Is Hypoxia a Factor Influencing PSMA-Directed Radioligand Therapy?—An In Silico Study on the Role of Chronic Hypoxia in Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
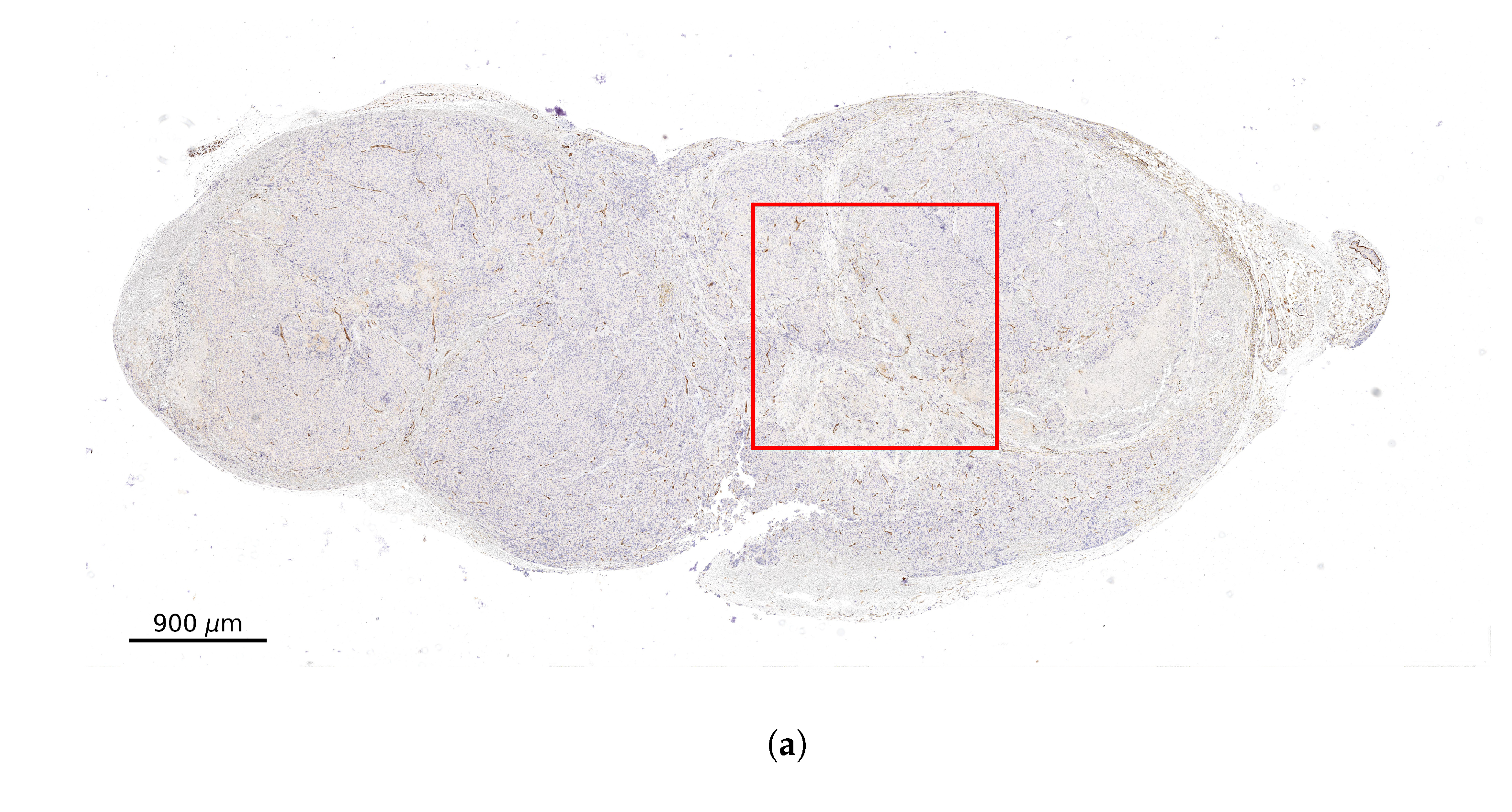
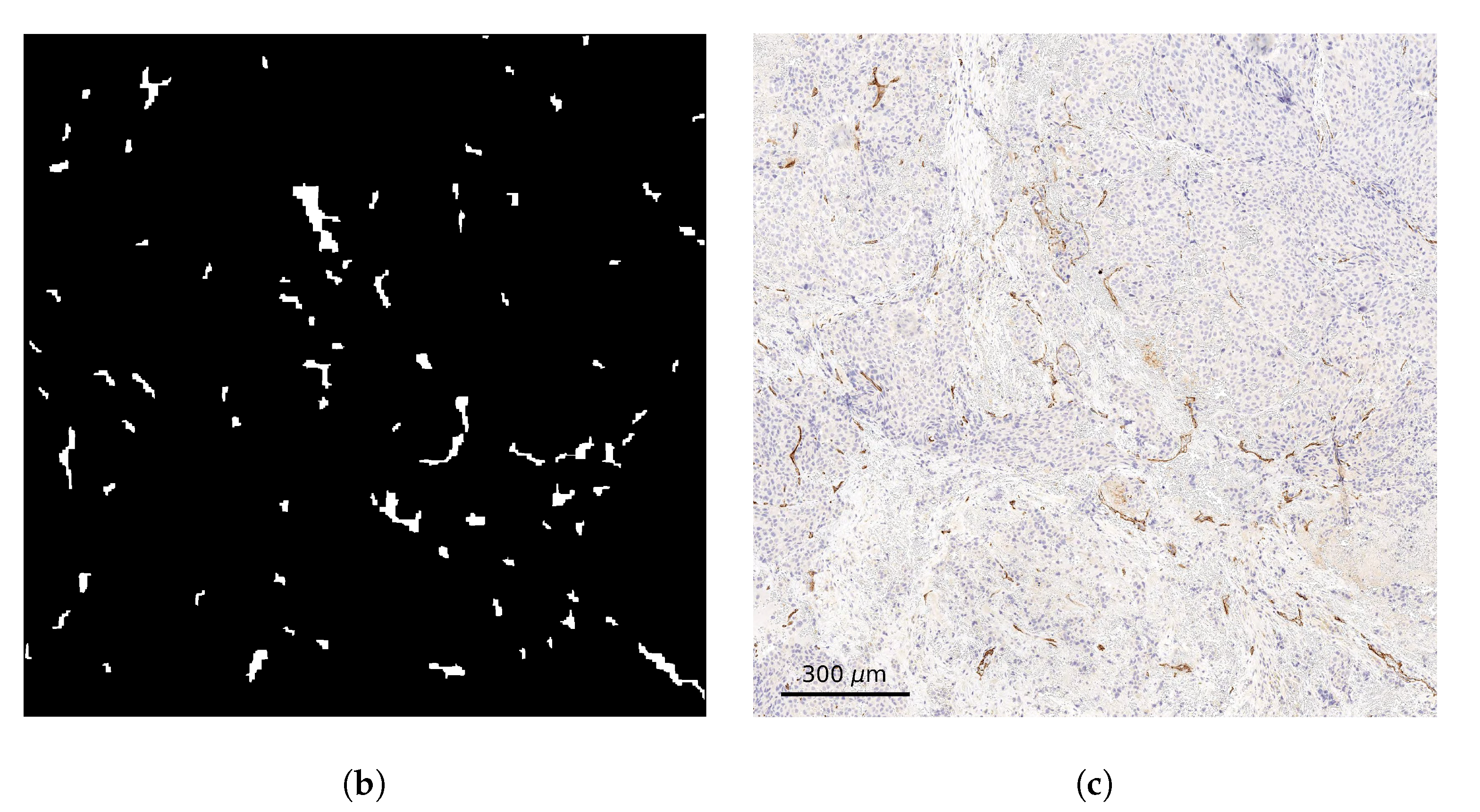
2.2. Stained Tumor Sections
2.3. Computational Domain and Vessel Map Generation
2.4. Multi-Scale Spatial-Temporal Models of PSMA-Ligands Dynamics
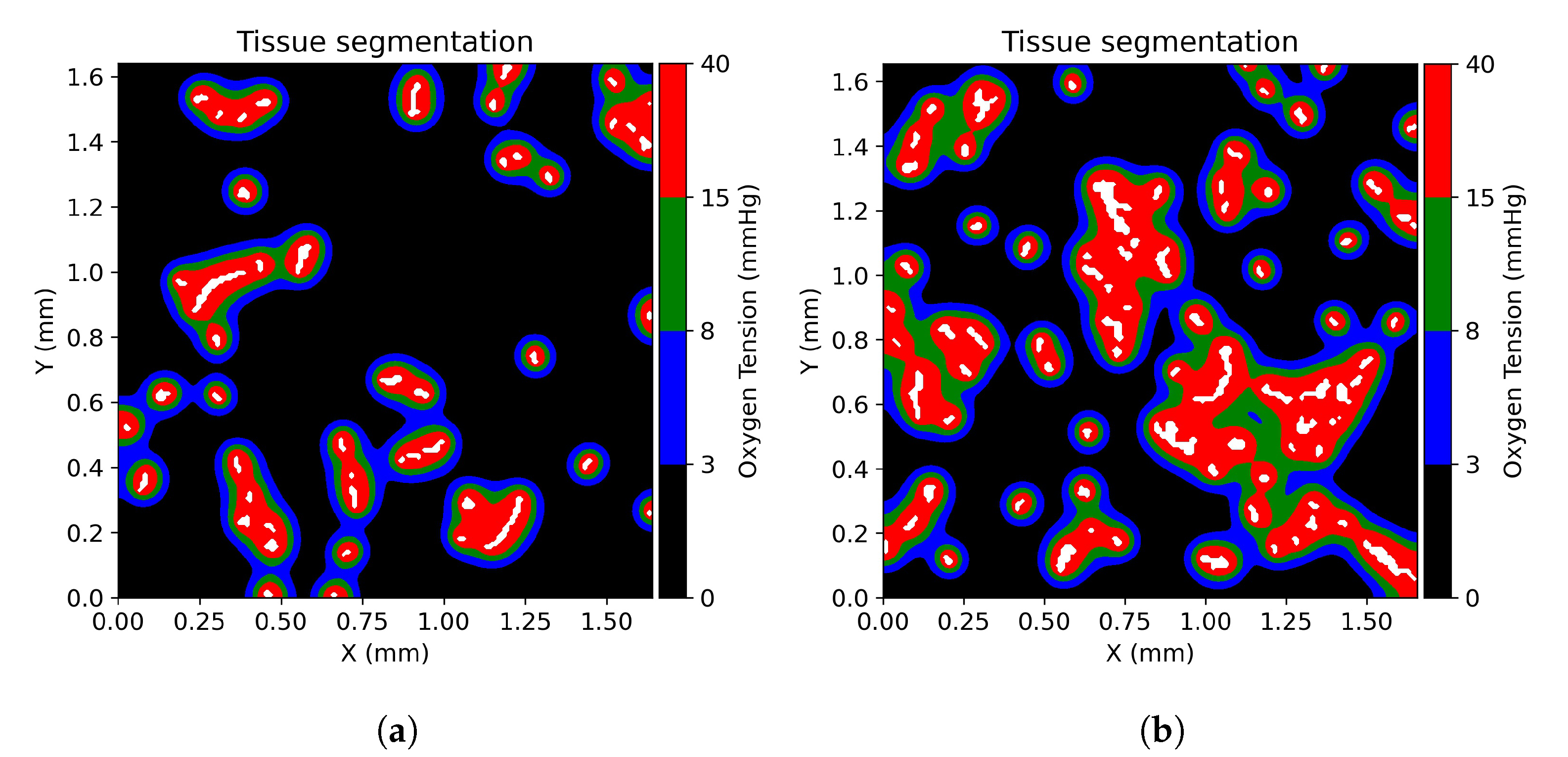
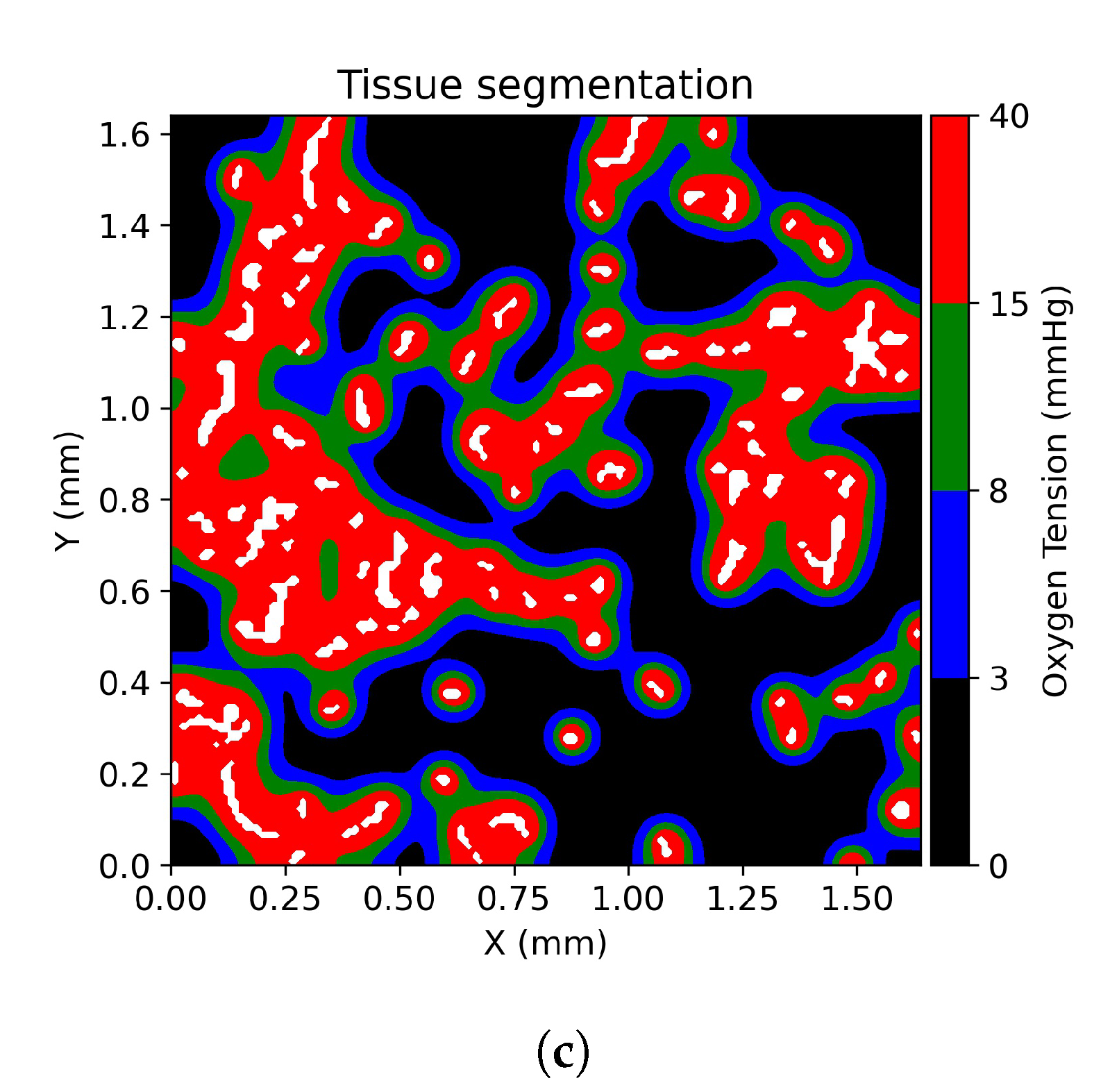
2.5. Tissue Oxygenation Models and Oxygen Dependent Tissue Segmentation
2.6. Physiologically Based Pharmacokinetic Model
2.7. Absorbed Dose and Cell Survival Probability
3. Results
3.1. Tissue Oxygenation and Oxygen Dependent Tissue Segmentation
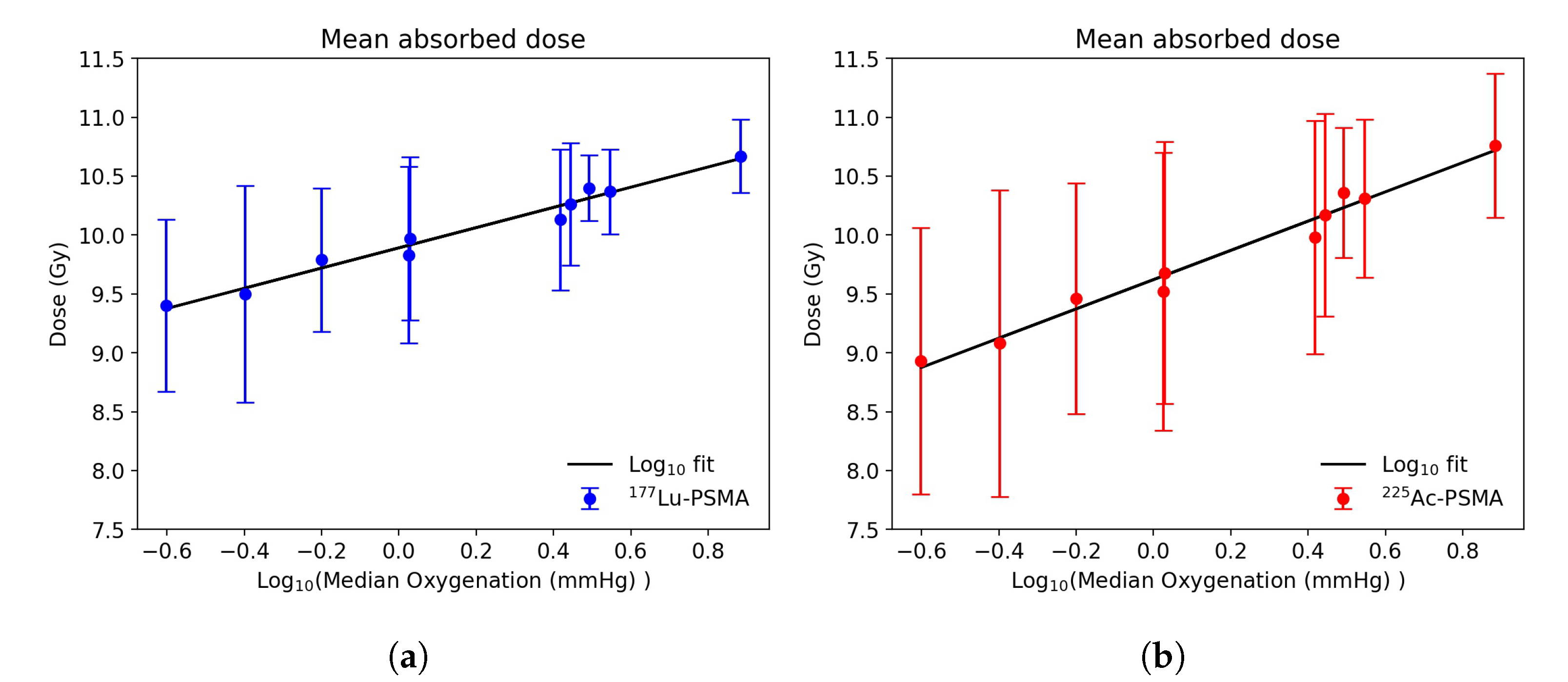
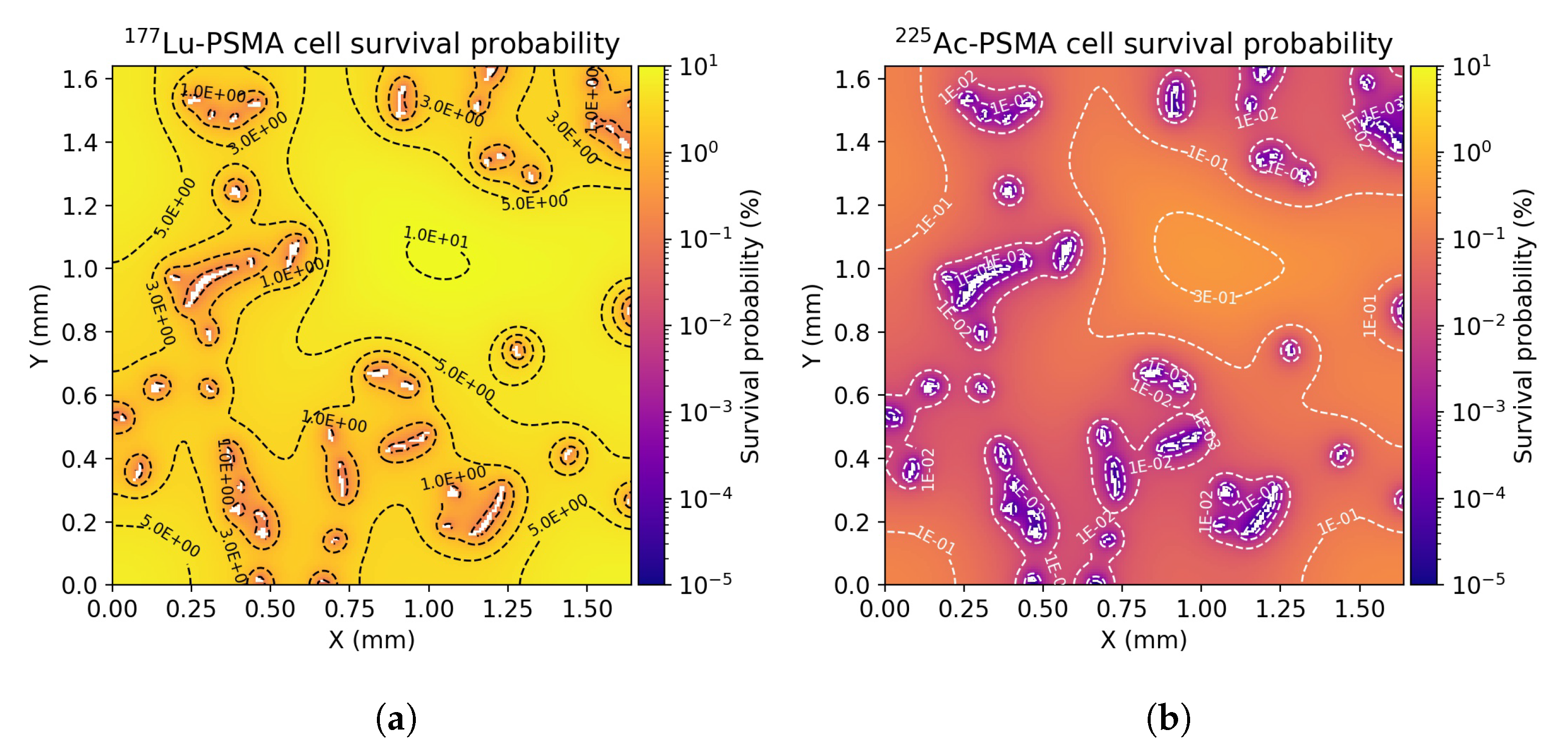
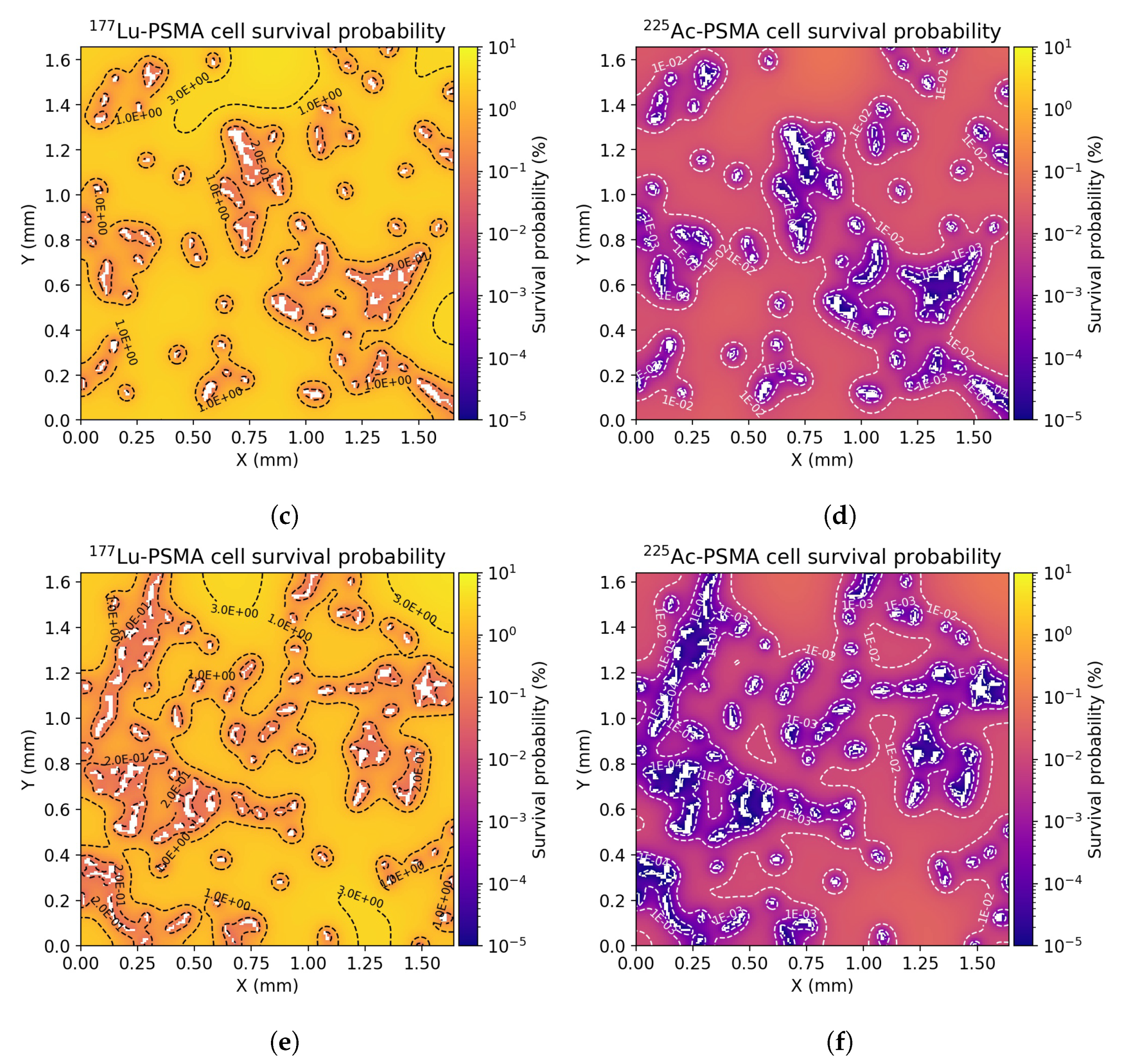
3.2. Dose Distribution in Tumor Microenvironment
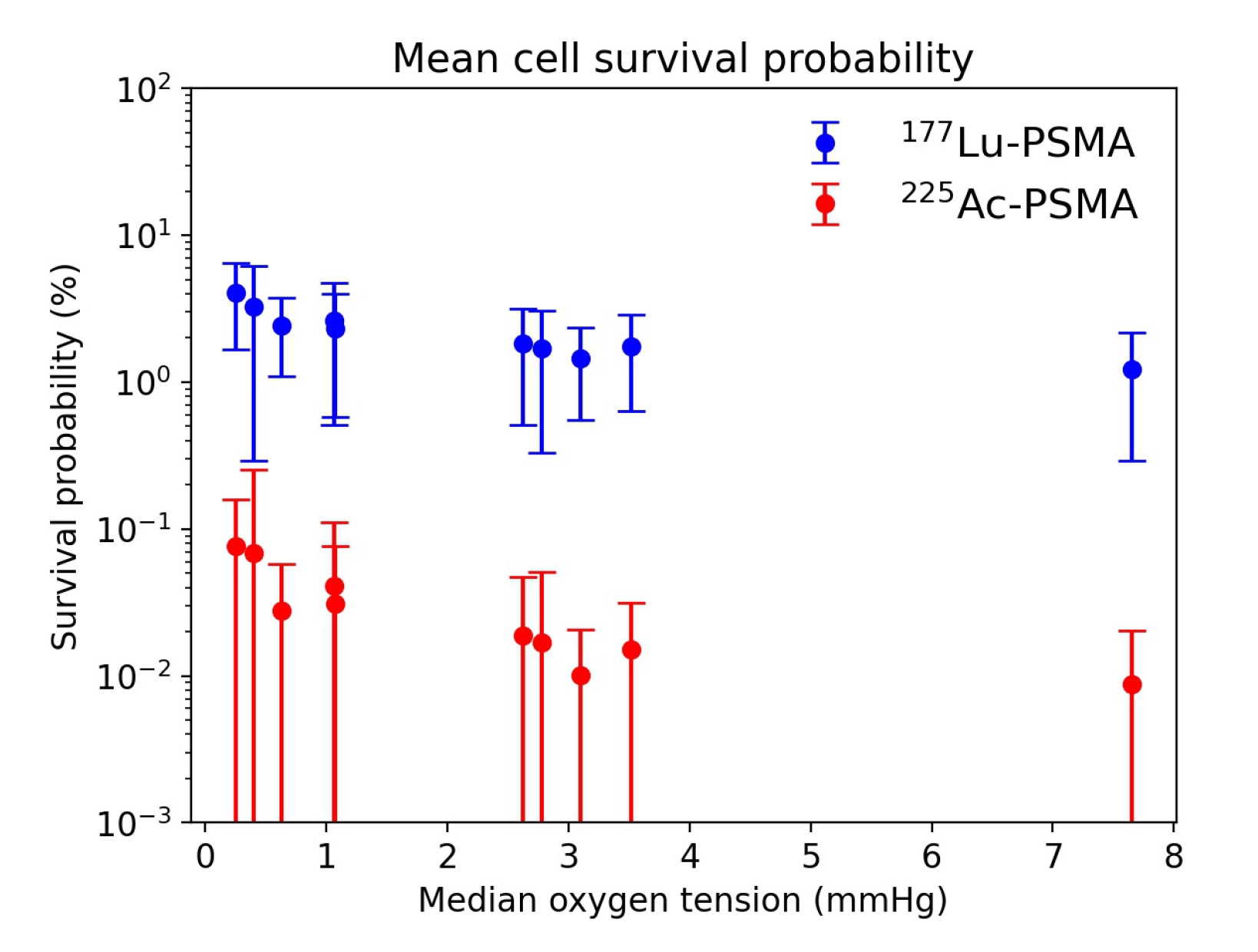
3.3. Radiobiological Efficacy Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michiels, C. Physiological and Pathological Responses to Hypoxia. Am. J. Pathol. 2004, 164, 1875–1882. [Google Scholar] [CrossRef] [Green Version]
- Vaupel, P. Hypoxia and Aggressive Tumor Phenotype: Implications for Therapy and Prognosis. Oncologist 2008, 13, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Wilson, W.; Hay, M. Targeting Hypoxia in Cancer Therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Moeller, B.; Richardson, R.; Dewhirst, M. Hypoxia and Radiotherapy: Opportunities for Improved Outcomes in Cancer Treatment. Cancer Metastasis Rev. 2007, 2, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Löbrich, M.; Kühne, M.; Wetzel, J.; Rothkamm, K. Joining of Correct and Incorrect DNA Double-Strand Break Ends in Normal Human and Ataxia Telangiectasia Fibroblasts. Genes Chromosom. Cancer 2000, 1, 59–68. [Google Scholar] [CrossRef]
- Chaudhary, P.; Marshall, T.; Perozziello, F.; Manti, L.; Currell, F.; Hanton, F.; McMahon, S.; Kavanagh, J.; Cirrone, G.; Romano, F.; et al. Relative Biological Effectiveness Variation Along Monoenergetic and Modulated Bragg Peaks of A 62-Mev Therapeutic Proton Beam: A Preclinical Assessment. Int. J. Radiat. Oncol. Biol. Phys. 2014, 1, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tommasino, F.; Durante, M. Proton Radiobiology. Cancers 2015, 1, 353–381. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, H.; Van De Gucht, M.; De Ridder, M. Hypoxic Radioresistance: Can ROS Be the Key to Overcome It? Cancers 2019, 1, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzl, T.; Wilkens, J. Modelling of the Oxygen Enhancement Ratio for Ion Beam Radiation Therapy. Phys. Med. Biol. 2011, 11, 3251–3268. [Google Scholar] [CrossRef]
- Helgstrand, J.; Røder, M.; Klemann, N.; Toft, B.; Lichtensztajn, D.; Brooks, J.; Brasso, K.; Vainer, B.; Iversen, P. Trends in Incidence and 5-Year Mortality in Men with Newly Diagnosed, Metastatic Prostate Cancer-A Population-Based Analysis of 2 National Cohorts. Cancer 2018, 14, 2931–2938. [Google Scholar] [CrossRef] [Green Version]
- Sweat, S.; Pacelli, A.; Murphy, G.; Bostwick, D. Prostate-Specific Membrane Antigen Expression Is Greatest in Prostate Adenocarcinoma and Lymph Node Metastases. Urology 1998, 4, 637–640. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.; Stefanova, M.; Bene Ova, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 8, 1170–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofman, M.; Violet, J.; Hicks, R.; Ferdinandus, J.; Thang, S.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.; et al. [177 Lu]-PSMA-617 Radionuclide Treatment in Patients with Metastatic Castration-Resistant Prostate Cancer (Lupsma Trial): A Single-Centre, Single-Arm, Phase 2 Study. Lancet Oncol. 2018, 6, 825–833. [Google Scholar] [CrossRef]
- Yadav, M.; Ballal, S.; Sahoo, R.; Dwivedi, S.; Bal, C. Radioligand Therapy with 177lu-PSMA for Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2019, 2, 275–285. [Google Scholar] [CrossRef]
- Tafreshi, N.; Doligalski, M.; Tichacek, C.; Pandya, D.; Budzevich, M.; El-Haddad, G.; Khushalani, N.; Moros, E.; McLaughlin, M.; Wadas, T.; et al. Development of Targeted Alpha Particle Therapy for Solid Tumors. Molecules 2019, 23, 4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochwil, C.; Giesel, F.; Heussel, C.; Kazdal, D.; Endris, V.; Nientiedt, C.; Bruchertseifer, F.; Kippenberger, M.; Rathke, H.; Leichsenring, J.; et al. Patients Resistant Against PSMA-Targeting a Radiation Therapy often Harbor Mutations in DNA Damage-Repair-Associated Genes. J. Nucl. Med. 2020, 5, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.; Sood, A.; Das, C.; Mittal, B. Evolving Role of 225Ac-PSMA Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer—A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis. 2021, 1–11. [Google Scholar] [CrossRef]
- Movsas, B.; Chapman, J.; Horwitz, E.; Pinover, W.; Greenberg, R.; Hanlon, A.; Iyer, R.; Hanks, G. Hypoxic Regions Exist in Human Prostate Carcinoma. Urology 1999, 1, 11–18. [Google Scholar] [CrossRef]
- Movsas, B.; Chapman, J.; Hanlon, A.; Horwitz, E.; Pinover, W.; Greenberg, R.; Stobbe, C.; Hanks, G. Hypoxia in Human Prostate Carcinoma. Am. J. Clin. Oncol. 2001, 5, 458–461. [Google Scholar] [CrossRef]
- Parker, C.; Milosevic, M.; Toi, A.; Sweet, J.; Panzarella, T.; Bristow, R.; Catton, C.; Catton, P.; Crook, J.; Gospodarowicz, M.; et al. Polarographic Electrode Study of Tumor Oxygenation in Clinically Localized Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 3, 750–757. [Google Scholar] [CrossRef]
- Stewart, G.; Ross, J.; McLaren, D.; Parker, C.; Habib, F.; Riddick, A. The Relevance of a Hypoxic Tumour Microenvironment in Prostate Cancer. BJU Int. 2010, 1, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Vaupel, P.; Ziegler, S.; Shi, K. Exploring the Quantitative Relationship between Metabolism and Enzymatic Phenotype by Physiological Modeling of Glucose Metabolism and Lactate Oxidation in Solid Tumors. Phys. Med. Biol. 2015, 6, 2547–2571. [Google Scholar] [CrossRef] [PubMed]
- Secomb, T.; Hsu, R.; Dewhirst, M.; Klitzman, B.; Gross, J. Analysis of Oxygen Transport to Tumor Tissue by Microvascular Networks. Int. J. Radiat. Oncol. Biol. Phys. 1993, 3, 481–489. [Google Scholar] [CrossRef]
- Secomb, T.; Hsu, R.; Ong, E.; Gross, J.; Dewhirst, M. Analysis of the Effects of Oxygen Supply and Demand on Hypoxic Fraction in Tumors. Acta Oncol. 1995, 3, 313–316. [Google Scholar] [CrossRef]
- Daşu, A.; Toma-Daşu, I.; Karlsson, M. Theoretical Simulation of Tumour Oxygenation and Results from Acute and Chronic Hypoxia. Phys. Med. Biol. 2003, 17, 2829–2842. [Google Scholar]
- Kelly, C.; Brady, M. A Model to Simulate Tumour Oxygenation and Dynamic [18F]-Fmiso PET Data. Phys. Med. Biol. 2006, 22, 5859–5873. [Google Scholar] [CrossRef]
- Mönnich, D.; Troost, E.; Kaanders, J.; Oyen, W.; Alber, M.; Thorwarth, D. Modelling and Simulation of [18F]Fluoromisonidazole Dynamics Based on Histology-Derived Microvessel Maps. Phys. Med. Biol. 2011, 7, 2045–2057. [Google Scholar] [CrossRef]
- Powathil, G.; Kohandel, M.; Milosevic, M.; Sivaloganathan, S. Modeling the spatial distribution of chronic tumor hypoxia: Implications for experimental and clinical studies. Comput. Math. Methods Med. 2012, 2012, 410602. [Google Scholar] [CrossRef]
- Shi, K.; Bayer, C.; Gaertner, F.; Astner, S.; Wilkens, J.; Nüsslin, F.; Vaupel, P.; Ziegler, S. Matching the Reaction-Diffusion Simulation to Dynamic [18F]FMISO PET Measurements in Tumors: Extension to a Flow-Limited Oxygen-Dependent Model. Physiol. Meas. 2017, 2, 188–204. [Google Scholar] [CrossRef]
- Horoszewicz, J.S.; Leong, S.S.; Chu, T.M.; Wajsman, Z.L.; Friedman, M.; Papsidero, L.; Kim, U.; Chai, L.S.; Kakati, S.; Arya, S.K.; et al. The LNCaP cell line: A new model for studies on human prostatic carcinoma. Prog. Clin. Biol. Res. 1980, 37, 115–132. [Google Scholar]
- Russell, P.; Kingsley, E. Human Prostate Cancer Cell Lines. Methods Mol. Med. 2003, 81, 21–39. [Google Scholar] [PubMed]
- Abramoff, M. ImageJ as an Image Processing Tool and Library. Microsc. Microanal. 2007, 13, 1672–1673. [Google Scholar] [CrossRef]
- Łuczyńska, E.; Heinze-Paluchowska, S.; Blecharz, P.; Jereczek-Fossa, B.; Petralia, G.; Bellomi, M.; Stelmach, A. Correlation between CT Perfusion and Clinico-Pathological Features in Prostate Cancer: A Prospective Study. Med. Sci. Monit. 2015, 21, 153–162. [Google Scholar] [PubMed] [Green Version]
- Jain, R. Determinants of tumor blood flow: A review. Cancer Res. 1988, 10, 2641–2658. [Google Scholar]
- Liu, H.; Rajasekaran, A.; Moy, P.; Xia, Y.; Kim, S.; Navarro, V.; Rahmati, R.; Bander, N. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998, 18, 4055–4060. [Google Scholar]
- Swabb, E.; Wei, J.; Gullino, P. Diffusion and convection in normal and neoplastic tissues. Cancer Res. 1974, 10, 2814–2822. [Google Scholar]
- Jain, R. Transport of Molecules across Tumor Vasculature. Cancer Metastasis Rev. 1987, 4, 559–593. [Google Scholar] [CrossRef]
- Begum, N.; Glatting, G.; Wester, H.; Eiber, M.; Beer, A.; Kletting, P. The Effect of Ligand Amount, Affinity and Internalization on PSMA-Targeted Imaging and Therapy: A Simulation Study Using A PBPK Model. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Hecht, F. New Development in Freefem++. J. Numer. Math. 2012, 20, 251–266. [Google Scholar] [CrossRef]
- McKeown, S. Defining Normoxia, Physoxia and Hypoxia in Tumours—Implications for Treatment Response. Br. J. Radiol. 2014, 1035, 20130676. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.; Lu, C. PBPK Modeling and Simulation in Drug Research and Development. Acta Pharm. Sin. B 2016, 5, 430–440. [Google Scholar] [CrossRef] [Green Version]
- Lyons, M.; Reisfeld, B.; Yang, R.; Lenaerts, A. A Physiologically Based Pharmacokinetic Model of Rifampin in Mice. Antimicrob. Agents Chemother. 2013, 4, 1763–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, Y.; Deng, J.; Murry, D.; An, G. A Whole-Body Physiologically Based Pharmacokinetic Model Of Gefitinib In Mice And Scale-Up to Humans. AAPS J. 2016, 1, 228–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Pan, C.; Wood, R.; Yeh, C.; Yeh, S.; Sha, K.; Krolewski, J.; Nastiuk, K. Quantitative Volumetric Imaging of Normal, Neoplastic and Hyperplastic Mouse Prostate Using Ultrasound. BMC Urol. 2015, 1, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, S.; Ricklis, R.; Williams, S.; Denmeade, S. Comparative Study of PSMA Expression in the Prostate of Mouse, Dog, Monkey, and Human. Prostate 2006, 9, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Warner, B.; Basuli, F.; Williams, M.; Wong, K.; Ton, A.; Chiorini, J.; Choyke, P.; Lin, F.; Jagoda, E. Identifying an appropriate animal model to examine preservation of salivary function with PSMA targeted radiotherapies. J. Nucl. Med. 2018, 59 (Suppl. 1), 1255. [Google Scholar]
- Bolch, W.; Bouchet, L.; Robertson, J.; Wessels, B.; Siegel, J.; Howell, R.; Erdi, A.; Aydogan, B.; Costes, S.; Watson, E. MIRD Pamphlet No. 17: The Dosimetry of Nonuniform Activity Distributions—Radionuclide S Values at the Voxel Level. J. Nucl. Med. 1999, 1, 11S–36S. [Google Scholar]
- Vaziri, B.; Wu, H.; Dhawan, A.; Du, P.; Howell, R. MIRD Pamphlet No. 25: Mirdcell V2.0 Software Tool for Dosimetric Analysis of Biologic Response of Multicellular Populations. J. Nucl. Med. 2014, 9, 1557–1564. [Google Scholar] [CrossRef] [Green Version]
- McMahon, S. The Linear Quadratic Model: Usage, Interpretation and Challenges. Phys. Med. Biol. 2018, 1, 01TR01. [Google Scholar] [CrossRef]
- Sgouros, G.; Roeske, J.; McDevitt, M.; Palm, S.; Allen, B.; Fisher, D.; Brill, A.; Song, H.; Howell, R.; Akabani, G. MIRD Pamphlet No. 22 (Abridged): Radiobiology and Dosimetry of α-Particle Emitters for Targeted Radionuclide Therapy. J. Nucl. Med. 2010, 2, 311–328. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, X.; Mayr, N. Dose Escalation To Combat Hypoxia In Prostate Cancer: A Radiobiological Study On Clinical Data. Br. J. Radiol. 2006, 947, 905–911. [Google Scholar] [CrossRef]
- Barendsen, G.; Koot, C.; van Kersen, G.; Bewley, D.; Field, S.; Parnell, C. The Effect of Oxygen on Impairment of the Proliferative Capacity of Human Cells in Culture by Ionizing Radiations of Different LET. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1966, 4, 317–327. [Google Scholar] [CrossRef]
- Dewhirst, M.; Cao, Y.; Moeller, B. Cycling Hypoxia And Free Radicals Regulate Angiogenesis And Radiotherapy Response. Nat. Rev. Cancer 2008, 6, 425–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaplin, D.J.; Olive, P.L.; Durand, R.E. Intermittent blood flow in a murine tumor: Radiobiological effects. Cancer Res. 1987, 47, 597–601. [Google Scholar]
- Bader, S.B.; Dewhirst, M.W.; Hammond, E.M. Cyclic Hypoxia: An Update on Its Characteristics, Methods to Measure It and Biological Implications in Cancer. Cancers 2021, 13, 23. [Google Scholar] [CrossRef]
- Maftei, C.A.; Bayer, C.; Shi, K.; Astner, S.T.; Vaupel, P. Quantitative assessment of hypoxia subtypes in microcirculatory supply units of malignant tumors using (immuno-) fluorescence techniques. Strahlenther. Und Onkol. 2011, 187, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.; Gibaldi, M.; Jusko, W. Multicompartment Pharmacokinetic Models And Pharmacologic Effects. J. Pharm. Sci. 1969, 4, 422–424. [Google Scholar] [CrossRef]
- Gerlowski, L.; Jain, R. Physiologically Based Pharmacokinetic Modeling: Principles and Applications. J. Pharm. Sci. 1983, 10, 1103–1127. [Google Scholar] [CrossRef]
- Liu, D.; Chalkidou, A.; Landau, D.; Marsden, P.; Fenwick, J. Interstitial Diffusion and The Relationship between Compartment Modelling and Multi-Scale Spatial-Temporal Modelling of 18f-FLT Tumour Uptake Dynamics. Phys. Med. Biol. 2014, 17, 5175–5202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potiron, V.; Clément-Colmou, K.; Jouglar, E.; Pietri, M.; Chiavassa, S.; Delpon, G.; Paris, F.; Supiot, S. Tumor Vasculature Remodeling by Radiation Therapy Increases Doxorubicin Distribution and Efficacy. Cancer Lett. 2019, 457, 1–9. [Google Scholar] [CrossRef]
- Kohandel, M.; Kardar, M.; Milosevic, M.; Sivaloganathan, S. Dynamics of Tumor Growth and Combination of Anti-Angiogenic and Cytotoxic Therapies. Phys. Med. Biol. 2007, 13, 3665–3677. [Google Scholar] [CrossRef]
- Vujaskovic, Z.; Anscher, M.; Feng, Q.; Rabbani, Z.; Amin, K.; Samulski, T.; Dewhirst, M.; Haroon, Z. Radiation-Induced Hypoxia May Perpetuate Late Normal Tissue Injury. Int. J. Radiat. Oncol. Biol. Phys. 2001, 4, 851–855. [Google Scholar] [CrossRef]
- Moeller, B.; Dewhirst, M. HIF-1 and Tumour Radiosensitivity. Br. J. Cancer 2006, 1, 1–5. [Google Scholar] [CrossRef]
- Kabakov, A.; Yakimova, A. Hypoxia-Induced Cancer Cell Responses Driving Radioresistance of Hypoxic Tumors: Approaches to Targeting and Radiosensitizing. Cancers 2021, 5, 1102. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, D.; Martin, L.; Atzberger, A.; Lynch, T.; Hollywood, D.; Marignol, L. Exposure to Hypoxia Following Irradiation Increases Radioresistance in Prostate Cancer Cells. Urol. Oncol. Semin. Orig. Investig. 2013, 7, 1106–1116. [Google Scholar] [CrossRef]
- Nesbitt, H.; Byrne, N.; Williams, S.; Ming, L.; Worthington, J.; Errington, R.; Patterson, L.; Smith, P.; McKeown, S.; McKenna, D. Targeting Hypoxic Prostate Tumors Using the Novel Hypoxia-Activated Prodrug OCT1002 Inhibits Expression of Genes Associated with Malignant Progression. Clin. Cancer Res. 2016, 7, 1797–1808. [Google Scholar] [CrossRef] [Green Version]
- Solon, E. Autoradiography Techniques and Quantification of Drug Distribution. Cell Tissue Res. 2015, 1, 87–107. [Google Scholar] [CrossRef]
- Tönnesmann, R.; Meyer, P.; Eder, M.; Baranski, A. [177Lu]Lu-PSMA-617 Salivary Gland Uptake Characterized by Quantitative in vitro Autoradiography. Pharmaceuticals 2019, 1, 18. [Google Scholar] [CrossRef] [Green Version]


| Symbol | Parameter | Value | Reference |
|---|---|---|---|
| Vessel wall permeability | cm s | [37] | |
| Diffusivity | cm s | [36] | |
| Molecule/Carrier movement coefficient | 1 | [36] | |
| Receptor density | nmol ml | fitted | |
| Association rate | ml nmol s | [38] | |
| Dissociation rate | s | [38] | |
| Internalization rate | s | [38] | |
| Release rate | s | [38] | |
| Fractional interstitial volume | 39% | [38] | |
| Fractional cellular volume | 61% | [38] | |
| Lu decay constant | s | ||
| Ac decay constant | s |
| Symbol | Parameter | Value | Reference |
|---|---|---|---|
| Vessel wall permeability to O | cm s | [27,29] | |
| Oxygen diffusivity | cm s | [27,29] | |
| Intraerythrocyte pO in tumors | 40 mmHg | [27,29] | |
| Maximum O consumption rate | 15 mmHg s | [27,29] | |
| Michaelis-Menten coefficient of oxygen consumption | 2.0 mmHg | [27,29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birindelli, G.; Drobnjakovic, M.; Morath, V.; Steiger, K.; D’Alessandria, C.; Gourni, E.; Afshar-Oromieh, A.; Weber, W.; Rominger, A.; Eiber, M.; et al. Is Hypoxia a Factor Influencing PSMA-Directed Radioligand Therapy?—An In Silico Study on the Role of Chronic Hypoxia in Prostate Cancer. Cancers 2021, 13, 3429. https://doi.org/10.3390/cancers13143429
Birindelli G, Drobnjakovic M, Morath V, Steiger K, D’Alessandria C, Gourni E, Afshar-Oromieh A, Weber W, Rominger A, Eiber M, et al. Is Hypoxia a Factor Influencing PSMA-Directed Radioligand Therapy?—An In Silico Study on the Role of Chronic Hypoxia in Prostate Cancer. Cancers. 2021; 13(14):3429. https://doi.org/10.3390/cancers13143429
Chicago/Turabian StyleBirindelli, Gabriele, Milos Drobnjakovic, Volker Morath, Katja Steiger, Calogero D’Alessandria, Eleni Gourni, Ali Afshar-Oromieh, Wolfgang Weber, Axel Rominger, Matthias Eiber, and et al. 2021. "Is Hypoxia a Factor Influencing PSMA-Directed Radioligand Therapy?—An In Silico Study on the Role of Chronic Hypoxia in Prostate Cancer" Cancers 13, no. 14: 3429. https://doi.org/10.3390/cancers13143429
APA StyleBirindelli, G., Drobnjakovic, M., Morath, V., Steiger, K., D’Alessandria, C., Gourni, E., Afshar-Oromieh, A., Weber, W., Rominger, A., Eiber, M., & Shi, K. (2021). Is Hypoxia a Factor Influencing PSMA-Directed Radioligand Therapy?—An In Silico Study on the Role of Chronic Hypoxia in Prostate Cancer. Cancers, 13(14), 3429. https://doi.org/10.3390/cancers13143429








