological Aspects of Silent Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2019, 104, 2473–2489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebersold, M.J.; Quast, L.M.; Laws, E.R., Jr.; Scheithauer, B.; Randall, R.V. Long-term results in transsphenoidal removal of non-functioning pituitary adenomas. J. Neurosurg. 1986, 64, 713–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenman, Y.; Melmed, S. Diagnosis and management of nonfunctioning pituitary tumors. Annu. Rev. Med. 1996, 47, 95–106. [Google Scholar] [CrossRef]
- Øystese, K.A.; Evang, J.A.; Bollerslev, J. Non-functioning pituitary adenomas: Growth and aggressiveness. Endocrine 2016, 53, 28–34. [Google Scholar] [CrossRef]
- Cortet-Rudelli, C.; Bonneville, J.-F.; Borson-Chazot, F.; Clavier, L.; Dequéant, B.C.; Desailloud, R.; Maiter, D.; Rohmer, V.; Sadoul, J.L.; Sonnet, E.; et al. Post-surgical management of non-functioning pituitary adenoma. Ann. d’Endocrinol. 2015, 76, 228–238. [Google Scholar] [CrossRef] [Green Version]
- Kanner, A.A.; Corn, B.W.; Greenman, Y. Radiotherapy of nonfunctioning and gonadotroph adenomas. Pituitary 2008, 12, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Raverot, G.; Vasiljevic, A.; Jouanneau, E. Prognostic factors of regrowth in nonfunctioning pituitary tumors. Pituitary 2017, 21, 176–182. [Google Scholar] [CrossRef]
- Kopczak, A.; Renner, U.; Stalla, G.K. Advances in understanding pituitary tumors. F1000Prime Rep. 2014, 6, 5. [Google Scholar] [CrossRef]
- Theodoropoulou, M.; Stalla, G.K. Somatostatin receptors: From signaling to clinical practice. Front. Neuroendocr. 2013, 34, 228–252. [Google Scholar] [CrossRef]
- Günther, T.; Tulipano, G.; Dournaud, P.; Bousquet, C.; Csaba, Z.; Kreienkamp, H.-J.; Lupp, A.; Korbonits, M.; Castaño, J.P.; Wester, H.-J.; et al. International Union of Basic and Clinical Pharmacology. CV. Somatostatin Receptors: Structure, Function, Ligands, and New Nomenclature. Pharmacol. Rev. 2018, 70, 763–835. [Google Scholar] [CrossRef] [Green Version]
- Bruns, C.; Lewis, I.; Briner, U.; Meno-Tetang, G.; Weckbecker, G. SOM230: A novel somatostatin peptidomimetic with broad so-matotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur. J. Endocrinol. 2002, 146, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lupp, A.; Mendoza, N.; Martin, N.; Beschorner, R.; Honegger, J.; Schlegel, J.; Shively, T.; Pulz, E.; Schulz, S.; et al. SSTR3 is a putative target for the medical treatment of gonadotroph adenomas of the pituitary. Endocr. Relat. Cancer 2014, 22, 111–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieria, N.L.; Wildemberg, L.E.; Colli, L.M.; Kasuki, L.; Marques, N.V.; Moraes, A.B.; Gasparetto, E.L.; Takiya, C.M.; Castro, M.; Gadelha, M.R. ZAC1 and SSTR2 are downregulated in non-functioning pituitary adenomas but not in somatotropinomas. PLoS ONE 2013, 8, 77406. [Google Scholar] [CrossRef]
- Ibáñez-Costa, A.; Rivero-Cortés, E.; Vázquez-Borrego, M.C.; Gahete, M.D.; Jiménez-Reina, L.; Venegas-Moreno, E.; De La Riva, A.; Arráez, M.Á.; González-Molero, I.; Schmid, H.A.; et al. Octreotide and pasireotide (dis)similarly inhibit pituitary tumor cells in vitro. J. Endocrinol. 2016, 231, 135–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez-Borrego, M.C.; Gupta, V.; Ibáñez-Costa, A.; Gahete, M.D.; Venegas-Moreno, E.; Toledano-Delgado, Á.; Cano, D.A.; Blanco-Acevedo, C.; Ortega-Salas, R.; Japón, M.A.; et al. A Somatostatin Receptor Subtype-3 (SST3) Peptide Agonist Shows Antitumor Effects in Experimental Models of Nonfunctioning Pituitary Tumors. Clin. Cancer Res. 2019, 26, 957–969. [Google Scholar] [CrossRef] [Green Version]
- Marinoni, I.; Lee, M.; Mountford, S.; Perren, A.; Bravi, I.; Jennen, L.; Feuchtinger, A.; Drouin, J.; Roncaroli, F.; Pellegata, N.S. Characteri-zation of MENX-associated pituitary tumours. Neuropathol. Appl. Neurobiol. 2013, 39, 256–269. [Google Scholar] [CrossRef]
- Lee, M.; Marinoni, I.; Irmler, M.; Psaras, T.; Honegger, J.B.; Beschorner, R.; Anastasov, N.; Beckers, J.; Theodoropoulous, M.; Roncaroli, F.; et al. Transcriptome analysis of MENX-associated rat pi-tuitary adenomas identifies novel molecular mechanisms involved in the pathogenesis of human pituitary gonadotroph ade-nomas. Acta Neuropathol. 2013, 126, 137–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmetzer, O.; Flörcken, A. Sex Differences in the Drug Therapy for Oncologic Diseases. Handb. Exp. Pharmacol. 2012, 214, 411–442. [Google Scholar] [CrossRef]
- Fougner, S.L.; Casar-Borota, O.; Berg, J.P.; Hald, J.K.; Ramm-Pettersen, J.; Bollerslev, J. The clinical response to somatostatin analogues in acromegaly correlates to the somatostatin receptor subtype 2a protein expression of the adenoma. Clin. Endocrinol. 2007, 68, 458–465. [Google Scholar] [CrossRef] [Green Version]
- Plöckinger, U.; Albrecht, S.; Mawrin, C.; Saeger, W.; Buchfelder, M.; Petersenn, S.; Schulz, S. Selective Loss of Somatostatin Receptor 2 in Octreotide-Resistant Growth Hormone-Secreting Adenomas. J. Clin. Endocrinol. Metab. 2008, 93, 1203–1210. [Google Scholar] [CrossRef] [Green Version]
- Casar-Borota, O.; Heck, A.; Schulz, S.; Nesland, J.M.; Ramm-Pettersen, J.; Lekva, T.; Alafuzoff, I.; Bollerslev, J. Expression of SSTR2a, but not of SSTRs 1, 3, or 5 in Somatotroph Adenomas Assessed by Monoclonal Antibodies Was Reduced by Octreotide and Correlated With the Acute and Long-Term Effects of Octreotide. J. Clin. Endocrinol. Metab. 2013, 98, E1730–E1739. [Google Scholar] [CrossRef] [Green Version]
- War, S.A.; Somvanshi, R.; Kumar, U. Somatostatin receptor-3 mediated intracellular signaling and apoptosis is regulated by its cytoplasmic terminal. Biochim. Biophys. Acta 2011, 1813, 390–402. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.; Patel, Y.C.; Srikant, C.B. Subtype-selective induction of wild-type p53 and apoptosis, but not cell cycle arrest, by human somatostatin receptor 3. Mol. Endocrinol. 1996, 10, 1688–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatto, F.; Feelders, R.A.; Franck, S.E.; Van Koetsveld, P.M.; Dogan, F.; Kros, J.M.; Neggers, S.J.C.M.M.; Van Der Lely, A.-J.; Lamberts, S.W.J.; Ferone, D.; et al. In Vitro Head-to-Head Comparison Between Octreotide and Pasireotide in GH-Secreting Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2017, 102, 2009–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Pas, R.; Feelders, R.A.; Gatto, F.; de Bruin, C.; Pereira, A.M.; van Koetsveld, P.M.; Sprij-Mooij, D.M.; Waaijers, A.M.; Dogan, F.; Schulz, S.; et al. Preoperative normalization of cortisol levels in Cushing’s disease after medical treatment: Consequences for somatostatin and dopamine receptor subtype expression and in vitro response to somatostatin analogs and dopamine agonists. J. Clin. Endocrinol. Metab. 2013, 98, 1880–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colao, A.; Boscaro, M.; Ferone, D.; Casanueva, F.F. Managing Cushing’s disease: The state of the art. Endocrine 2014, 47, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papotti, M.; Bongiovanni, M.; Volante, M.; Allìa, E.; Landolfi, S.; Helboe, L.; Schindler, M.; Cole, S.L.; Bussolati, G. Expression of soma-tostatin receptor types 1-5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. 2002, 440, 461–475. [Google Scholar] [CrossRef]
- Ibáñez-Costa, A.; López-Sánchez, L.M.; Gahete, M.D.; Rivero-Cortés, E.; Vázquez-Borrego, M.C.; Gálvez, M.A.; de la Riva, A.; Vene-gas-Moreno, E.; Jiménez-Reina, L.; Moreno-Carazo, A.; et al. BIM-23A760 influences key functional endpoints in pituitary adenomas and normal pituitaries: Molecular mechanisms underlying the differential response in adenomas. Sci. Rep. 2017, 7, 2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imhof, A.-K.; Glück, L.; Gajda, M.; Lupp, A.; Bräuer, R.; Schaible, H.-G.; Schulz, S. Differential antiinflammatory and antinociceptive effects of the somatostatin analogs octreotide and pasireotide in a mouse model of immune-mediated arthritis. Arthritis Rheum. 2011, 63, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Doll, C.; Jacobs, S.; Kolodziej, A.; Stumm, R.; Schulz, S. Reassessment of sst2Somatostatin Receptor Expression in Human Normal and Neoplastic Tissues Using the Novel Rabbit Monoclonal Antibody UMB-1. J. Clin. Endocrinol. Metab. 2008, 93, 4519–4524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Händel, M.; Schulz, S.; Stanarius, A.; Schreff, M.; Erdtmann-Vourliotis, M.; Schmidt, H.; Wolf, G.; Höllt, V. Selective targeting of so-matostatin receptor 3 to neuronal cilia. Neuroscience 1999, 89, 909–926. [Google Scholar] [CrossRef]
- Schulz, S.; Händel, M.; Schreff, M.; Schmidt, H.; Höllt, V. Localization of five somatostatin receptors in the rat central nervous system using subtype-specific antibodies. J. Physiol. 2000, 94, 259–264. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulde, S.; Wiedemann, T.; Schillmaier, M.; Valença, I.; Lupp, A.; Steiger, K.; Yen, H.-Y.; Bäuerle, S.; Notni, J.; Luque, R.; et al. Gender-Specific Efficacy Revealed by Head-to-Head Comparison of Pasireotide and Octreotide in a Representative In Vivo Model of Nonfunctioning Pituitary Tumors. Cancers 2021, 13, 3097. https://doi.org/10.3390/cancers13123097
Gulde S, Wiedemann T, Schillmaier M, Valença I, Lupp A, Steiger K, Yen H-Y, Bäuerle S, Notni J, Luque R, et al. Gender-Specific Efficacy Revealed by Head-to-Head Comparison of Pasireotide and Octreotide in a Representative In Vivo Model of Nonfunctioning Pituitary Tumors. Cancers. 2021; 13(12):3097. https://doi.org/10.3390/cancers13123097
Chicago/Turabian StyleGulde, Sebastian, Tobias Wiedemann, Mathias Schillmaier, Isabel Valença, Amelie Lupp, Katja Steiger, Hsi-Yu Yen, Stephen Bäuerle, Johannes Notni, Raul Luque, and et al. 2021. "Gender-Specific Efficacy Revealed by Head-to-Head Comparison of Pasireotide and Octreotide in a Representative In Vivo Model of Nonfunctioning Pituitary Tumors" Cancers 13, no. 12: 3097. https://doi.org/10.3390/cancers13123097





