Late Neurological and Cognitive Sequelae and Long-Term Monitoring of Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors: A Systematic Review by the Fondazione Italiana Linfomi
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Identification
2.2. Eligibility Criteria
2.3. Risk of Bias and Quality of Evidence Assessment
2.4. Study Selection and Data Extraction
2.5. Data Synthesis
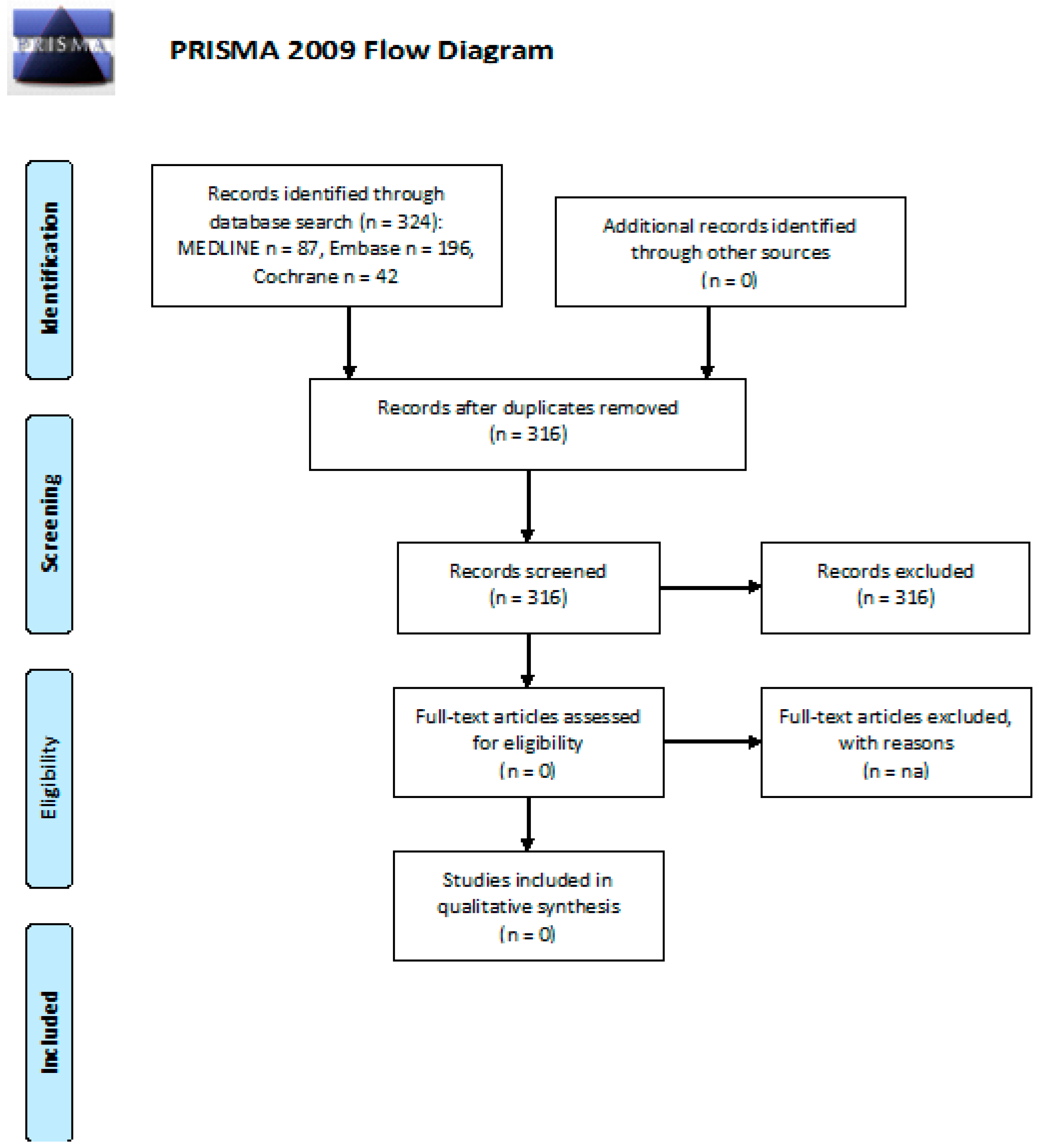
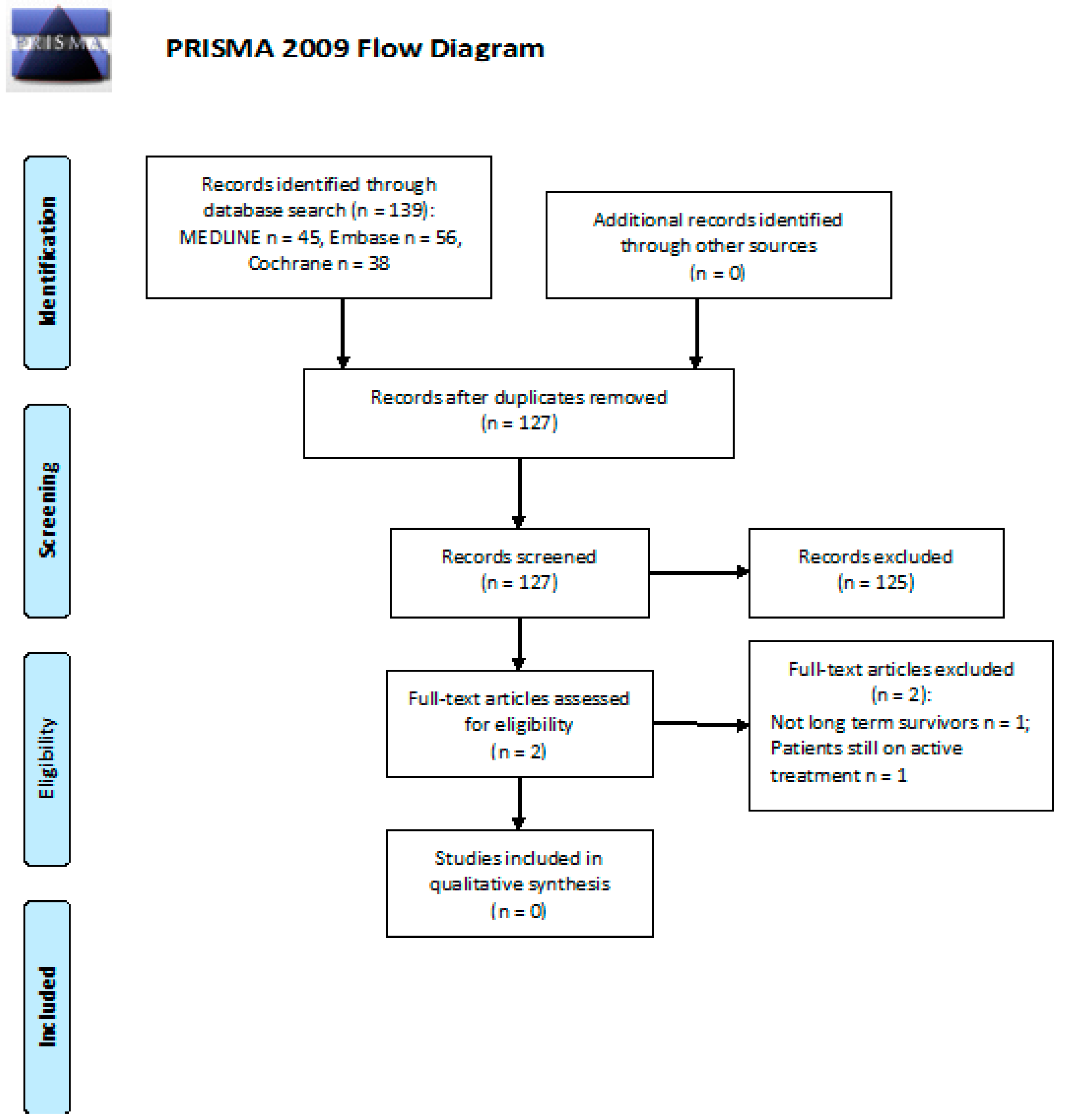
3. Results
3.1. Neurotoxicity: What Is the Incidence of PN in Long-Term cHL or DLBCL Survivors after First- and/or Second-Line CT or RT?
3.2. Neurotoxicity: Efficacy of Follow-Up Programs for Diagnosis and Management of PN in cHL or DLBCL Long-Term Survivors after First and/or Second Line CT or RT
3.3. Cognitive Impairment: What Is the Incidence of Cognitive Impairment in Long-Term cHL or DLBCL Survivors after First- and/or Second-Line CT or RT?
3.4. Cognitive Impairment: Efficacy of Follow-Up Programs for Diagnosis and Management of Cognitive Impairment in cHL or DLBCL Long-Term Survivors after First and/or Second Line CT or RT
3.5. Fatigue: What Is the Incidence of Fatigue in Long-Term cHL or DLBCL Survivors after First- and/or Second-Line CT or RT?
3.6. Anxiety and Depression: What Is the Incidence of Anxiety and Depression in Long-Term cHL or DLBCL Survivors after First- and/or Second-Line CT or RT?
3.7. Anxiety and Depression: Efficacy of Follow-Up Programs for Diagnosis and Management of Anxiety and Depression in cHL or DLBCL Long-Term Survivors after First and/or Second Line CT or RT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [Green Version]
- Ciavarella, S.; Minoia, C.; Quinto, A.M.; Oliva, S.; Carbonara, S.; Cormio, C.; Cox, M.C.; Bravo, E.; Santoro, F.; Napolitano, M.; et al. Improving Provision of Care for Long-term Survivors of Lymphoma. Clin. Lymphoma Myeloma Leuk. 2017, 17, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Stubblefield, M.D.; Burstein, H.J.; Burton, A.W.; Custodio, C.M.; Deng, G.E.; Ho, M.; Junck, L.; Morris, G.S.; Paice, J.A.; Tummala, S.; et al. NCCN task force report: Management of neuropathy in cancer. J. Natl. Compr. Cancer Netw. 2009, 7 (Suppl. S5), S1–S26. [Google Scholar] [CrossRef]
- Nademanee, A.; Sureda, A.; Stiff, P.; Holowiecki, J.; Abidi, M.; Hunder, N.; Pecsok, M.; Uttarwar, M.; Purevjal, I.; Sweetenham, J. Safety Analysis of Brentuximab Vedotin from the Phase III AETHERA Trial in Hodgkin Lymphoma in the Post-Transplant Consolidation Setting. Biol. Blood Marrow Transpl. 2018, 24, 2354–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Health Conference. Constitution of the World Health Organization. 1946. Bull. World Health Organ. 2002, 80, 983–984. [Google Scholar]
- Linendoll, N.; Saunders, T.; Burns, R.; Nyce, J.D.; Wendell, K.B.; Evens, A.M.; Parsons, S.K. Health-related quality of life in Hodgkin lymphoma: A systematic review. Health Qual. Life Outcomes 2016, 14, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wefel, J.S.; Schagen, S.B. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J. Clin. 2015, 65, 123–138. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.M.; Zent, C.S.; Janelsins, M.C. What is known and unknown about chemotherapy-related cognitive impairment in patients with haematological malignancies and areas of needed research. Br. J. Haematol. 2016, 174, 835–846. [Google Scholar] [CrossRef] [Green Version]
- Cuijpers, P.; Smit, F. Excess mortality in depression: A meta-analysis of community studies. J. Affect. Disord. 2002, 72, 227–236. [Google Scholar] [CrossRef]
- Pinquart, M.; Duberstein, P.R. Depression and cancer mortality: A meta-analysis. Psychol. Med. 2010, 40, 1797–1810. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Guidelines Version 2.2020 “Survivorship”. Available online: https://www.nccn.org/professionals/physician_gls/pdf/survivorship/pdf (accessed on 15 October 2020).
- The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Available online: https://osf.io/preprints/metaaxiv/v7gm2/ (accessed on 15 October 2020).
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Altman, D.G. The Cochrane Collaboration’s tool for assessing risk of bias. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P., Green, S., Eds.; John Wiley and Sons: New York, NY, USA, 2010; pp. 194–202. [Google Scholar]
- Wells Ga, S.B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses; Ottawa Health Research Institute: Ottawa, ON, Canada, 1999; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 15 March 2021).
- Oerlemans, S.; Issa, D.E.; van den Broek, E.C.; Nijziel, M.R.; Coebergh, J.W.W.; Huijgens, P.C.; Mols, F.; van de Poll-Franse, L.V. Health-related quality of life and persistent symptoms in relation to (R-)CHOP14, (R)CHOP21, and other therapies among patients with diffuse large B-cell lymphoma: Results of the population-based PHAROS-registry. Ann. Hematol. 2014, 93, 1705–1715. [Google Scholar] [CrossRef]
- Joly, F.; Henry-Amar, M.; Arveux, P.; Reman, O.; Tanguy, A.; Peny, A.M.; LeBailly, P.; Macé-Lesec’H, J.; Vié, B.; Génot, J.Y.; et al. Late psychosocial sequelae in Hodgkin’s disease survivors: A French population-based case-control study. J. Clin. Oncol. 1996, 14, 2444–2453. [Google Scholar] [CrossRef] [PubMed]
- Greil, R.; Holzner, B.; Kemmler, G.; Kopp, M.; Buchowski, A.; Oberaigner, W.; Fritsch, E.; Dirnhofer, S.; RueVer, U.; Diehl, V.; et al. Retrospective Assessment of Quality of Life and Treatment Outcome in Patients with Hodgkin’s Disease from 1969 to 1994. Eur. J. Cancer 1999, 35, 698–706. [Google Scholar] [CrossRef]
- Gil-Fernández, J.; Ramos, C.; Tamayo, T.; Tomás, F.; Figuera, A.; Arranz, R.; Martínez-Chamorro, C.; Fernández-Rañada, M. Quality of life and psychological well-being in Spanish long-term survivors of Hodgkin’s disease: Results of a controlled pilot study. Ann. Hematol. 2003, 82, 14–18. [Google Scholar] [CrossRef]
- Goodman, K.A.; Riedel, E.; Serrano, V.; Gulati, S.; Moskowitz, C.H.; Yahalom, J. Long-Term Effects of High-Dose Chemotherapy and Radiation for Relapsed and Refractory Hodgkin’s Lymphoma. J. Clin. Oncol. 2008, 26, 5240–5247. [Google Scholar] [CrossRef]
- Heutte, N.; Flechtner, H.H.; Mounier, N.; Mellink, W.A.M.; Meerwaldt, J.H.; Eghbali, H.; van’t Veer, M.B.; Noordijk, E.M.; Kluin-Nelemans, J.C.; Lampka, E.; et al. Quality of life after successful treatment of early-stage Hodgkin’s lymphoma: 10-year follow-up of the EORTC–GELA H8 randomised controlled trial. Lancet Oncol. 2009, 10, 1160–1170. [Google Scholar] [CrossRef]
- Brandt, J.; Dietrich, S.; Meissner, J.; Neben, K.; Ho, A.D.; Witzens-Harig, M. Quality of life of long-term survivors with Hodgkin lymphoma after high-dose chemotherapy, autologous stem cell transplantation, and conventional chemotherapy. Leuk. Lymphoma 2010, 51, 2012–2020. [Google Scholar] [CrossRef]
- Minn, A.Y.; Riedel, E.; Halpern, J.; Johnston, L.J.; Horning, S.J.; Hoppe, R.T.; Goodman, K.A. Long-term outcomes after high dose therapy and autologous haematopoietic cell rescue for refractory/relapsed Hodgkin Lymphoma. Br. J. Haematol. 2012, 159, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.A.; Oerlemans, S.; Krol, A.D.G.; Creutzberg, C.L.; Van De Poll-Franse, L.V. Chronic fatigue in Hodgkin lymphoma survivors and associations with anxiety, depression and comorbidity. Br. J. Cancer 2014, 110, 868–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Kim, I.R.; Kim, S.H.; Lee, S.; Ok, O.; Kim, W.S.; Suh, C.; Lee, M.H. Health-related quality of life in Korean lymphoma survivors compared with the general population. Ann. Hematol. 2014, 93, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Husson, O.; Oerlemans, S.; Mols, F.; Schep, G.; Van De Poll-Franse, L.V. High levels of physical activity are associated with lower levels of fatigue among lymphoma patients: Results from the longitudinal PROFILES registry. Acta Oncol. 2015, 54, 678–684. [Google Scholar] [CrossRef] [Green Version]
- Hjermstad, M.J.; Oldervoll, L.; Fosså, S.D.; Holte, H.; Jacobsen, A.B.; Loge, J.H. Quality of life in long-term Hodgkin’s disease survivors with chronic fatigue. Eur. J. Cancer 2006, 42, 327–333. [Google Scholar] [CrossRef]
- Hjermstad, M.J.; Fosså, S.D.; Oldervoll, L.; Holte, H.; Jacobsen, A.B.; Loge, J.H. Fatigue in Long-Term Hodgkin’s Disease Survivors: A Follow-Up Study. J. Clin. Oncol. 2005, 23, 6587–6595. [Google Scholar] [CrossRef] [PubMed]
- Kiserud, C.E.; Seland, M.; Holte, H.; Fosså, A.; Fosså, S.D.; Bollerslev, J.; Bjøro, T.; Loge, J.H. Fatigue in male lymphoma survivors differs between diagnostic groups and is associated with latent hypothyroidism. Acta Oncol. 2015, 54, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Kaasa, S.; Loge, J.H.; Knobel, H.; Jordhøy, M.S.; Brenne, E. Fatigue. Measures and relation to pain. Acta Anaesthesiol. Scand. 1999, 43, 939–947. [Google Scholar] [CrossRef]
- Loge, J.H.; Abrahamsen, A.F.; Ekeberg, O.; Kaasa, S. Fatigue and Psychiatric Morbidity Among Hodgkin’s Disease Survivors. J. Pain Symptom Manag. 2000, 19, 91–99. [Google Scholar] [CrossRef]
- Loge, J.H.; Abrahamsen, A.F.; Ekeberg, O.; Kaasa, S. Reduced health-related quality of life among Hodgkin’s disease survivors: A comparative study with general population norms. Ann. Oncol. 1999, 10, 71–77. [Google Scholar] [CrossRef]
- Loge, J.H.; Abrahamsen, A.F.; Ekeberg, O.; Kaasa, S. Hodgkin’s disease survivors more fatigued than the general population. J. Clin. Oncol. 1999, 17, 253–261. [Google Scholar] [CrossRef]
- Knobel, H.; Loge, J.H.; Lund, M.B.; Forfang, K.; Nome, O.; Kaasa, S. Late medical complications and fatigue in Hodgkin’s disease survivors. J. Clin. Oncol. 2001, 19, 3226–3233. [Google Scholar] [CrossRef] [PubMed]
- Miltényi, Z.; Magyari, F.; Simon, Z.; Illés, A. Quality of life and fatigue in Hodgkin’s lymphoma patients. Tumori J. 2010, 96, 594–600. [Google Scholar] [CrossRef]
- Mols, F.; Vingerhoets, A.J.; Coebergh, J.W.; Vreugdenhil, G.; Aaronson, N.K.; Lybeert, M.L.; van de Poll-Franse, L.V. Better quality of life among 10–15 year survivors of Hodgkin’s lymphoma compared to 5–9 year survivors: A population-based study. Eur. J. Cancer 2006, 42, 2794–2801. [Google Scholar] [CrossRef] [Green Version]
- Oldervoll, L.M.; Loge, J.H.; Kaasa, S.; Lydersen, S.; Hjermstad, M.J.; Thorsen, L.; Holte, H., Jr.; Jacobsen, A.B.; Fosså, S.D. Physical activity in Hodgkin’s lymphoma survivors with and without chronic fatigue compared with the general population—A cross-sectional study. BMC Cancer 2007, 7, 210. [Google Scholar] [CrossRef] [PubMed]
- Oldervoll, L.M.; Kaasa, S.; Knobel, H.; Loge, J.H. Exercise reduces fatigue in chronic fatigued Hodgkin’s disease survivors—Results from a pilot study. Eur. J. Cancer 2003, 39, 57–63. [Google Scholar] [CrossRef]
- Van Tulder, M.W.; Aaronson, N.K.; Bruning, P.F. The quality of life of long-term survivors of Hodgkin’s disease. Ann. Oncol. 1994, 5, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.; Biasoli, I.; Scheliga, A.; Baptista, R.L.; Brabo, E.P.; Morais, J.C.; Werneck, G.L.; Spector, N. Association of social network and social support with health-related quality of life and fatigue in long-term survivors of Hodgkin lymphoma. Support. Care Cancer 2013, 21, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Rüffer, J.U.; Flechtner, H.; Tralls, P.; Josting, A.; Sieber, M.; Lathan, B.; Diehl, V.; German Hodgkin Lymphoma Study Group. Fatigue in long-term survivors of Hodgkin‘s lymphoma; a report from the German Hodgkin Lymphoma Study Group (GHSG). Eur. J. Cancer 2003, 39, 2179–2186. [Google Scholar] [CrossRef]
- Wettergren, L.; Björkholm, M.; Axdorph, U.; Langius-Eklöf, A. Determinants of health-related quality of life in long-term survivors of Hodgkin’s lymphoma. Qual. Life Res. 2004, 13, 1369–1379. [Google Scholar] [CrossRef]
- Vissers, P.A.; Thong, M.S.; Pouwer, F.; Zanders, M.M.; Coebergh, J.W.; van de Poll-Franse, L.V. The impact of comorbidity on Health-Related Quality of Life among cancer survivors: Analyses of data from the PROFILES registry. Cancer Surviv. 2013, 7, 602–613. [Google Scholar] [CrossRef]
- Behringer, K.; Goergen, H.; Müller, H.; Thielen, I.; Brillant, C.; Kreissl, S.; Halbsguth, T.V.; Meissner, J.; Greil, R.; Moosmann, P.; et al. Cancer-Related Fatigue in Patients with and Survivors of Hodgkin Lymphoma: The Impact on Treatment Outcome and Social Reintegration. J. Clin. Oncol. 2016, 34, 4329–4337. [Google Scholar] [CrossRef]
- Kreissl, S.; Mueller, H.; Goergen, H.; Mayer, A.; Brillant, C.; Behringer, K.; Halbsguth, T.V.; Hitz, F.; Soekler, M.; Shonukan, O.; et al. Cancer-related fatigue in patients with and survivors of Hodgkin’s lymphoma: A longitudinal study of the German Hodgkin Study Group. Lancet Oncol. 2016, 17, 1453–1462. [Google Scholar] [CrossRef]
- Korszun, A.; Sarker, S.J.; Chowdhury, K.; Clark, C.; Greaves, P.; Johnson, R.; Kingston, J.; Levitt, G.; Matthews, J.; White, P.; et al. Psychosocial factors associated with impact of cancer in longterm haematological cancer survivors. Br. J. Haematol. 2014, 164, 790–803. [Google Scholar] [CrossRef]
- Loge, J.H.; Abrahamsen, A.F.; Ekeberg, O.; Hannisdal, E.; Kaasa, S. Psychological distress after cancer cure: A survey of 459 Hodgkin’s disease survivors. Br. J. Cancer 1997, 76, 791–796. [Google Scholar] [CrossRef] [Green Version]
- Magyari, F.; Kósa, K.; Berecz, R.; Illés, A.; Miltényi, Z.; Simon, Z.; Illés, Á. Employment status and health related quality of life among Hodgkin-lymphoma survivors’—Results based on data from a major treatment center in Hungary. Health Qual. Life Outcomes 2017, 15, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksnes, L.H.; Hall, K.S.; Jebsen, N.; Fosså, S.D.; Dahl, A.A. Young survivors of malignant bone tumours in the extremities: A comparative study of quality of life, fatigue and mental distress. Support. Care Cancer 2007, 15, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Rowland, J.H.; Gallicchio, L.; Mollica, M.; Saiontz, N.; Falisi, A.L.; Tesauro, G. Survivorship Science at the NIH: Lessons Learned from Grants Funded in Fiscal Year 2016. J. Natl. Cancer Inst. 2019, 111, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Haematological Malignancy Research Network (HMRN). Available online: https://www.hmrn.org/statistics/survival (accessed on 15 January 2021).
- Chen, R.; Gopal, A.K.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Connors, J.M.; Engert, A.; Larsen, E.K.; Huebner, D.; et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood 2016, 128, 1562–1566. [Google Scholar] [CrossRef]
- Moskowitz, C.H.; Walewski, J.; Nademanee, A.; Masszi, T.; Agura, E.; Holowiecki, J.; Abidi, M.H.; Chen, A.I.; Stiff, P.; Viviani, S.; et al. Five-year PFS from the AETHERA thrial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood 2018, 132, 2639–2642. [Google Scholar] [CrossRef] [Green Version]
- Delanian, S.; Lefaix, J.L.; Pradat, P.F. Radiation-induced neuropathy in cancer survivors. Radiother. Oncol. 2012, 105, 273–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidel, C.; Kuhnt, T.; Kortmann, R.D.; Hering, K. Radiation-induced camptocormia and dropped head syndrome Review and case report of radiation-induced movement disorders. Strahlenther. Onkol. 2015, 191, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Micaily, B.; Brady, L.W. Hodgkin’s disease—Results from a program in radiation therapy. J. Clin. Oncol. 1984, 7, 159–171. [Google Scholar]
- Frick, M.A.; Vachani, C.C.; Hampshire, M.K.; Bach, C.; Arnold-Korzeniowski, K.; Metz, J.M.; Hill-Kayser, C.E. Patient-Reported Survivorship Care Practices and Late Effects After Treatment of Hodgkin and Non-Hodgkin Lymphoma. JCO Clin. Cancer Inf. 2018, 2, 1–10. [Google Scholar]
- Trachtenberg, E.; Mashiach, T.; Ben Hayun, R.; Tadmor, T.; Fisher, T.; Aharon-Peretz, J.; Dann, E.J. Cognitive impairment in Hodgkin lymphoma survivors. Br. J. Haematol. 2018, 182, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Wouters, H.; Baars, J.W.; Schagen, S.B. Neurocognitive function of lymphoma patients after treatment with chemotherapy. Acta Oncol. 2016, 55, 1121–1125. [Google Scholar] [CrossRef] [Green Version]





| Clinical Question | PICOs |
|---|---|
| What is the incidence of PN in long-term cHL or DLBCL survivors after first- and/or second-line CT or RT? | P: long-term cHL or DLBCL survivor (≥5 years disease- or treatment-free) adults (≥18-year-old at diagnosis) treated with first-line therapy or second-line therapy including ASCT I: chemotherapy (e.g., ABVD for cHL or R-CHOP for DLBCL), radiotherapy C: none/age- and sex-matched general population/other chemotherapy or radiotherapy regimen O: incidence of sensory/motor neuropathy cases, other neurotoxicities S: cohort, controlled cohort, RCT, reviews of those studies |
| Efficacy of follow-up programs for diagnosis and management of PN in cHL or DLBCL long-term survivors after first and/or second line CT or RT | P: long-term cHL or DLBCL survivor (≥5 years disease- or treatment-free) adults (≥18 years at diagnosis) treated with first-line therapy or second-line therapy including ASCT I: chemotherapy (e.g., ABVD for cHL or R-CHOP for DLBCL), radiotherapy C: scheduled follow-up program/no scheduled follow-up program/scheduled follow-up program with different timing (frequency or other variables) O: incidence of sensory/motor neuropathy cases, other neurotoxicities, QoL S: cohort, controlled cohort, RCT, reviews of those studies |
| What is the incidence of cognitive impairment in long-term cHL or DLBCL survivors after first- and/or second-line CT or RT? | P: long-term cHL or DLBCL survivor (≥5 years disease- or treatment-free) adults (≥18 years at diagnosis) treated with first-line therapy or second-line therapy including ASCT I: chemotherapy (e.g., ABVD for cHL or R-CHOP for DLBCL), radiotherapy C: none/age- and sex-matched general population/other chemotherapy or radiotherapy regimen O: incidence of cognitive impairment (e.g., lack of memory, concentration, attention) S: cohort, controlled cohort, RCT, reviews of those studies |
| Efficacy of follow-up programs for diagnosis and management of cognitive impairment in cHL or DLBCL long-term survivors after first and/or second line CT or RT | P: long-term cHL or DLBCL survivor (≥5 years disease- or treatment-free) adults (≥18 years at diagnosis) treated with first-line therapy or second-line therapy including ASCT I: chemotherapy (e.g., ABVD for cHL or R-CHOP for DLBCL), radiotherapy C: scheduled follow-up program/no scheduled follow-up program/scheduled follow-up program with different timing (frequency or other variables) O: incidence of cognitive impairment, QoL S: cohort, controlled cohort, RCT, reviews of those studies |
| What is the incidence of fatigue in long-term cHL or DLBCL survivors after first and/or second line CT or RT? | P: long-term cHL or DLBCL survivor (≥5 years disease- or treatment-free) adults (≥18 years at diagnosis) treated with first-line therapy or second-line therapy including ASCT I: chemotherapy (e.g., ABVD for cHL or R-CHOP for DLBCL), radiotherapy C: none/age- and sex-matched general population/other chemotherapy or radiotherapy regimen O: incidence/prevalence of fatigue S: cohort, controlled cohort, RCT, reviews of those studies |
| What is the incidence of anxiety and depression in long-term cHL or DLBCL survivors after first- and/or second-line CT or RT? | P: long-term cHL or DLBCL survivor (≥5 years disease- or treatment-free) adults (≥18 years at diagnosis) treated with first-line therapy or second-line therapy including ASCT I: chemotherapy (e.g., ABVD for cHL or R-CHOP for DLBCL), radiotherapy C: none/age- and sex-matched general population/other chemotherapy or radiotherapy regimen O: incidence of anxiety and depression S: cohort, controlled cohort, RCT, reviews of those studies |
| Efficacy of follow-up programs for diagnosis and management of anxiety and depression in cHL or DLBCL long-term survivors after first and/or second line CT or RT | P: long-term cHL or DLBCL survivor (≥5 years disease or treatment-free) adults (≥18 years at diagnosis) treated with first-line therapy or second-line therapy including ASCT I: chemotherapy (e.g., ABVD for cHL or R-CHOP for DLBCL), radiotherapy C: scheduled follow-up program/no scheduled follow-up program/scheduled follow-up program with different timing (frequency or other variables) O: incidence of anxiety and depression, QoL S: cohort, controlled cohort, RCT, reviews of those studies |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franceschetti, S.; Annunziata, M.A.; Agostinelli, G.; Gerardi, C.; Allocati, E.; Minoia, C.; Guarini, A. Late Neurological and Cognitive Sequelae and Long-Term Monitoring of Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors: A Systematic Review by the Fondazione Italiana Linfomi. Cancers 2021, 13, 3401. https://doi.org/10.3390/cancers13143401
Franceschetti S, Annunziata MA, Agostinelli G, Gerardi C, Allocati E, Minoia C, Guarini A. Late Neurological and Cognitive Sequelae and Long-Term Monitoring of Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors: A Systematic Review by the Fondazione Italiana Linfomi. Cancers. 2021; 13(14):3401. https://doi.org/10.3390/cancers13143401
Chicago/Turabian StyleFranceschetti, Silvia, Maria Antonietta Annunziata, Giulia Agostinelli, Chiara Gerardi, Eleonora Allocati, Carla Minoia, and Attilio Guarini. 2021. "Late Neurological and Cognitive Sequelae and Long-Term Monitoring of Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors: A Systematic Review by the Fondazione Italiana Linfomi" Cancers 13, no. 14: 3401. https://doi.org/10.3390/cancers13143401







