Lattice or Oxygen-Guided Radiotherapy: What If They Converge? Possible Future Directions in the Era of Immunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Lattice Radiotherapy: Concept
3. Clinical Use of LATTICE Radiotherapy
4. Oxygen-Guided Radiotherapy: Oxygen Is the Needed Comburent for Radiotherapy, Not Only for Fire
5. OGRT in Clinical Practice
6. High Dose per Fraction Radiotherapy and Immunotherapy: The Most Recent Evidence for a Successful Cooperation
7. A Look to the Future and Open Questions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wu, X.; Ahmed, M.M.; Wright, J.; Gupta, S.; Pollack, A. On modern technical approaches of three-dimensional high-dose lattice radiotherapy (lrt). Cureus 2010, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Liberson, F. The Value of a Multi-perforated Screen in Deep X-ray Therapy. Radiology 1933, 20, 186–195. [Google Scholar] [CrossRef]
- Pokhrel, D.; Halfman, M.; Sanford, L.; Chen, Q.; Kudrimoti, M. A novel, yet simple MLC-based 3D-crossfire technique for spatially fractionated GRID therapy treatment of deep-seated bulky tumors. J. Appl. Clin. Med. Phys. 2020, 21, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Sindoni, A.; Severo, C.; Vadala’, R.E.; Ferini, G.; Mazzei, M.M.; Vaccaro, M.; Iatì, G.; Pontoriero, A.; Pergolizzi, S. Levetiracetam-induced radiation recall dermatitis in a patient undergoing stereotactic radiotherapy. J. Dermatol. 2016, 43, 1440–1441. [Google Scholar] [CrossRef]
- Schapira, E.L.; Hubbeling, H.; Yeap, B.Y.; Mehan, W.A.; Shaw, A.T.; Oh, K.; Gainor, J.F.; Shih, H.A. Improved Overall Survival and Locoregional Disease Control with Concurrent PD-1 Pathway Inhibitors and Stereotactic Radiosurgery for Lung Cancer Patients with Brain Metastases. Int. J. Radiat. Oncol. 2018, 101, 624–629. [Google Scholar] [CrossRef]
- Wu, X.; Perez, N.C.; Zheng, Y.; Li, X.; Jiang, L.; Amendola, B.E.; Xu, B.; Mayr, N.A.; Lu, J.J.; Hatoum, G.F.; et al. The Technical and Clinical Implementation of LATTICE Radiation Therapy (LRT). Radiat. Res. 2020, 194, 737–746. [Google Scholar] [CrossRef]
- Crosbie, J.; Anderson, R.; Rothkamm, K.; Restall, C.M.; Cann, L.; Ruwanpura, S.; Meachem, S.; Yagi, N.; Svalbe, I.; Lewis, R.A.; et al. Tumor Cell Response to Synchrotron Microbeam Radiation Therapy Differs Markedly from Cells in Normal Tissues. Int. J. Radiat. Oncol. 2010, 77, 886–894. [Google Scholar] [CrossRef]
- Bouchet, A.; Lemasson, B.; Christen, T.; Potez, M.; Rome, C.; Coquery, N.; Le Clec’H, C.; Moisan, A.; Bräuer-Krisch, E.; Leduc, G.; et al. Synchrotron microbeam radiation therapy induces hypoxia in intracerebral gliosarcoma but not in the normal brain. Radiother. Oncol. 2013, 108, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Ibahim, M.J.; Crosbie, J.C.; Yang, Y.; Zaitseva, M.; Stevenson, A.W.; Rogers, P.A.W.; Paiva, P. An Evaluation of Dose Equivalence between Synchrotron Microbeam Radiation Therapy and Conventional Broadbeam Radiation Using Clonogenic and Cell Impedance Assays. PLoS ONE 2014, 9, e100547. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Fujita, M.; Regine, W.F.; Megooni, A.S.; Ibbott, G.S.; Ahmed, M.M. High-dose spatially-fractionated radiation (GRID): A new paradigm in the management of advanced cancers. Int. J. Radiat. Oncol. 1999, 45, 721–727. [Google Scholar] [CrossRef]
- Pellizzon, A.C.A. Lattice radiation therapy—its concept and impact in the immunomodulation cancer treatment era. Rev. Assoc. Médica Bras. 2020, 66, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Kanagavelu, S.; Gupta, S.; Wu, X.; Philip, S.; Wattenberg, M.; Hodge, J.W.; Couto, M.D.; Chung, K.D.; Ahmed, M.M. In VivoEffects of Lattice Radiation Therapy on Local and Distant Lung Cancer: Potential Role of Immunomodulation. Radiat. Res. 2014, 182, 149–162. [Google Scholar] [CrossRef]
- Kwan, D.K.; Norman, A. Radiosensitivity of Human Lymphocytes and Thymocytes. Radiat. Res. 1977, 69, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, X.; Zhang, X.; Chang, S.X.; Megooni, A.; Donnelly, E.D.; Ahmed, M.M.; Griffin, R.J.; Welsh, J.S.; Ii, C.B.S.; et al. Photon GRID Radiation Therapy: A Physics and Dosimetry White Paper from the Radiosurgery Society (RSS) GRID/LATTICE, Microbeam and FLASH Radiotherapy Working Group. Radiat. Res. 2020, 194, 665–677. [Google Scholar] [CrossRef] [PubMed]
- E Amendola, B.; Perez, N.; Amendola, M.; Wu, X.; Ahmed, M.M.; Iglesias, A.J.; Estape, R.; Lambrou, N.; Bortoletto, P. LATTICE Radiotherapy with RapidArc for Treatment of Gynecological Tumors:Dosimetric and Early Clinical Evaluations. Cureus 2010, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Suarez, J.M.B.; E Amendola, B.; Perez, N.; Amendola, M.; Wu, X. The Use of Lattice Radiation Therapy (LRT) in the Treatment of Bulky Tumors: A Case Report of a Large Metastatic Mixed Mullerian Ovarian Tumor. Cureus 2015, 7. [Google Scholar] [CrossRef] [Green Version]
- Amendola, B.E.; Perez, N.; Wu, X.; Suarez, J.M.B.; Lu, J.J.; Amendola, M. Improved outcome of treating locally advanced lung cancer with the use of Lattice Radiotherapy (LRT): A case report. Clin. Transl. Radiat. Oncol. 2018, 9, 68–71. [Google Scholar] [CrossRef] [Green Version]
- E Amendola, B.; Perez, N.C.; Wu, X.; Amendola, M.A.; Qureshi, I.Z. Safety and Efficacy of Lattice Radiotherapy in Voluminous Non-small Cell Lung Cancer. Cureus 2019, 11, e4263. [Google Scholar] [CrossRef] [Green Version]
- Amendola, B.E.; Perez, N.C.; Mayr, N.A.; Wu, X.; Amendola, M. Spatially Fractionated Radiation Therapy Using Lattice Radiation in Far-advanced Bulky Cervical Cancer: A Clinical and Molecular Imaging and Outcome Study. Radiat. Res. 2020, 194, 724–736. [Google Scholar] [CrossRef]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef] [Green Version]
- Duriseti, S.; Kavanaugh, J.; Goddu, S.; Price, A.; Knutson, N.; Reynoso, F.; Michalski, J.; Mutic, S.; Robinson, C.; Spraker, M.B. Spatially fractionated stereotactic body radiation therapy (Lattice) for large tumors. Adv. Radiat. Oncol. 2021, 6. [Google Scholar] [CrossRef]
- Pollack, A.; Chinea, F.M.; Bossart, E.; Kwon, D.; Abramowitz, M.C.; Lynne, C.; Jorda, M.; Marples, B.; Patel, V.N.; Wu, X.; et al. Phase I Trial of MRI-Guided Prostate Cancer Lattice Extreme Ablative Dose (LEAD) Boost Radiation Therapy. Int. J. Radiat. Oncol. 2020, 107, 305–315. [Google Scholar] [CrossRef]
- Ferini, G.; Pergolizzi, S. A Ten-year-long Update on Radiation Proctitis Among Prostate Cancer Patients Treated With Curative External Beam Radiotherapy. In Vivo 2021, 35, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Kopchick, B.; Xu, H.; Niu, Y.; Becker, S.; Qiu, X.; Yu, C. Technical Note: Dosimetric feasibility of lattice radiotherapy for breast cancer using GammaPod. Med. Phys. 2020, 47, 3928–3934. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, X.; Zhang, J.; Li, W.; Dong, F.; Chen, C.; Lin, Q.; Zhang, C.; Zheng, F.; Yan, W.; et al. Combined High-Dose LATTICE Radiation Therapy and Immune Checkpoint Blockade for Advanced Bulky Tumors: The Concept and a Case Report. Front. Oncol. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Castorina, P.; Castorina, L.; Ferini, G. Non-Homogeneous Tumor Growth and Its Implications for Radiotherapy: A Phenomenological Approach. J. Pers. Med. 2021, 11, 527. [Google Scholar] [CrossRef]
- Borkenstein, K.; Levegrün, S.; Peschke, P. Modeling and computer simulations of tumor growth and tumor response to radiotherapy. Radiat. Res. 2004, 162, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Epel, B.; Maggio, M.C.; Barth, E.D.; Miller, R.C.; Pelizzari, C.A.; Krzykawska-Serda, M.; Sundramoorthy, S.V.; Aydogan, B.; Weichselbaum, R.R.; Tormyshev, V.M.; et al. Oxygen-Guided Radiation Therapy. Int. J. Radiat. Oncol. 2019, 103, 977–984. [Google Scholar] [CrossRef]
- Prasanna, A.; Ahmed, M.M.; Mohiuddin, M.; Coleman, C.N. Exploiting sensitization windows of opportunity in hyper and hypo-fractionated radiation therapy. J. Thorac. Dis. 2014, 6, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Kempf, H.; Bleicher, M.; Meyer-Hermann, M. Spatio-Temporal Dynamics of Hypoxia during Radiotherapy. PLoS ONE 2015, 10, e0133357. [Google Scholar] [CrossRef]
- Carlson, D.J.; Keall, P.; Loo, B.W.; Chen, Z.J.; Brown, J.M. Hypofractionation Results in Reduced Tumor Cell Kill Compared to Conventional Fractionation for Tumors with Regions of Hypoxia. Int. J. Radiat. Oncol. 2011, 79, 1188–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamis, S.; Kohandel, M.; Dubois, L.; Yaromina, A.; Lambin, P.; Powathil, G.G. Combining hypoxia-activated prodrugs and radiotherapy in silico: Impact of treatment scheduling and the intra-tumoural oxygen landscape. PLoS Comput. Biol. 2020, 16, e1008041. [Google Scholar] [CrossRef] [PubMed]
- Bolli, E.; D’Huyvetter, M.; Murgaski, A.; Berus, D.; Stange, G.; Clappaert, E.J.; Arnouk, S.; Antunes, A.R.P.; Krasniqi, A.; Lahoutte, T.; et al. Stromal-targeting radioimmunotherapy mitigates the progression of therapy-resistant tumors. J. Control. Release 2019, 314, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Šentjurc, M.; Čemažar, M.; Serša, G. EPR oximetry of tumors in vivo in cancer therapy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Epel, B.; Redler, G.; Tormyshev, V.; Halpern, H.J. Towards Human Oxygen Images with Electron Paramagnetic Resonance Imaging. Single Mol. Single Cell Seq. 2016, 876, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.B.; Khan, N.; Zaki, B.; Hartford, A.; Ernstoff, M.S.; Swartz, H.M. Clinical Electron Paramagnetic Resonance (EPR) Oximetry Using India Ink. Adv. Exp. Med. Biol. 2010, 662, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Epel, B.; Krzykawska-Serda, M.; Tormyshev, V.; Maggio, M.C.; Barth, E.D.; Pelizzari, C.A.; Halpern, H.J. Spin Lattice Relaxation EPR pO2 Images May Direct the Location of Radiation Tumor Boosts to Enhance Tumor Cure. Cell Biophys. 2017, 75, 295–298. [Google Scholar] [CrossRef]
- Hou, H.; Abramovic, Z.; Lariviere, J.P.; Sentjurc, M.; Swartz, H.; Khan, N. Effect of a Topical Vasodilator on Tumor Hypoxia and Tumor Oxygen Guided Radiotherapy using EPR Oximetry. Radiat. Res. 2010, 173, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Servagi-Vernat, S.; Differding, S.; Sterpin, E.; Hanin, F.-X.; LaBar, D.; Bol, A.; Lee, J.A.; Grégoire, V. Hypoxia-guided adaptive radiation dose escalation in head and neck carcinoma: A planning study. Acta Oncol. 2015, 54, 1008–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzeroni, M.; Toma-Dasu, I.; Ureba, A.; Schiavo, F.; Wiedenmann, N.; Bunea, H.; Thomann, B.; Baltas, D.; Mix, M.; Stoykow, C.; et al. Quantification of Tumor Oxygenation Based on FMISO PET: Influence of Location and Oxygen Level of the Well-Oxygenated Reference Region. Adv. Exp. Med. Biol. 2020, 1232, 177–182. [Google Scholar] [CrossRef]
- Lee, N.Y.; Mechalakos, J.G.; Nehmeh, S.; Lin, Z.; Squire, O.D.; Cai, S.; Chan, K.; Zanzonico, P.B.; Greco, C.; Ling, C.C.; et al. Fluorine-18-Labeled Fluoromisonidazole Positron Emission and Computed Tomography-Guided Intensity-Modulated Radiotherapy for Head and Neck Cancer: A Feasibility Study. Int. J. Radiat. Oncol. 2008, 70, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Epel, B.; Redler, G.; Pelizzari, C.; Tormyshev, V.M.; Halpern, H.J. Approaching Oxygen-Guided Intensity-Modulated Radiation Therapy. Single Mol. Single Cell Seq. 2016, 876, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Epel, B.; Maggio, M.; Pelizzari, C.; Halpern, H.J. Electron Paramagnetic Resonance pO2 Image Tumor Oxygen-Guided Radiation Therapy Optimization. Chem. Biol. Pteridines Folates 2017, 977, 287–296. [Google Scholar] [CrossRef]
- Redler, G.; Pearson, E.; Liu, X.; Gertsenshteyn, I.; Epel, B.; Pelizzari, C.; Aydogan, B.; Weichselbaum, R.; Halpern, H.J.; Wiersma, R.D. Small Animal IMRT Using 3D-Printed Compensators. Int. J. Radiat. Oncol. 2021, 110, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Lariviere, J.P.; Demidenko, E.; Gladstone, D.; Swartz, H.; Khan, N. Repeated tumor pO2 measurements by multi-site EPR oximetry as a prognostic marker for enhanced therapeutic efficacy of fractionated radiotherapy. Radiother. Oncol. 2009, 91, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Hou, H.; Mupparaju, S.P.; Lariviere, J.P.; Hodge, S.; Gui, J.; Swartz, H.M.; Khan, N. Assessment of the Changes in 9L and C6 Glioma pO2by EPR Oximetry as a Prognostic Indicator of Differential Response to Radiotherapy. Radiat. Res. 2013, 179, 343–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahle, T.J.; Rusten, E.; Stokkevåg, C.H.; Silvoniemi, A.; Mairani, A.; Fjæra, L.F.; Rørvik, E.; Henjum, H.; Wright, P.; Boer, C.G.; et al. The FLUKA Monte Carlo code coupled with an OER model for biologically weighted dose calculations in proton therapy of hypoxic tumors. Phys. Med. 2020, 76, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Bonnitcha, P.; Grieve, S.; Figtree, G. Clinical imaging of hypoxia: Current status and future directions. Free. Radic. Biol. Med. 2018, 126, 296–312. [Google Scholar] [CrossRef]
- Busk, M.; Overgaard, J.; Horsman, M.R. Imaging of Tumor Hypoxia for Radiotherapy: Current Status and Future Directions. Semin. Nucl. Med. 2020, 50, 562–583. [Google Scholar] [CrossRef]
- Welz, S.; Mönnich, D.; Pfannenberg, C.; Nikolaou, K.; Reimold, M.; la Fougère, C.; Reischl, G.; Mauz, P.-S.; Paulsen, F.; Alber, M.; et al. Prognostic value of dynamic hypoxia PET in head and neck cancer: Results from a planned interim analysis of a randomized phase II hypoxia-image guided dose escalation trial. Radiother. Oncol. 2017, 124, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, E.; Dasu, A.; Uhrdin, J.; Even, A.; Van Elmpt, W.; Lambin, P.; Wersäll, P.; Toma-Dasu, I. Defining the hypoxic target volume based on positron emission tomography for image guided radiotherapy—the influence of the choice of the reference region and conversion function. Acta Oncol. 2017, 56, 819–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, L.-B.-A.; Bol, A.; Labar, D.; Cao-Pham, T.-T.; Jordan, B.; Grégoire, V.; Gallez, B. Predictive value of 18F-FAZA PET imaging for guiding the association of radiotherapy with nimorazole: A preclinical study. Radiother. Oncol. 2015, 114, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.-B.-A.; Bol, A.; Labar, D.; Karroum, O.; Bol, V.; Jordan, B.; Grégoire, V.; Gallez, B. Potential role of hypoxia imaging using 18F-FAZA PET to guide hypoxia-driven interventions (carbogen breathing or dose escalation) in radiation therapy. Radiother. Oncol. 2014, 113, 204–209. [Google Scholar] [CrossRef]
- Bollineni, V.R.; Kerner, G.S.; Pruim, J.; Steenbakkers, R.J.H.M.; Wiegman, E.M.; Koole, M.; De Groot, E.H.; Willemsen, A.T.; Luurtsema, G.; Widder, J.; et al. PET Imaging of Tumor Hypoxia Using 18F-Fluoroazomycin Arabinoside in Stage III-IV Non-Small Cell Lung Cancer Patients. J. Nucl. Med. 2013, 54, 1175–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.H.; Wada, M.; Anderson, N.J.; Joon, D.L.; Lee, S.T.; Gong, S.J.; Gunawardana, D.H.; Sachinidis, J.; O’Keefe, G.; Gan, H.K.; et al. Hypoxia-targeted radiotherapy dose painting for head and neck cancer using18F-FMISO PET: A biological modeling study. Acta Oncol. 2013, 52, 1723–1729. [Google Scholar] [CrossRef] [Green Version]
- De Figueiredo, B.H.; Zacharatou, C.; Galland-Girodet, S.; Benech, J.; De Clermont-Gallerande, H.; Lamare, F.; Hatt, M.; Digue, L.; Pujol, E.D.M.D.; Fernandez, P. Hypoxia imaging with [18F]-FMISO-PET for guided dose escalation with intensity-modulated radiotherapy in head-and-neck cancers. Strahlenther. Und Onkol. 2014, 191, 217–224. [Google Scholar] [CrossRef]
- Hendrickson, K.; Phillips, M.; Smith, W.; Peterson, L.; Krohn, K.; Rajendran, J. Hypoxia imaging with [F-18] FMISO-PET in head and neck cancer: Potential for guiding intensity modulated radiation therapy in overcoming hypoxia-induced treatment resistance. Radiother. Oncol. 2011, 101, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.; Schoder, H.; Beattie, B.; Lanning, R.; Riaz, N.; McBride, S.; Katabi, N.; Li, D.; Yarusi, B.; Chan, S.; et al. Strategy of Using Intratreatment Hypoxia Imaging to Selectively and Safely Guide Radiation Dose De-escalation Concurrent With Chemotherapy for Locoregionally Advanced Human Papillomavirus–Related Oropharyngeal Carcinoma. Int. J. Radiat. Oncol. 2016, 96, 9–17. [Google Scholar] [CrossRef]
- Saksø, M.; Primdahl, H.; Johansen, J.; Nowicka-Matus, K.; Overgaard, J.; Dahanca, O.B.O. DAHANCA 33: Functional image-guided dose-escalated radiotherapy to patients with hypoxic squamous cell carcinoma of the head and neck (NCT02976051). Acta Oncol. 2019, 59, 208–211. [Google Scholar] [CrossRef]
- Busk, M.; Horsman, M.R.; Overgaard, J.; Jakobsen, S. Dual-tracer PET of viable tumor volume and hypoxia for identification of necrosis-containing radio-resistant Sub-volumes. Acta Oncol. 2019, 58, 1476–1482. [Google Scholar] [CrossRef]
- Van der Heide, U.A.; Houweling, A.C.; Groenendaal, G.; Beets-Tan, R.G.; Lambin, P. Functional MRI for radiotherapy dose painting. Magn. Reson. Imaging 2012, 30, 1216–1223. [Google Scholar] [CrossRef] [Green Version]
- Krishna, M.C.; Matsumoto, S.; Saito, K.; Matsuo, M.; Mitchell, J.B.; Ardenkjaer-Larsen, J.H. Magnetic resonance imaging of tumor oxygenation and metabolic profile. Acta Oncol. 2013, 52, 1248–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, A.; Engineer, R.; Chopra, S.; Mahanshetty, U.; Juvekar, S.; Shrivastava, S.; Desekar, N.; Thakur, M. Role of 3T multiparametric-MRI with BOLD hypoxia imaging for diagnosis and post therapy response evaluation of postoperative recurrent cervical cancers. Eur. J. Radiol. Open 2016, 3, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, A.; Little, R.A.; Latif, A.; Featherstone, A.K.; Babur, M.; Peset, I.; Cheung, S.; Watson, Y.; Tessyman, V.; Mistry, H.; et al. Oxygen-enhanced MRI Is Feasible, Repeatable, and Detects Radiotherapy-induced Change in Hypoxia in Xenograft Models and in Patients with Non-small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 3818–3829. [Google Scholar] [CrossRef] [Green Version]
- Hallac, R.R.; Zhou, H.; Pidikiti, R.; Song, K.; Stojadinovic, S.; Zhao, D.; Solberg, T.; Peschke, P.; Mason, R.P. Correlations of noninvasive BOLD and TOLD MRI with pO2 and relevance to tumor radiation response. Magn. Reson. Med. 2014, 71, 1863–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gkagkanasiou, M.; Ploussi, A.; Gazouli, M.; Efstathopoulos, E.P. USPIO-Enhanced MRI Neuroimaging: A Review. J. Neuroimaging 2015, 26, 161–168. [Google Scholar] [CrossRef]
- Bennani-Baiti, B.; Pinker, K.; Zimmermann, M.; Helbich, T.H.; Baltzer, P.A.; Clauser, P.; Kapetas, P.; Bago-Horvath, Z.; Stadlbauer, A. Non-Invasive Assessment of Hypoxia and Neovascularization with MRI for Identification of Aggressive Breast Cancer. Cancers 2020, 12, 2024. [Google Scholar] [CrossRef]
- He, J.; Hu, Y.; Hu, M.; Li, B. Development of PD-1/PD-L1 Pathway in Tumor Immune Microenvironment and Treatment for Non-Small Cell Lung Cancer. Sci. Rep. 2015, 5, srep13110. [Google Scholar] [CrossRef] [Green Version]
- Protopapa, M.; Kouloulias, V.; Kougioumtzopoulou, A.; Liakouli, Z.; Papadimitriou, C.; Zygogianni, A. Novel treatment planning approaches to enhance the therapeutic ratio: Targeting the molecular mechanisms of radiation therapy. Clin. Transl. Oncol. 2019, 22, 447–456. [Google Scholar] [CrossRef]
- Sindoni, A.; Minutoli, F.; Ascenti, G.; Pergolizzi, S. Combination of immune checkpoint inhibitors and radiotherapy: Review of the literature. Crit. Rev. Oncol. 2017, 113, 63–70. [Google Scholar] [CrossRef]
- Owen, D.; Sio, T.T. Stereotactic body radiotherapy (SBRT) for central and ultracentral node-negative lung tumors. J. Thorac. Dis. 2020, 12, 7024–7031. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, R.E.; Santacaterina, A.; Sindoni, A.; Platania, A.; Arcudi, A.; Ferini, G.; Mazzei, M.M.; Marletta, D.; Rifatto, C.; Risoleti, E.V.I.; et al. Stereotactic body radiotherapy in non-operable lung cancer patients. Clin. Transl. Oncol. 2016, 18, 1158–1159. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, A.; Parisi, S.; Tamburella, C.; Lillo, S.; Ferini, G.; Molino, L.; Iatì, G.; Pontoriero, A.; Bottari, A.; Mazziotti, S.; et al. Stereotactic body radiation therapy and radiofrequency ablation for the treatment of liver metastases: How and when? Rep. Pr. Oncol. Radiother. 2020, 25, 299–306. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Q.; Luo, Y.; Gao, C.; Zhuang, X.; Xu, G.; Qaio, T. High-dose irradiation in combination with toll-like receptor 9 agonist CpG oligodeoxynucleotide 7909 downregulates PD-L1 expression via the NF-κB signaling pathway in non-small cell lung cancer cells. OncoTargets Ther. 2016, 9, 6511–6518. [Google Scholar] [CrossRef] [Green Version]
- Muraro, E.; Furlan, C.; Avanzo, M.; Martorelli, D.; Comaro, E.; Rizzo, A.; Fae’, D.A.; Berretta, M.; Militello, L.; Del Conte, A.; et al. Local High-Dose Radiotherapy Induces Systemic Immunomodulating Effects of Potential Therapeutic Relevance in Oligometastatic Breast Cancer. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Falcke, S.E.; Rühle, P.F.; Deloch, L.; Fietkau, R.; Frey, B.; Gaipl, U.S. Clinically Relevant Radiation Exposure Differentially Impacts Forms of Cell Death in Human Cells of the Innate and Adaptive Immune System. Int. J. Mol. Sci. 2018, 19, 3574. [Google Scholar] [CrossRef] [Green Version]
- Melo, A.M.; Maher, S.G.; O’Leary, S.; Doherty, D.G.; Lysaght, J. Selective effects of radiotherapy on viability and function of invariant natural killer T cells in vitro. Radiother. Oncol. 2020, 145, 128–136. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Ding, Y.; Huang, F.; Huang, W.; Lan, R.; Chen, R.; Wu, B.; Fu, L.; Yang, Y.; et al. Hypofractionated Irradiation Suppressed the Off-Target Mouse Hepatocarcinoma Growth by Inhibiting Myeloid-Derived Suppressor Cell-Mediated Immune Suppression. Front. Oncol. 2020, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Kane, N.; Kobayashi, N.; Kono, E.A.; Yamashiro, J.M.; Nickols, N.G.; Reiter, R.E. High-dose per Fraction Radiotherapy Induces Both Antitumor Immunity and Immunosuppressive Responses in Prostate Tumors. Clin. Cancer Res. 2021, 27, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Kang, J.; Zhao, R. Abscopal effect of radiation on lymph node metastasis in esophageal carcinoma: A case report and literature review. Oncol. Lett. 2018, 16, 3555–3560. [Google Scholar] [CrossRef]
- Trommer, M.; Yeo, S.Y.; Persigehl, T.; Bunck, A.; Grüll, H.; Schlaak, M.; Theurich, S.; Von Bergwelt-Baildon, M.; Morgenthaler, J.; Herter, J.M.; et al. Abscopal Effects in Radio-Immunotherapy—Response Analysis of Metastatic Cancer Patients With Progressive Disease Under Anti-PD-1 Immune Checkpoint Inhibition. Front. Pharmacol. 2019, 10, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theelen, W.S.M.E.; Peulen, H.M.U.; Lalezari, F.; Van Der Noort, V.; De Vries, J.F.; Aerts, J.G.J.V.; Dumoulin, D.W.; Bahce, I.; Niemeijer, A.-L.N.; De Langen, A.J.; et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients with Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2019, 5, 1276–1282. [Google Scholar] [CrossRef]
- Mujoo, K.; Hunt, C.R.; Pandita, R.K.; Ferrari, M.; Krishnan, S.; Cooke, J.P.; Hahn, S.; Pandita, T.K. Harnessing and Optimizing the Interplay between Immunotherapy and Radiotherapy to Improve Survival Outcomes. Mol. Cancer Res. 2018, 16, 1209–1214. [Google Scholar] [CrossRef] [Green Version]
- Lehrer, E.J.; McGee, H.M.; Peterson, J.L.; Vallow, L.; Ruiz-Garcia, H.; Zaorsky, N.G.; Sharma, S.; Trifiletti, D.M. Stereotactic Radiosurgery and Immune Checkpoint Inhibitors in the Management of Brain Metastases. Int. J. Mol. Sci. 2018, 19, 3054. [Google Scholar] [CrossRef] [Green Version]
- Sundahl, N.; Vandekerkhove, G.; Decaestecker, K.; Meireson, A.; De Visschere, P.; Fonteyne, V.; De Maeseneer, D.; Reynders, D.; Goetghebeur, E.; Van Dorpe, J.; et al. Randomized Phase 1 Trial of Pembrolizumab with Sequential Versus Concomitant Stereotactic Body Radiotherapy in Metastatic Urothelial Carcinoma. Eur. Urol. 2019, 75, 707–711. [Google Scholar] [CrossRef]
- Bates, J.E.; Morris, C.G.; Milano, M.T.; Yeung, A.R.; Hoppe, B.S. Immunotherapy with hypofractionated radiotherapy in metastatic non-small cell lung cancer: An analysis of the National Cancer Database. Radiother. Oncol. 2019, 138, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Parisi, S.; Lillo, S.; Cacciola, A.; Santacaterina, A.; Palazzolo, C.; Platania, A.; Settineri, N.; Franchina, T.; Tamburella, C.; Pergolizzi, S. Vaginal Mucosal Melanoma: A Complete Remission after Immunotherapy and ‘0-7-21’ Radiotherapy Regimen (24 Gy/3 fractions/21 days). Folia Med. 2020, 62, 605–609. [Google Scholar] [CrossRef]
- Stessin, A.M.; Clausi, M.G.; Zhao, Z.; Lin, H.; Hou, W.; Jiang, Z.; Duong, T.Q.; Tsirka, S.E.; Ryu, S. Repolarized macrophages, induced by intermediate stereotactic dose radiotherapy and immune checkpoint blockade, contribute to long-term survival in glioma-bearing mice. J. Neuro-Oncol. 2020, 147, 547–555. [Google Scholar] [CrossRef]
- Riva, M.; Wouters, R.; Nittner, D.; Ceuster, J.; Sterpin, E.; Giovannoni, R.; Himmelreich, U.; Gsell, W.; Van Ranst, M.; Coosemans, A. Radiation dose-escalation and dose-fractionation modulate the immune microenvironment, cancer stem cells and vasculature in experimental high-grade gliomas. J. Neurosurg Sci. 2020. [Google Scholar] [CrossRef]
- Sahebjam, S.; A Forsyth, P.; Tran, N.D.; A Arrington, J.; Macaulay, R.; Etame, A.B.; Walko, C.M.; Boyle, T.; Peguero, E.N.; Jaglal, M.; et al. Hypofractionated stereotactic re-irradiation with pembrolizumab and bevacizumab in patients with recurrent high-grade gliomas: Results from a phase I study. Neuro-Oncology 2021, 23, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Rolig, A.S.; Charych, D.H.; Hoch, U.; Kasiewicz, M.J.; Rose, D.C.; McNamara, M.J.; Hilgart-Martiszus, I.F.; Redmond, W.L. NKTR-214 immunotherapy synergizes with radiotherapy to stimulate systemic CD8+T cell responses capable of curing multi-focal cancer. J. Immunother. Cancer 2019, 8, e000464. [Google Scholar] [CrossRef]
- Keam, S.P.; Halse, H.; Nguyen, T.; Wang, M.; Losio, N.V.K.; Mitchell, C.; Caramia, F.; Byrne, D.J.; Haupt, S.; Ryland, G.; et al. High dose-rate brachytherapy of localized prostate cancer converts tumors from cold to hot. J. Immunother. Cancer 2020, 8, e000792. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Fernandez, D.; Dhillon, J.; Abraham-Miranda, J.; Awasthi, S.; Kim, Y.; Zhang, J.; Jain, R.; Serna, A.; Pow-Sang, J.M.; et al. Proof-of-principle Phase I results of combining nivolumab with brachytherapy and external beam radiation therapy for Grade Group 5 prostate cancer: Safety, feasibility, and exploratory analysis. Prostate Cancer Prostatic Dis. 2020, 24, 140–149. [Google Scholar] [CrossRef]
- Linares-Galiana, I.; Berenguer-Frances, M.A.; Cañas-Cortés, R.; Pujol-Canadell, M.; Comas-Antón, S.; Martínez, E.; Laplana, M.; Pérez-Montero, H.; Pla-Farnós, M.J.; Navarro-Martin, A.; et al. Changes in peripheral immune cells after intraoperative radiation therapy in low-risk breast cancer. J. Radiat. Res. 2021, 62, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.-Y.; Pan, C.-K.; Huang, Y.-S.; Tsai, C.-Y.; Wang, C.-W.; Lin, Y.-L.; Kuo, S.-H.; Shieh, M.-J. Evaluation of antitumor immunity by a combination treatment of high-dose irradiation, anti-PDL1, and anti-angiogenic therapy in murine lung tumors. Cancer Immunol. Immunother. 2021, 70, 391–404. [Google Scholar] [CrossRef]
- Reiss, K.A.; Wattenberg, M.M.; Damjanov, N.; Dunphy, E.P.; Jacobs-Small, M.; Lubas, M.J.; Robinson, J.; DiCicco, L.; Garcia-Marcano, L.; Giannone, M.A.; et al. A Pilot Study of Galunisertib plus Stereotactic Body Radiotherapy in Patients with Advanced Hepatocellular Carcinoma. Mol. Cancer Ther. 2021, 20, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.I.; Barsoumian, H.B.; Sezen, D.; Verma, V.; Patel, R.; Wasley, M.; Hu, Y.; Dunn, J.D.; He, K.; Chen, D.; et al. Addition of TLR9 agonist immunotherapy to radiation improves systemic antitumor activity. Transl. Oncol. 2021, 14, 100983. [Google Scholar] [CrossRef]
- Wei, Q.; He, H.; Lv, L.; Xu, X.; Sun, W. The promising role of radiotherapy in the treatment of advanced or metastatic renal cell carcinoma: A narrative review. Transl. Androl. Urol. 2020, 9, 2821–2830. [Google Scholar] [CrossRef]
- Milhem, C.; Moralès, O.; Ingelaere, C.; Pasquier, D.; Mordon, S.; Mortier, L.; Mirabel, X.; Delhem, N. Combination of High Dose Hypofractionated Radiotherapy with Anti-PD1 Single Dose Immunotherapy Leads to a Th1 Immune Activation Resulting in a Complete Clinical Response in a Melanoma Patient. Int. J. Mol. Sci. 2020, 21, 6772. [Google Scholar] [CrossRef]
- Asur, R.; Butterworth, K.T.; Penagaricano, J.A.; Prise, K.M.; Grifn, R.J. High dose bystander efects in spatially fractionated radia-tion therapy. Cancer Lett. 2015, 356, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Khan, M.K.; Wu, X.; Simone, C.B.; Fan, J.; Gressen, E.; Zhang, X.; Limoli, C.L.; Bahig, H.; Tubin, S.; et al. Spatially fractionated radiation therapy: History, present and the future. Clin. Transl. Radiat. Oncol. 2020, 20, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Tubin, S.; Khan, M.K.; Salerno, G.; Mourad, W.F.; Yan, W.; Jeremic, B.; Tubin, S. Mono-institutional phase 2 study of innovative Stereotactic Body RadioTherapy targeting PArtial Tumor HYpoxic (SBRT-PATHY) clonogenic cells in unresectable bulky non-small cell lung cancer: Profound non-targeted effects by sparing peri-tumoral immune microenvironment. Radiat. Oncol. 2019, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- De Olza, M.O.; Bourhis, J.; Irving, M.; Coukos, G.; Herrera, F.G. High versus low dose irradiation for tumor immune reprogramming. Curr. Opin. Biotechnol. 2020, 65, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Menon, H.; Chen, D.; Ramapriyan, R.; Verma, V.; Barsoumian, H.B.; Cushman, T.R.; Younes, A.; Cortez, M.A.; Erasmus, J.J.; De Groot, P.; et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J. Immunother. Cancer 2019, 7, 237. [Google Scholar] [CrossRef] [Green Version]
- Barsoumian, H.B.; Ramapriyan, R.; I Younes, A.; Caetano, M.S.; Menon, H.; I Comeaux, N.; Cushman, T.R.; E Schoenhals, J.; Cadena, A.P.; Reilly, T.P.; et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J. Immunother. Cancer 2020, 8, e000537. [Google Scholar] [CrossRef]
- Sang, W.; Xie, L.; Wang, G.; Li, J.; Zhang, Z.; Li, B.; Guo, S.; Deng, C.X.; Dai, Y. Oxygen-Enriched Metal-Phenolic X-Ray Nanopro-cessor for Cancer Radio-Radiodynamic Therapy in Combination with Checkpoint Blockade Immunotherapy. Adv. Sci. (Weinh) 2020, 8, 2003338. [Google Scholar] [CrossRef] [PubMed]
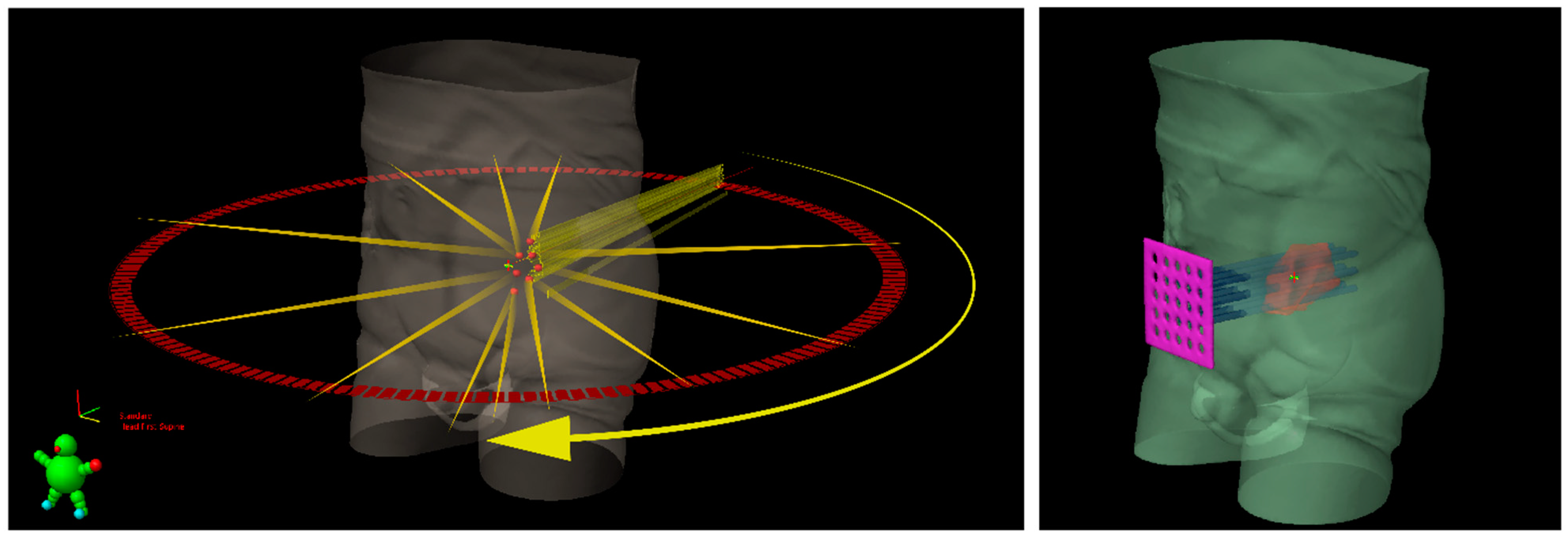
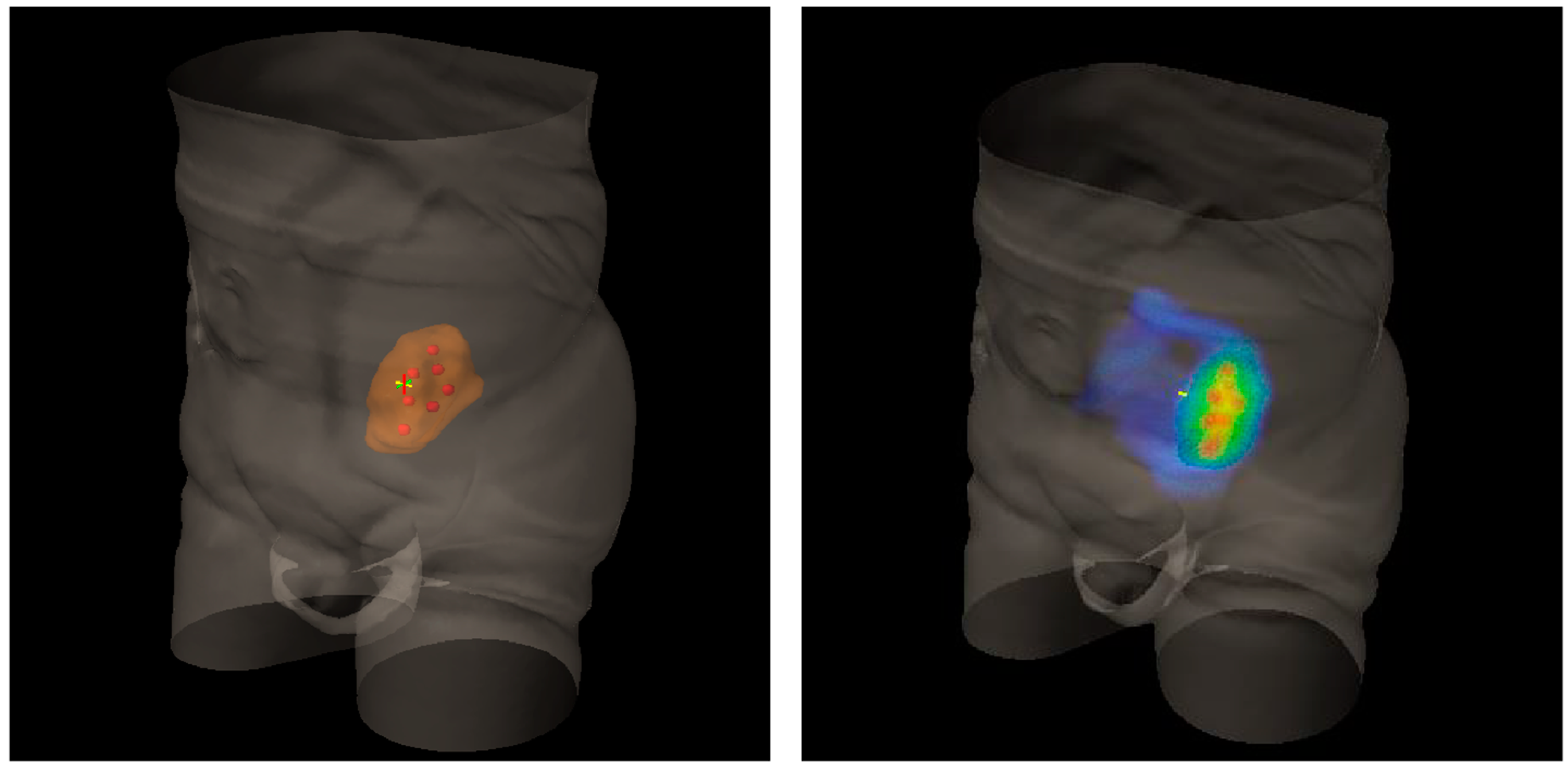
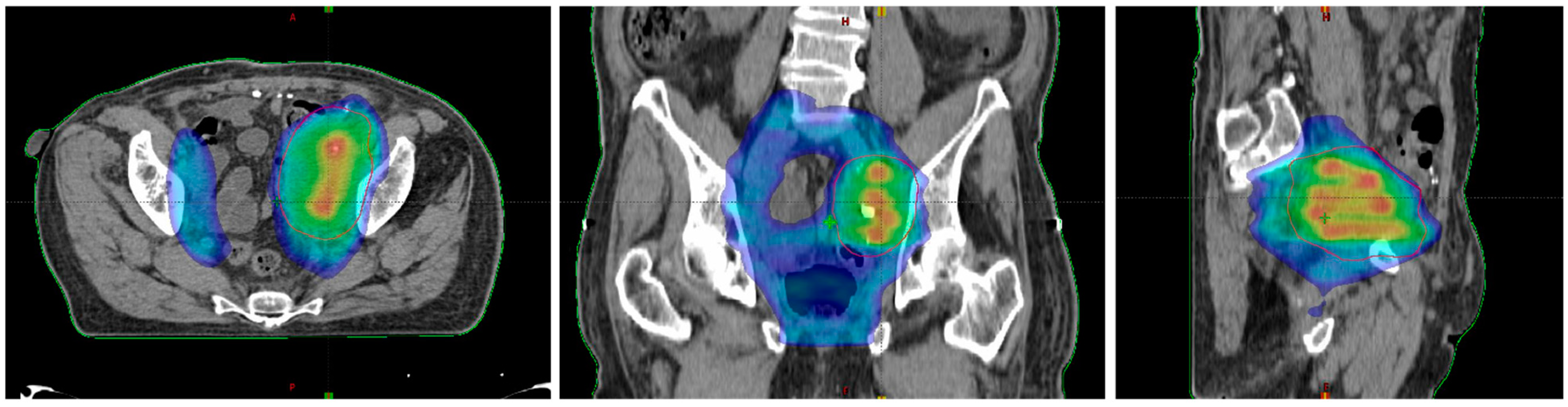
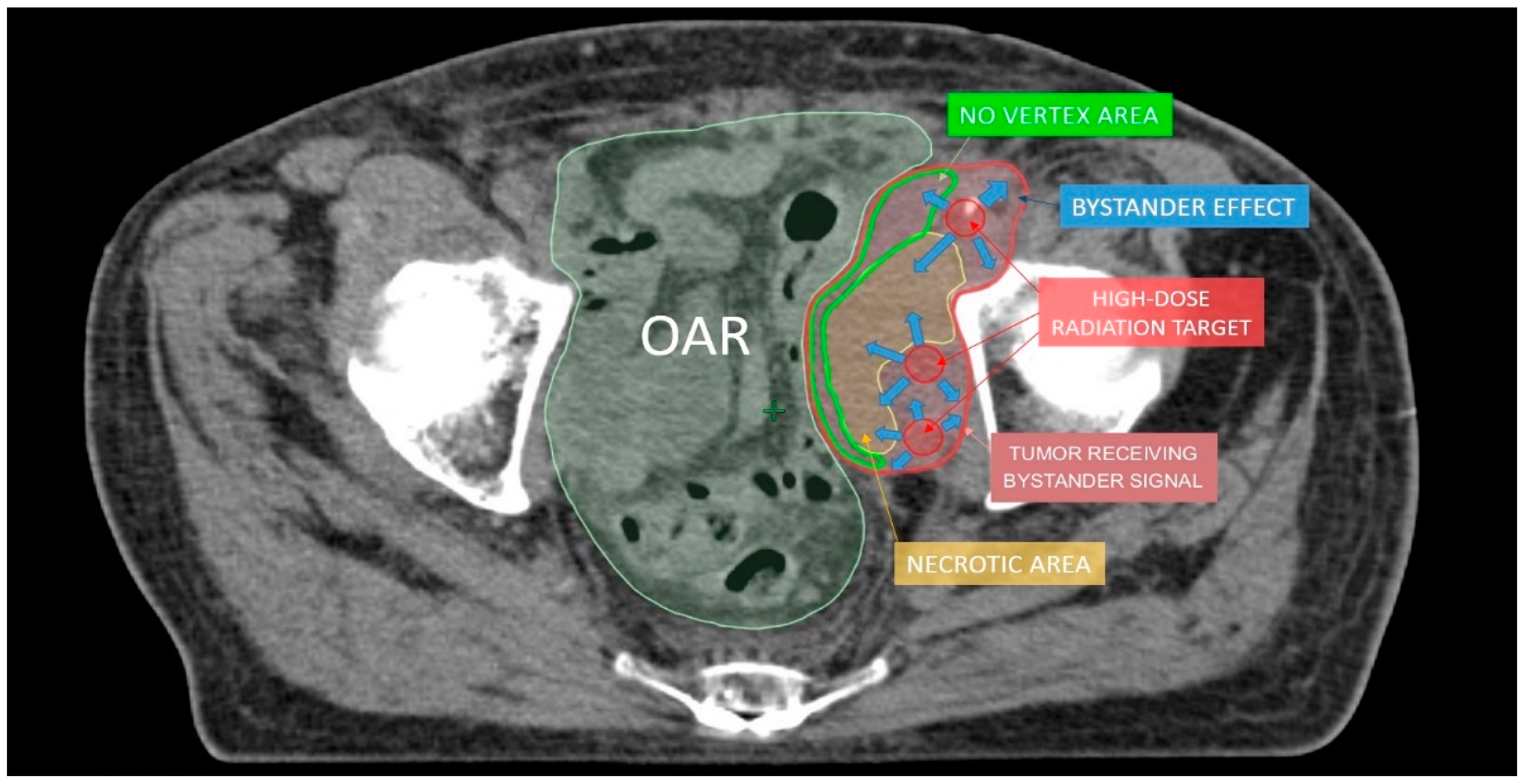
| Authors | Treated Sites (n) | Median Volume (Range) (cc) | Vertices (n) | Patients (n) | Follow-Up Median (Range) (mo) | Histology | Lattice RT Dose/fx (cGy) (Total Dose, Gy) | Further EBRT | Volume Reduction (Range, %) | Side Effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Amendola et al. [15], 2010 | Pelvis | 915 | 15 | 1 | 1 | Cervix squamous cell carcinoma | 240 (48) | Yes (prior) | 70% | Diarrhea (G1) |
| Suarez et al. [16], 2015 | Pelvis | 1495 | 12 | 1 | 20 | Ovarian carcinosarcoma | 900 (27) | Yes (post) | 70% | None |
| Amendola et al. [18], 2019 | Thorax | 175 (46–487) | 3 | 10 | 6 (1–71) | Non-small cell lung cancer | 1800 (18) | Yes (post) | 64% (15–83) | Radiation pneumonitis (G1) |
| Amendola et al. [19], 2020 | Pelvis | 200.35 (74.1–412.4) | 2–11 | 10 | 28.5 (4–77) | Squamous cell, adeno/adenosquamous carcinomas | 800 (24) | Yes (post) | 48% (6–91%) | Diarrhea G1, G2 cystitis |
| Duriseti S. et al. [21], 2021 | Thorax/abdomen/pelvis | 687.5 (350–4440) | Ordered threedimensional spatial arrangement | 11 | - | Various histologies | 1334 (66.7) | Yes (simultaneous) | Dosimetric feasibility | |
| Pollack A et al. [22], 2020 | Prostate | - | 1–3 (cylinders) | 25 | 66 (21–7) | High-risk prostate cancer | 1200–1400 (12–14 Gy) | Yes (post) | - | No acute G3 GU/GI; G1 (15), G2 (4) and G4 (1) (sepsis after a post-treatment transurethral resection) of late GU toxicity; G1 (11) and G2 (4) of late GI toxicity. |
| Kopchick B. et al. [24], 2020 | Breast | - | 22–172 (shots) | - | - | - | 2000 (20) | - | Dosimetric feasibility | |
| Jiang L. et al. [25], 2021 | Posterior chest wall | 63.2 | 6 | 1 | 7 | Non-small cell lung cancer | 2000 cGy at 69% isodose line | - | None |
| Authors | Tracer | Technique | Site | Histology | Pt | Aims | FU (Median mo) | Results/Toxicity |
|---|---|---|---|---|---|---|---|---|
| Williams B. B. et al., 2010 [36] | India ink as an O2 reporter | EPR | Different tumor locations | Various histologies | 10 | Direct measurements of absolute pO2 of tumors and other tissues in human subjects | - | - |
| Stefan Welz et al., 2017 [50] | 18F-fluoromisonidazole (FMISO) | dynFMISO PET-CT | H&N | Locally advanced HNSCC | 25 | Standard radiochemotherapy (stdRT) (70 Gy/35 fractions) vs. DE (77 Gy/35 fractions) with SIB to hypoxic tumor volume (HV) | 27 | Acute and late toxicity did not show significant differences between the two arms |
| Lindblom E. et al., 2017 [51] | 18F-flortanidazole (18F-HX4) | 18F-FMISO-PET | Thorax | Non-small cell lung cancer | 10 | Delineate hypoxic sub-volumes | - | - |
| Bollineni, V. R. et al., 2013 [54] | 18F-fluoroazomycin arabinoside (18F-FAZA) | PET-CT | Thorax | Advanced-stage non-small cell lung cancer (NSCLC) | 11 | Detect heterogeneous distributions of hypoxic subvolumes even within homogeneous 18F-FDG background | - | - |
| Chang, J. H et al., 2013 [55] | 18F-fluoromisonidazole (FMISO) | 18F-FMISO-PET | H&N | HNSCC | 8 | PET-guided radiotherapy dose painting to potentially overcome the radioresistant effects of hypoxia in HNSCC | - | Increases the TCP without increasing the NTCP, and increases the UTCP |
| B. Henriques de Figueiredo et al., 2014 [56] | 18F-fluoromisonidazole (FMISO) | 18F-FMISO-PET | H&N | III and IV H&N | 10 | Non-invasive assessment of hypoxia and dose escalation with [18F]-FMISO-PET-guided radiotherapy for head and neck cancers (HNC) | - | Improvement in TCP without excessive increase in NTCP for parotids |
| Kristi Hendrickson et al., 2011 [57] | 18F-fluoromisonidazole (FMISO) | 18F-FMISO-PET | H&N | HNSCC | 10 | PET-guided radiotherapy for boost planning (SIB) to the hypoxic subvolumes | 23 | Increasing the predicted TCP (mean 17%) without increasing expected complications |
| Nancy Lee et al., 2016 [58] | 18F-FDG and 18F-FMISO | 18FDG-PET and dynFMISO PET-CT | OPC | HPV-positive oropharyngeal carcinoma | 33 | Reducing the dose of radiation based on hypoxia imaging response | 32 (21–61) |
Intratreatment functional imaging is safe but requires further studies to determine its ultimate role in de-escalation treatment strategies |
| Abhishek Mahajan et al., 2016 [63] | mpMRI parameters | MRI | Pelvis | Cervix carcinoma | 30 | Characterizing and detecting vaginal vault/local recurrence | 6 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferini, G.; Valenti, V.; Tripoli, A.; Illari, S.I.; Molino, L.; Parisi, S.; Cacciola, A.; Lillo, S.; Giuffrida, D.; Pergolizzi, S. Lattice or Oxygen-Guided Radiotherapy: What If They Converge? Possible Future Directions in the Era of Immunotherapy. Cancers 2021, 13, 3290. https://doi.org/10.3390/cancers13133290
Ferini G, Valenti V, Tripoli A, Illari SI, Molino L, Parisi S, Cacciola A, Lillo S, Giuffrida D, Pergolizzi S. Lattice or Oxygen-Guided Radiotherapy: What If They Converge? Possible Future Directions in the Era of Immunotherapy. Cancers. 2021; 13(13):3290. https://doi.org/10.3390/cancers13133290
Chicago/Turabian StyleFerini, Gianluca, Vito Valenti, Antonella Tripoli, Salvatore Ivan Illari, Laura Molino, Silvana Parisi, Alberto Cacciola, Sara Lillo, Dario Giuffrida, and Stefano Pergolizzi. 2021. "Lattice or Oxygen-Guided Radiotherapy: What If They Converge? Possible Future Directions in the Era of Immunotherapy" Cancers 13, no. 13: 3290. https://doi.org/10.3390/cancers13133290
APA StyleFerini, G., Valenti, V., Tripoli, A., Illari, S. I., Molino, L., Parisi, S., Cacciola, A., Lillo, S., Giuffrida, D., & Pergolizzi, S. (2021). Lattice or Oxygen-Guided Radiotherapy: What If They Converge? Possible Future Directions in the Era of Immunotherapy. Cancers, 13(13), 3290. https://doi.org/10.3390/cancers13133290







