Alpha-Fetoprotein- and CD40Ligand-Expressing Dendritic Cells for Immunotherapy of Hepatocellular Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Cell Lines
2.2. Plasmids and Adenoviral Vectors
2.3. Stable Transfection of Hepa129-Cells
2.4. Western Blot of mAFP
2.5. Bone Marrow Derived DC and Adenoviral Transduction
2.6. Tumor Induction
2.7. DC Treatment Experiments
2.8. ELISA
2.9. Flow Cytometry
2.10. Isolation of Splenocytes and IFNγ-Secretion Assay
2.11. Caspase Detection in Tumors
2.12. Statistical Analysis
3. Results
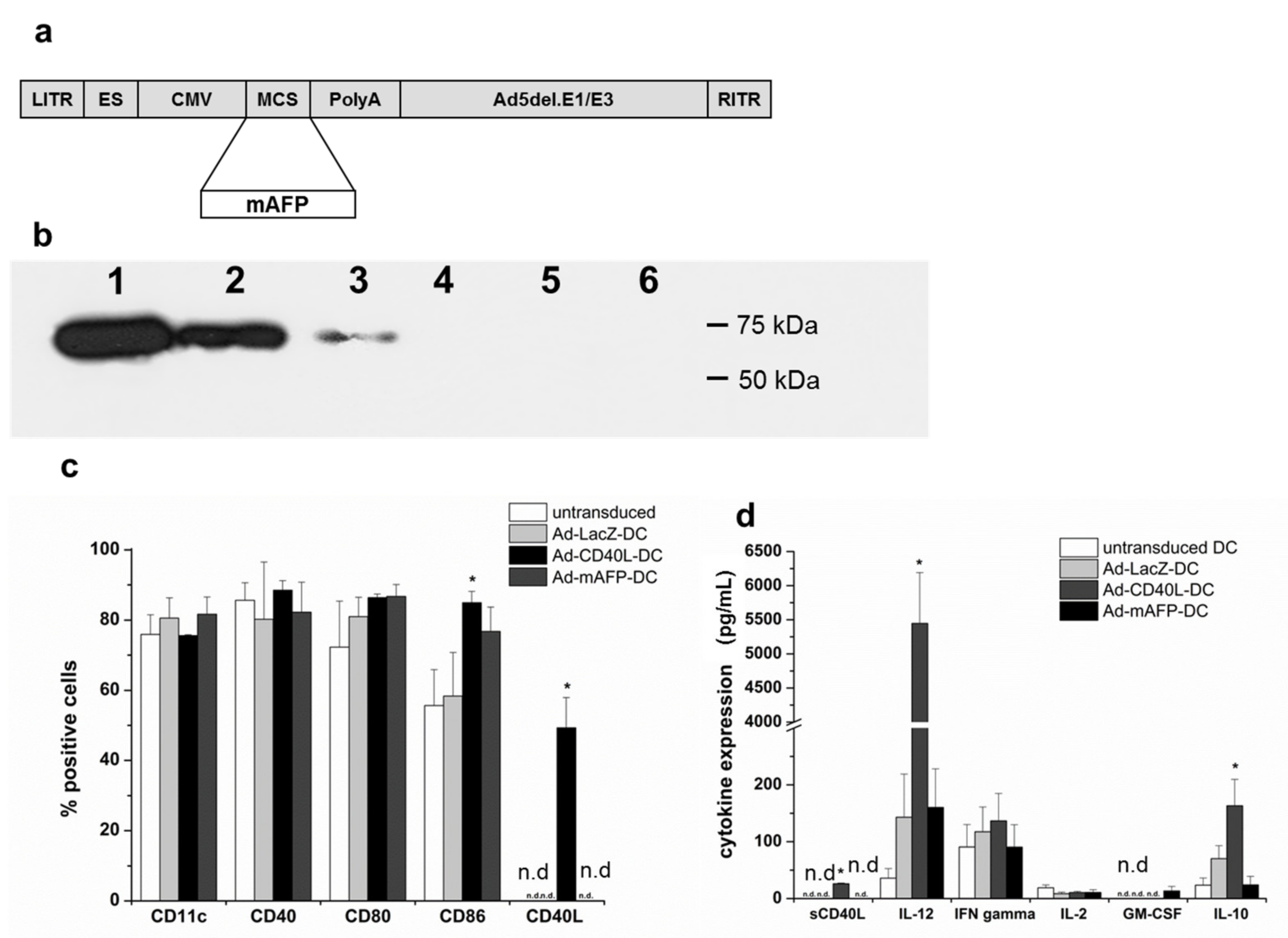
3.1. Adenoviral Transduction of DC with Ad-mAFP and Ad-CD40L
3.2. Induction of mAFP-Positive Hepatocellular Carcinoma in C3H Mice
3.3. Antitumor Effects of s.c. Vaccination with Ad-mAFP-DC on Pre-Established HCC
3.4. Antitumor Effects of s.c. Vaccination with Ad-mAFP-DC in Combination with i.t. Application of Ad-CD40L-DC in Pre-Established HCC
3.5. Antitumor Effects in Pre-Established Orthotopic HCC
3.6. Changes in the Intratumoral Cytokine Amounts
3.7. Recruitment of Immune Cell Populations in the Tumors
3.8. Induction of Tumor-Specific Effector Cells
3.9. Induction of Tumor Cell Apoptosis by Ad-CD40L-DC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mc Glynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef]
- Dasgupta, P.; Henshaw, C.; Youlden, D.R.; Clark, P.J.; Aitken, J.F.; Baade, P.D. Global trends in incidence rates of primary adult liver cancers: A systematic review and meta-analysis. Front. Oncol. 2020, 10, 171. [Google Scholar] [CrossRef] [Green Version]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Medavaram, S.; Zhang, Y. Emerging therapies in advanced hepatocellular carcinoma. Exp. Hematol. Oncol. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Mahn, R.; Vogt, A.; Kupczyk, P.; Sadeghlar, F.; van Beekum, K.; Hüneburg, R.; Meyer, C.; Toma, M.; Ahmadzadehfar, H.; Essler, M.; et al. Programmed cell death protein 1 (PD-1)-inhibition in hepatocellular carcinoma (HCC): A single center experience. Scand. J. Gastroenterol. 2020, 55, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I. Dendritic cells: Master regulators of the immune response. Cancer Immunol. Res. 2013, 1, 145–149. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Peng, Y.; Wang, L.; Hong, Y.; Jiang, X.; Li, Q.; Liu, H.; Huang, L.; Wu, J.; Celis, E.; et al. Identification of α-fetoprotein-specific T-cell receptors for hepatocellular carcinoma immunotherapy. Hepatology 2018, 68, 574–589. [Google Scholar] [CrossRef] [Green Version]
- Flecken, T.; Schmidt, N.; Hild, S.; Gostick, E.; Drognitz, O.; Zeiser, R.; Schemmer, P.; Bruns, H.; Eiermann, T.; Price, D.A.; et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8(+) T-cell responses in hepatocellular carcinoma. Hepatology 2014, 59, 1415–1426. [Google Scholar] [CrossRef] [Green Version]
- Hanke, P.; Rabe, C.; Serwe, M.; Böhm, S.; Pagenstecher, C.; Sauerbruch, T.; Caselmann, W.H. Cirrhotic patients with or without hepatocellular carcinoma harbour AFP-specific T-lymphocytes that can be activated in vitro by human alpha-fetoprotein. Scand. J. Gastroenterol. 2002, 37, 949–955. [Google Scholar] [CrossRef]
- He, Y.; Hong, Y.; Mizejewski, G.J. Engineering α-fetoprotein-based gene vaccines to prevent and treat hepatocellular carcinoma review and future prospects. Immunotherapy 2014, 6, 725–736. [Google Scholar] [CrossRef] [Green Version]
- González-Carmona, M.A.; Märten, A.; Hoffmann, P.; Schneider, C.; Sievers, E.; Schmidt-Wolf, I.G.; Sauerbruch, T.; Caselmann, W.H. Patient-derived dendritic cells transduced with an a-fetoprotein-encoding adenovirus and co-cultured with autologous cytokine-induced lymphocytes induce a specific and strong immune response against hepatocellular carcinoma cells. Liver Int. 2006, 26, 369–379. [Google Scholar] [CrossRef]
- Butterfield, L.H.; Ribas, A.; Dissette, V.B.; Lee, Y.; Yang, J.Q.; De la Rocha, P.; Duran, S.D.; Hernandez, J.; Seja, E.; Potter, D.M.; et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin. Cancer Res. 2006, 12, 2817–2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, D.H.; Midgley, R.S.; Mirza, N.; Torr, E.E.; Ahmed, F.; Steele, J.C.; Steven, N.M.; Kerr, D.J.; Young, L.S.; Adams, D.H. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology 2009, 49, 124–132. [Google Scholar] [CrossRef]
- Chen, C.; Ma, Y.-H.; Zhang, Y.-T.; Zhang, F.; Zhou, N.; Wang, X.; Liu, T.; Li, Y.-M. Effect of dendritic cell-based immunotherapy on hepatocellular carcinoma: A systematic review and meta-analysis. Cytotherapy 2018, 20, 975–989. [Google Scholar] [CrossRef]
- Beckebaum, S.; Zhang, X.; Chen, X.; Yu, Z.; Frilling, A.; Dworacki, G.; Grosse-Wilde, H.; Broelsch, C.E.; Gerken, G.; Cicinnati, V.R. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin. Cancer Res. 2004, 10, 7260–7269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaraj, S.; Gupta, K.; Pisarev, V.; Kinarsky, L.; Sherman, S.; Kang, L.; Herber, D.L.; Schneck, J.; Gabrilovich, D.I. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 2007, 13, 828–835. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Rong, D.; Zhang, B.; Zheng, W.; Wang, X.; Chen, Z.; Tang, W. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: Challenges and opportunities. Mol. Cancer 2019, 18, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto, J.; Melero, I.; Sangro, B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat. Rev. Gastroenterol Hepatol. 2015, 12, 681–700. [Google Scholar] [CrossRef]
- Schoenberger, S.P.; Toes, R.E.; van der Voort, E.I.; Offringa, R.; Melief, C.J. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 1998, 393, 480–483. [Google Scholar] [CrossRef]
- Elgueta, R.; Benson, M.J.; De Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [Green Version]
- Sadeghlar, F.; Vogt, A.; Mohr, R.U.; Mahn, R.; van Beekum, K.; Kornek, M.; Weismüller, T.J.; Branchi, V.; Matthaei, H.; Toma, M.; et al. Induction of cytotoxic effector cells towards cholangiocellular, pancreatic, and colorectal tumor cells by activation of the immune checkpoint CD40/CD40L on dendritic cells. Cancer Immunol. Immunother. 2020, 70, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Fallaux, F.J.; Kranenburg, O.; Cramer, S.J.; Houweling, A.; Van Ormondt, H.; Hoeben, R.C.; Van Der Eb, A.J. Characterization of 911: A new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum. Gene Ther. 1996, 7, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Hanke, P.; Serwe, M.; Dombrowski, F.; Sauerbruch, T.; Caselmann, W.H. DNA vaccination with AFP-encoding plasmid DNA prevents growth of subcutaneous AFP-expressing tumors and does not interfere with liver regeneration in mice. Cancer Gene Ther. 2002, 9, 346–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Carmona, M.A.; Lukacs-Kornek, V.; Timmerman, A.; Shabani, S.; Kornek, M.; Vogt, A.; Yildiz, Y.; Sievers, E.; Schmidt-Wolf, I.G.; Caselmann, W.H.; et al. CD40ligand-expressing dendritic cells induce regression of hepatocellular carcinoma by activating innate and acquired immunity in vivo. Hepatology 2008, 48, 157–168. [Google Scholar] [CrossRef]
- Schmitz, V.; Barajas, M.; Wang, L.; Peng, D.; Duarte, M.; Prieto, J.; Qian, C. Adenovirus-mediated CD40 ligand gene therapy in a rat model of orthotopic hepatocellular carcinoma. Hepatology 2001, 34, 72–81. [Google Scholar] [CrossRef]
- Vogt, A.; Sievers, E.; Lukacs-Kornek, V.; Decker, G.; Raskopf, E.; Meumann, N.; Büning, H.; Sauerbruch, T.; Strassburg, C.P.; Schmidt-Wolf, I.G.; et al. Improving immunotherapy of hepatocellular carcinoma (HCC) using dendritic cells (DC) engineered to express IL-12 in vivo. Liver Int. 2013, 34, 447–461. [Google Scholar] [CrossRef]
- Kalathil, S.; Lugade, A.A.; Miller, A.; Iyer, R.; Thanavala, Y. Higher frequencies of GARP (+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013, 73, 2435–2444. [Google Scholar] [CrossRef] [Green Version]
- Palucka, K.; Ueno, H.; Fay, J.; Banchereau, J. Dendritic cells and immunity against cancer. J. Intern. Med. 2011, 269, 64–73. [Google Scholar] [CrossRef] [Green Version]
- L Sabado, R.; Balan, S.; Bhardwaj, N. Dendritic cell-based immunotherapy. Cell Res. 2017, 27, 74–95. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.-T.; Nie, Y.; Sun, S.-N.; Lin, T.; Han, R.-J.; Jiang, J.; Li, Z.; Li, J.-Q.; Xiao, Y.-P.; Fan, Y.-Y.; et al. Tumor-associated antigen-based personalized dendritic cell vaccine in solid tumor patients. Cancer Immunol. Immunother. 2020, 69, 1375–1387. [Google Scholar] [CrossRef]
- Molinier-Frenkel, V.; Prévost-Blondel, A.; Hong, S.S.; Lengagne, R.; Boudaly, S.; Magnusson, M.K.; Boulanger, P.; Guillet, J.G. The maturation of murine dendritic cells induced by human adenovirus is mediated by the fiber knob domain. J. Biol. Chem. 2003, 278, 37175–37182. [Google Scholar] [CrossRef] [Green Version]
- Basner-Tschakarjan, E.; Gaffal, E.; O’Keeffe, M.; Tormo, D.; Limmer, A.; Wagner, H.; Hochrein, H.; Tüting, T. Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-alpha production. J. Gene. Med. 2006, 8, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, R.F.; Biagi, E.; Dutour, A.; Yvon, E.S.; Brown, M.P.; Lin, T.; Mei, Z.; Grilley, B.; Popek, E.; Heslop, H.E.; et al. Immunotherapy of high-risk acute leukemia with a recipient (autologous) vaccine expressing transgenic human CD40L and IL-2 after chemotherapy and allogeneic stem cell transplantation. Blood 2006, 107, 1332–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vonderheide, R.H.; Flaherty, K.T.; Khalil, M.; Stumacher, M.S.; Bajor, D.L.; Hutnick, N.A.; Sullivan, P.; Mahany, J.J.; Gallagher, M.; Kramer, A.; et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J. Clin. Oncol. 2007, 25, 876–883. [Google Scholar] [CrossRef]
- Wierda, W.G.; Cantwell, M.J.; Woods, S.J.; Rassenti, L.Z.; Prussak, C.E.; Kipps, T.J. CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood 2000, 96, 2917–2924. [Google Scholar] [CrossRef] [PubMed]
- Unitt, E.; Rushbrook, S.M.; Marshall, A.; Davies, S.; Gibbs, P.; Morris, L.S.; Coleman, N.; Alexander, G.J. Compromised lymphocytes infiltrate hepatocellular carcinoma: The role of T-regulatory cells. Hepatology 2005, 41, 722–730. [Google Scholar] [CrossRef]
- Granito, A.; Muratori, L.; Lalanne, C.; Quarneti, C.; Ferri, S.; Guidi, M.; Lenzi, M.; Muratori, P. Hepatocellular carcinoma in viral and autoimmune liver diseases: Role of CD4+ CD25+ Foxp3+ regulatory T cells in the immune microenvironment. World J. Gastroenterol 2021, 27, 2994–3009. [Google Scholar] [CrossRef]
- Langhans, B.; Nischalke, H.D.; Krämer, B.; Dold, L.; Lutz, P.; Mohr, R.; Vogt, A.; Toma, M.; Eis-Hübinger, A.M.; Nattermann, J.; et al. Role of regulatory T cells and checkpoint inhibition in hepatocellular carcinoma. Cancer Immunol. Immunother. 2019, 68, 2055–2066. [Google Scholar] [CrossRef]
- Loskog, A.; Totterman, T.H. CD40L—A multipotent molecule for tumor therapy. Endocr. Metab. Immune Disord. Drug Targets 2007, 7, 23–28. [Google Scholar] [CrossRef]
- Piechutta, M.; Berghoff, A.S. New emerging targets in cancer immunotherapy: The role of Cluster of Differentiation 40 (CD40/TNFR5). ESMO Open 2019, 4, e000510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quezada, S.A.; Peggs, K.S.; Curran, M.A.; Allison, J.P. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Investig. 2006, 116, 1935–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, P.-Y.; Ma, G.; Weber, K.J.; Ozao-Choy, J.; Wang, G.; Yin, B.; Divino, C.M.; Chen, S.-H. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010, 70, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Georgopoulos, N.T.; Steele, L.P.; Thomson, M.J.; Selby, P.J.; Southgate, J.; Trejdosiewicz, L.K. A novel mechanism of CD40-induced apoptosis of carcinoma cells involving TRAF3 and JNK/AP-1 activation. Cell Death Differ. 2006, 13, 1789–1801. [Google Scholar] [CrossRef]
- Elmetwali, T.; Searle, P.F.; McNeish, I.; Young, L.S.; Palmer, D.H. CD40 ligand induced cytotoxicity in carcinoma cells is enhanced by inhibition of metalloproteinase cleavage and delivery via a conditionally-replicating adenovirus. Mol. Cancer 2010, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, N.F.; Purdon, T.J.; van Leeuwen, D.G.; Lopez, A.V.; Curran, K.J.; Daniyan, A.F.; Brentjens, R.J. CD40 Ligand-Modified Chimeric Antigen Receptor T Cells Enhance Antitumor Function by Eliciting an Endogenous Antitumor Response. Cancer Cell. 2019, 35, 473–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komita, H.; Homma, S.; Saotome, H.; Zeniya, M.; Ohno, T.; Toda, G. Interferon-gamma produced by interleukin-12-activated tumor infiltrating CD8+T cells directly induces apoptosis of mouse hepatocellular carcinoma. J. Hepatol. 2006, 45, 662–672. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogt, A.; Sadeghlar, F.; Ayub, T.H.; Schneider, C.; Möhring, C.; Zhou, T.; Mahn, R.; Bartels, A.; Praktiknjo, M.; Kornek, M.T.; et al. Alpha-Fetoprotein- and CD40Ligand-Expressing Dendritic Cells for Immunotherapy of Hepatocellular Carcinoma. Cancers 2021, 13, 3375. https://doi.org/10.3390/cancers13133375
Vogt A, Sadeghlar F, Ayub TH, Schneider C, Möhring C, Zhou T, Mahn R, Bartels A, Praktiknjo M, Kornek MT, et al. Alpha-Fetoprotein- and CD40Ligand-Expressing Dendritic Cells for Immunotherapy of Hepatocellular Carcinoma. Cancers. 2021; 13(13):3375. https://doi.org/10.3390/cancers13133375
Chicago/Turabian StyleVogt, Annabelle, Farsaneh Sadeghlar, Tiyasha H. Ayub, Carlo Schneider, Christian Möhring, Taotao Zhou, Robert Mahn, Alexandra Bartels, Michael Praktiknjo, Miroslaw T. Kornek, and et al. 2021. "Alpha-Fetoprotein- and CD40Ligand-Expressing Dendritic Cells for Immunotherapy of Hepatocellular Carcinoma" Cancers 13, no. 13: 3375. https://doi.org/10.3390/cancers13133375
APA StyleVogt, A., Sadeghlar, F., Ayub, T. H., Schneider, C., Möhring, C., Zhou, T., Mahn, R., Bartels, A., Praktiknjo, M., Kornek, M. T., Toma, M., Schmidt-Wolf, I. G. H., Branchi, V., Matthaei, H., Kalff, J. C., Strassburg, C. P., & Gonzalez-Carmona, M. A. (2021). Alpha-Fetoprotein- and CD40Ligand-Expressing Dendritic Cells for Immunotherapy of Hepatocellular Carcinoma. Cancers, 13(13), 3375. https://doi.org/10.3390/cancers13133375






