Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review
Abstract
:Simple Summary
Abstract
1. Introduction
- PDT biological effects may be at least partially localised to the tumour, resulting in a higher concentration of the PS within the tumour in comparison to healthy cells.
- PDT uses non-ionizing radiation (in most cases) and its cytotoxic mechanisms produce limited damage to DNA and connective tissue structures (i.e., collagen), which after the treatment act as a scaffold enabling, potentially, the healing of the treated volume [2].
- Considering the previous point, this treatment could be used as many times as required by clinicians, something that is not possible with the current established treatments (surgery, chemotherapy, and radiotherapy). PDT has no “memory effect” as radiotherapy.
- There is also a rapidly increasing body of evidence that the damage and unique mechanism of PDT treatment on tumours and their microenvironments could inhibit drug resistance pathways and re-sensitize resistant cells to standard therapies [3].
- Superficial PDT: involves skin treatments with low light penetration depth (typically <2 mm). It is also usually referred to as external PDT.
- Interstitial PDT (I-PDT): can treat tumours beyond 1 cm assisted by the use of needles, catheters, and optical fibres, but using conventional light sources—with its light penetration limits—similarly as superficial PDT.
- Deep PDT: includes a wide variety of technologies aiming at deeper penetration beyond what is achieved by conventional light sources. This section includes NIR radiation of upconversion materials, advanced PSs excited with novel nonlinear optical techniques, ionising radiation, self-illuminated compounds, and emerging implants.
2. Light Technology for PDT
2.1. Background
2.1.1. Light Absorption in Biological Tissues
2.1.2. PDT Mechanism of Action upon Absorption
2.1.3. Dose and Beam Parameters
2.2. Conventional PDT: Superficial and Interstitial
2.2.1. Light Source Types
2.2.2. Penetration Depth and Light Source Characteristics
2.2.3. Pulsed, Continuous, and Other Light Waveforms for PDT
2.2.4. Delivery Devices
2.3. Deep PDT
2.3.1. NIR Radiation
2.3.2. Ionising Radiation
2.3.3. Self-Illuminated Systems
2.3.4. Implants
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 6 April 2021).
- Barr, H.; Tralau, C.J.; Boulos, P.B.; MacRobert, A.J.; Tilly, R.; Bown, S.G. The contrasting mechanisms of colonic collagen damage between photodynamic therapy and thermal injury. Photochem. Photobiol. 1987, 46, 795–800. [Google Scholar] [CrossRef]
- Aniogo, E.C.; Plackal Adimuriyil George, B.; Abrahamse, H. The role of photodynamic therapy on multidrug resistant breast cancer. Cancer Cell Int. 2019, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Kleinovink, J.W.; Van Driel, P.B.; Snoeks, T.J.; Prokopi, N.; Fransen, M.F.; Cruz, L.J.; Mezzanotte, L.; Chan, A.; Löwik, C.W.; Ossendorp, F. Combination of photodynamic therapy and specific immunotherapy efficiently eradicates established tumors. Clin. Cancer Res. 2016, 22, 1459–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamblin, M.R.; Huang, Y. Imaging in Photodynamic Therapy; Taylor & Francis: London, UK, 2017. [Google Scholar]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogue, B.W.; Elliott, J.T.; Kanick, S.C.; Davis, S.C.; Samkoe, K.S.; Maytin, E.V.; Pereira, S.P.; Hasan, T. Revisiting photodynamic therapy dosimetry: Reductionist & surrogate approaches to facilitate clinical success. Phys. Med. Biol. 2016, 61, R57–R89. [Google Scholar] [PubMed] [Green Version]
- Nowis, D.; Makowski, M.; Stokłosa, T.; Legat, M.; Issat, T.; Gołąb, J. Direct tumor damage mechanisms of photodynamic therapy. Acta Biochim. Pol. 2005, 52, 339–352. [Google Scholar] [CrossRef] [Green Version]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two—Cellular signaling, cell metabolism and modes of cell death. Photodiagn. Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell death pathways in photodynamic therapy of cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.O.; Ha, K.S. New Insights into the Mechanisms for Photodynamic Therapy-Induced Cancer Cell Death. In International Review of Cell and Molecular Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 295, pp. 139–174. [Google Scholar]
- Kushibiki, T.; Hirasawa, T.; Okawa, S.; Ishihara, M. Responses of cancer cells induced by photodynamic therapy. J. Healthc. Eng. 2013, 4, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Photodynamic therapy (PDT): PDT mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Alghamdi, M.S.; Alotaibi, A.S.; Alzhrani, R.; Alwuthaynani, F.; Althobaiti, Y.S.; Almalki, A.H.; Sau, S.; Iyer, A.K. Progress in clinical trials of photodynamic therapy for solid tumors and the role of nanomedicine. Cancers 2020, 12, 2793. [Google Scholar] [CrossRef] [PubMed]
- Brancaleon, L.; Moseley, H. Laser and non-laser light sources for photodynamic therapy. Lasers Med. Sci. 2002, 17, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Mang, T.S. Lasers and light sources for PDT: Past, present and future. Photodiagn. Photodyn. Ther. 2004, 1, 43–48. [Google Scholar] [CrossRef]
- Finlay, J.C.; Darafsheh, A. Light sources, drugs, and dosimetry. In Biomedical Optics in Otorhinolaryngology: Head and Neck Surgery; Wong, B.J.-F., Ilgner, J., Eds.; Springer: New York, NY, USA, 2016; pp. 311–336. ISBN 9781493917587. [Google Scholar]
- Mallidi, S.; Anbil, S.; Bulin, A.L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the barriers of light penetration: Strategies, perspectives and possibilities for photodynamic therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuchin, V.V. Optical Properties of Tissues with Strong (Multiple) Scattering. In Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnostics; SPIE: Bellingham, DC, USA; Washington, DC, USA, 2015; p. 4. [Google Scholar]
- Jacques, S.L.; Prahl, S.A. Available online: https://omlc.org/spectra/ (accessed on 6 June 2021).
- van Veen, R.L.P.; Sterenborg, H.J.C.M.; Pifferi, A.; Torricelli, A.; Cubeddu, R. Determination of VIS-NIR absorption coefficients of mammalian fat, with time- and spatially resolved diffuse reflectance and transmission spectroscopy. In Proceedings of the Biomedical Topical Meeting (2004), Miami Beach, FL, USA, 14–17 April 2004; p. SF4. [Google Scholar]
- Tsai, C.L.; Chen, J.C.; Wang, W.J. Near-infrared absorption property of biological soft tissue constituents. J. Med. Biol. Eng. 2001, 21, 7–14. [Google Scholar]
- Hale, G.M.; Querry, M.R. Optical Constants of Water in the 200-nm to 200-μm Wavelength Region. Appl. Opt. 1973, 12, 555. [Google Scholar] [CrossRef]
- Suzaki, H.; Kobayashi, N.; Nagaoka, T.; Iwasaki, K.; Umezu, M.; Takeda, S.; Togawa, T. Noninvasive measurement of total hemoglobin and hemoglobin derivatives using multiwavelength pulse spectrophotometry—In vitro study with a mock circulatory system. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 799–802. [Google Scholar]
- Allison, R.R. Photodynamic therapy: Oncologic horizons. Future Oncol. 2014, 10, 123–142. [Google Scholar] [CrossRef]
- Starkey, J.R.; Rebane, A.K.; Drobizhev, M.A.; Meng, F.; Gong, A.; Elliott, A.; McInnerney, K.; Spangler, C.W. New two-photon activated photodynamic therapy sensitizers induce xenograft tumor regressions after near-IR laser treatment through the body of the host mouse. Clin. Cancer Res. 2008, 14, 6564–6573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welsher, K.; Sherlock, S.P.; Dai, H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc. Natl. Acad. Sci. USA 2011, 108, 8943–8948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications, Second ed.; Wiley-VCH: Weinheim, Germany, 2012; ISBN 9783527328376. [Google Scholar]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Du, H.; Lindsey, J.S. PhotochemCAD 3: Diverse Modules for Photophysical Calculations with Multiple Spectral Databases. Photochem. Photobiol. 2018, 94, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Lindsey, J.S. Database of Absorption and Fluorescence Spectra of >300 Common Compounds for use in PhotochemCAD. Photochem. Photobiol. 2018, 94, 290–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovell, J.F.; Liu, T.W.B.; Chen, J.; Zheng, G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of Third Generation Photosensitizers Used in Anticancer Photodynamic Therapy: Review. Photodiagn. Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef]
- Zhu, T.C.; Finlay, J.C.; Wilson, B. TH-A-T-6C-01: Photodynamic Therapy: Fundamentals and Dosimetry. Med. Phys. 2005, 32, 2150. [Google Scholar] [CrossRef]
- Jenkins, P.A.; Carroll, J.D. How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed. Laser Surg. 2011, 29, 785–787. [Google Scholar] [CrossRef]
- Keiser, G. Light-Tissue Interactions. In Biophotonics; Springer: Singapore, 2016; pp. 147–196. [Google Scholar]
- Katarina, S.; Bendsoe, N.; Axelsson, J.; Andersson-Engels, S.; Svanberg, S. Photodynamic therapy: Superficial and interstitial illumination. J. Biomed. Opt. 2010, 15, 041502. [Google Scholar]
- Peng, Q.; Juzeniene, A.; Chen, J.; Svaasand, L.O.; Warloe, T.; Giercksky, K.E.; Moan, J. Lasers in medicine. Rep. Prog. Phys. 2008, 71, 56701. [Google Scholar] [CrossRef]
- Szeimies, R.-M.; Radny, P.; Sebastian, M.; Borrosch, F.; Dirschka, T.; Krähn-Senftleben, G.; Reich, K.; Pabst, G.; Voss, D.; Foguet, M.; et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: Results of a prospective, randomized, double-blind, placebo-controlled phase III study. Br. J. Dermatol. 2010, 163, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A. Methyl Aminolevulinate: Actinic Keratoses and Bowen’s Disease. Dermatol. Clin. 2007, 25, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Sibata, C.H. Photodynamic therapy: Mechanism of action and role in the treatment of skin disease. G. Ital. Dermatol. Venereol. Organo Uff. Soc. Ital. Dermatol. Sifilogr. 2010, 145, 491–507. [Google Scholar]
- Morton, C.; Horn, M.; Leman, J.; Tack, B.; Bedane, C.; Tjioe, M.; Ibbotson, S.; Khemis, A.; Wolf, P. Comparison of Topical Methyl Aminolevulinate Photodynamic Therapy With Cryotherapy or Fluorouracil for Treatment of Squamous Cell Carcinoma In Situ. Arch. Dermatol. 2006, 142, 729–735. [Google Scholar] [CrossRef]
- Basset-Seguin, N.; Ibbotson, S.H.; Emtestam, L.; Tarstedt, M.; Morton, C.; Maroti, M.; Calzavara-Pinton, P.; Varma, S.; Roelandts, R.; Wolf, P. Topical methyl aminolaevulinate photodynamic therapy versus cryotherapy for superficial basal cell carcinoma: A 5 year randomized trial. Eur. J. Dermatol. 2008, 18, 547–553. [Google Scholar]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef] [PubMed]
- Shafirstein, G.; Bellnier, D.; Oakley, E.; Hamilton, S.; Potasek, M.; Beeson, K.; Parilov, E. Interstitial photodynamic therapy—A focused review. Cancers 2017, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Swartling, J.; Axelsson, J.; Ahlgren, G.; Kälkner, K.M.; Nilsson, S.; Svanberg, S.; Svanberg, K.; Andersson-Engels, S. System for interstitial photodynamic therapy with online dosimetry: First clinical experiences of prostate cancer. J. Biomed. Opt. 2010, 15, 058003. [Google Scholar] [CrossRef]
- Shikunova, I.A.; Dolganova, I.N.; Dubyanskaya, E.N.; Mukhina, E.E.; Zaytsev, K.I.; Kurlov, V.N. Sapphire capillary interstitial irradiators for laser medicine. In Proceedings of the Saratov Fall Meeting 2017: Optical Technologies in Biophysics and Medicine XIX, Saratov, Russian, 26 April 2018; Volume 10716. [Google Scholar]
- Schmidt, M.H.; Bajic, D.M.; Reichert, K.W.; Martin, T.S.; Meyer, G.A.; Whelan, H.T. Light-emitting Diodes as a Light Source for Intraoperative Photodynamic Therapy. Neurosurgery 1996, 38, 552–557. [Google Scholar] [PubMed] [Green Version]
- Vu, H.; Kieu, N.M.; Gam, D.T.; Shin, S.; Tien, T.Q.; Vu, N.H. Design and Evaluation of Uniform LED Illumination Based on Double Linear Fresnel Lenses. Appl. Sci. 2020, 10, 3257. [Google Scholar] [CrossRef]
- Meulemans, J.; Delaere, P.; Vander Poorten, V. Photodynamic therapy in head and neck cancer: Indications, outcomes, and future prospects. Curr. Opin. Otolaryngol. Head Neck Surg. 2019, 27, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Bown, S.G. Photodynamic therapy for cancer of the pancreas—The story so far. Photonics Lasers Med. 2016, 5, 91–100. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Li, B. Photodynamic therapy for prostate cancer: A systematic review and meta-analysis. Prostate Int. 2019, 7, 83–90. [Google Scholar] [CrossRef]
- Wang, K.; Yu, B.; Pathak, J.L. An update in clinical utilization of photodynamic therapy for lung cancer. J. Cancer 2021, 12, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Quirk, B.J.; Brandal, G.; Donlon, S.; Vera, J.C.; Mang, T.S.; Foy, A.B.; Lew, S.M.; Girotti, A.W.; Jogal, S.; LaViolette, P.S.; et al. Photodynamic therapy (PDT) for malignant brain tumors—Where do we stand? Photodiagn. Photodyn. Ther. 2015, 12, 530–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostańska, E.; Aebisher, D.; Bartusik-Aebisher, D. The Potential of Photodynamic Therapy in Current Breast Cancer Treatment Methodologies; Elsevier: Roma, Italy, 2021; Volume 137. [Google Scholar]
- Banerjee, S.M.; El-Sheikh, S.; Malhotra, A.; Mosse, C.A.; Parker, S.; Williams, N.R.; MacRobert, A.J.; Hamoudi, R.; Bown, S.G.; Keshtgar, M.R.S. Photodynamic Therapy in Primary Breast Cancer. J. Clin. Med. 2020, 9, 483. [Google Scholar] [CrossRef] [Green Version]
- Trevisan, E.; Menegazzi, R.; Zabucchi, G.; Troian, B.; Prato, S.; Vita, F.; Rapozzi, V.; Grandolfo, M.; Borelli, V. Effect of methylene blue photodynamic therapy on human neutrophil functional responses. J. Photochem. Photobiol. B Biol. 2019, 199, 111605. [Google Scholar] [CrossRef] [PubMed]
- Kercher, E.M.; Zhang, K.; Waguespack, M.; Lang, R.T.; Olmos, A.; Spring, B.Q. High-power light-emitting diode array design and assembly for practical photodynamic therapy research. J. Biomed. Opt. 2020, 25, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hempstead, J.; Jones, D.P.; Ziouche, A.; Cramer, G.M.; Rizvi, I.; Arnason, S.; Hasan, T.; Celli, J.P. Low-cost photodynamic therapy devices for global health settings: Characterization of battery-powered LED performance and smartphone imaging in 3D tumor models. Sci. Rep. 2015, 5, 10093. [Google Scholar] [CrossRef] [Green Version]
- Daly, S.R.; Zheng, F.; Krouse, M.; Guo, Z.; Mahoney, P.; McIlroy, B.W. Novel LED array used for photodynamic therapy (PDT). Light Emit. Diodes Res. Manuf. Appl. VII 2003, 4996, 229. [Google Scholar]
- Kamanlı, A.F.; Yıldız, M.Z.; Özyol, E.; Deveci Ozkan, A.; Sozen Kucukkara, E.; Guney Eskiler, G. Investigation of LED-based photodynamic therapy efficiency on breast cancer cells. Lasers Med. Sci. 2020. [Google Scholar] [CrossRef]
- Duchi, S.; Ramos-Romero, S.; Dozza, B.; Guerra-Rebollo, M.; Cattini, L.; Ballestri, M.; Dambruoso, P.; Guerrini, A.; Sotgiu, G.; Varchi, G.; et al. Development of near-infrared photoactivable phthalocyanine-loaded nanoparticles to kill tumor cells: An improved tool for photodynamic therapy of solid cancers. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Tunér, J.; Jenkins, P. Complications in comparing lasers and LED. Comment on Esper MA, Nicolau RA, Arisawa EA (2011) the effect of two phototherapy protocols on pain control in orthodontic procedure—A preliminary clinical study. Lasers Med. Sci. 2012, 27, 1257–1258. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, K.W.; Young, S.W.; Qing, F.; Miles, D.R.; Thiemann, P.A. Light Emitting Diode versus Laser Irradiation Phototherapy with Lutetium Texaphyrin (PCI-0123). In Proceedings of the Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy VI, San Jose, CA, USA, 8 May 1997; Dougherty, T.J., Ed.; SPIE: Washington, DC, USA, 1997; Volume 2972, pp. 46–53. [Google Scholar]
- Yu, C.H.; Lin, H.P.; Chen, H.M.; Yang, H.; Wang, Y.P.; Chiang, C.P. Comparison of clinical outcomes of oral erythroleukoplakia treated with photodynamic therapy using either light-emitting diode or laser light. Lasers Surg. Med. 2009, 41, 628–633. [Google Scholar] [CrossRef] [PubMed]
- de Jode, M.L.; McGilligan, J.A.; Dilkes, M.G.; Cameron, I.; Hart, P.B.; Grahn, M.F. A comparison of novel light sources for photodynamic therapy. In Lasers Medical Science; Springer: London, UK, 1997; Volume 12, pp. 260–268. [Google Scholar]
- Jacques, S.L. How tissue optics affect dosimetry of photodynamic therapy. J. Biomed. Opt. 2010, 15, 051608. [Google Scholar] [CrossRef] [Green Version]
- Dimofte, A.; Finlay, J.C.; Zhu, T.C. A method for determination of the absorption and scattering properties interstitially in turbid media. Phys. Med. Biol. 2005, 50, 2291–2311. [Google Scholar] [CrossRef] [PubMed]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Bargo, P.R.; Prahl, S.A.; Goodell, T.T.; Sleven, R.A.; Koval, G.; Blair, G.; Jacques, S.L. In vivo determination of optical properties of normal and tumor tissue with white light reflectance and an empirical light transport model during endoscopy. J. Biomed. Opt. 2005, 10, 034018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef] [PubMed]
- Hode, T.; Jenkins, P.; Jordison, S.; Hode, L. To what extent is coherence lost in tissue? In Proceedings of the Mechanisms for Low-Light Therapy VI, San Francisco, CA, USA, 10 February 2011; Hamblin, M.R., Waynant, R.W., Anders, J., Eds.; SPIE: Washington, DC, USA, 2011; Volume 7887, p. 788703. [Google Scholar]
- Fixler, D.; Duadi, H.; Ankri, R.; Zalevsky, Z. Determination of coherence length in biological tissues. Lasers Surg. Med. 2011, 43, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Hode, T.; Duncan, D.; Kirkpatrick, S.; Jenkins, P.; Hode, L. The importance of coherence in phototherapy. In Proceedings of the Mechanisms for Low-Light Therapy IV, San Francisco, CA, USA, 10 February 2011; Hamblin, M.R., Waynant, R.W., Anders, J., Eds.; SPIE: Washington, DC, USA, 2009; Volume 7165, p. 716507. [Google Scholar]
- Rubinov, A.N. Physical grounds for biological effect of laser radiation. J. Phys. D Appl. Phys. 2003, 36, 2317–2330. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Factors Affecting Photodynamic Therapy and Anti-Tumor Immune Response. Anticancer. Agents Med. Chem. 2020, 21, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Almeida, D.R.Q.; Terra, L.F.; Wailemann, R.A.M.; Gomes, V.M.; Arini, G.S.; Ravagnani, F.G.; Baptista, M.S.; Labriola, L. Fluence Rate Determines PDT Efficiency in Breast Cancer Cells Displaying Different GSH Levels. Photochem. Photobiol. 2020, 96, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.J.; van Gemert, M.J.C.; Star, W.M.; Wilson, B.C. Definitions and Overview of Tissue Optics. In Optical-Thermal Response of Laser-Irradiated Tissue; Springer: Boston, MA, USA, 1995; pp. 15–46. [Google Scholar]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Yassine, A.A.; Lilge, L.; Betz, V. Optimizing interstitial photodynamic therapy with custom cylindrical diffusers. J. Biophotonics 2019, 12, e201800153. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Zhang, L.-P.; Wu, F.; Zhao, Y. Photosensitizers for Two-Photon Excited Photodynamic Therapy. Adv. Funct. Mater. 2017, 27, 1704079. [Google Scholar] [CrossRef]
- de Bruijn, H.S.; Kruijt, B.; van der Ploeg-van den Heuvel, A.; Sterenborg, H.J.C.M.; Robinson, D.J. Increase in protoporphyrin IX after 5-aminolevulinic acid based photodynamic therapy is due to local re-synthesis. Photochem. Photobiol. Sci. 2007, 6, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qiu, F.; Chen, R.; Yan, D.; Zhu, X. Fluorescence resonance energy transfer-based drug delivery systems for enhanced photodynamic therapy. J. Mater. Chem. B 2020, 8, 3772–3788. [Google Scholar] [CrossRef]
- Inglut, C.T.; Gaitan, B.; Najafali, D.; Lopez, I.A.; Connolly, N.P.; Orsila, S.; Perttilä, R.; Woodworth, G.F.; Chen, Y.; Huang, H. Predictors and Limitations of the Penetration Depth of Photodynamic Effects in the Rodent Brain. Photochem. Photobiol. 2020, 96, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Cincotta, L.; Szeto, D.; Lampros, E.; Hasan, T.; Cincotta, A.H. Benzophenothiazine and Benzoporphyrin Derivative Combination Phototherapy Effectively Eradicates Large Murine Sarcomas. Photochem. Photobiol. 1996, 63, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Upconversion in photodynamic therapy: Plumbing the depths. Dalton Trans. 2018, 47, 8571–8580. [Google Scholar] [CrossRef] [PubMed]
- Bashkatov, A.N.; Berezin, K.V.; Dvoretskiy, K.N.; Chernavina, M.L.; Genina, E.A.; Genin, V.D.; Kochubey, V.I. Measurement of tissue optical properties in the context of tissue optical clearing. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Vargas, G.; Chan, E.K.; Barton, J.K.; Rylander, H.G.; Welch, A.J. Use of an agent to reduce scattering in skin. Lasers Surg. Med. 1999, 24, 133–141. [Google Scholar] [CrossRef]
- Sterenborg, H.J.C.M.; Gemert, M.J.C. van Photodynamic therapy with pulsed light sources: A theoretical analysis. Phys. Med. Biol. 1996, 41, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Pogue, B.W.; Lilge, L.; Patterson, M.S.; Wilson, B.C.; Hasan, T. Absorbed photodynamic dose from pulsed versus continuous wave light examined with tissue-simulating dosimeters. Appl. Opt. 1997, 36, 7257. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, S.; Morimoto, Y.; Sato, S.; Arai, T.; Seguchi, K.; Asanuma, H.; Kikuchi, M. Differences between cytotoxicity in photodynamic therapy using a pulsed laser and a continuous wave laser: Study of oxygen consumption and photobleaching. Lasers Med. Sci. 2004, 18, 179–183. [Google Scholar] [CrossRef]
- Grecco, C.; Moriyama, L.T.; Cosci, A.; Pratavieira, S.; Bagnato, V.S.; Kurachi, C. Necrosis response to photodynamic therapy using light pulses in the femtosecond regime. Lasers Med. Sci. 2013, 28, 1177–1182. [Google Scholar] [CrossRef]
- Grecco, C.; Pratavieira, S.; Bagnato, V.; Kurachi, C. Comparison of two photosensitizers in photodynamic therapy using light pulses in femtosecond regime: An animal study. In Proceedings of the Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XXV, San Francisco, CA, USA, 1 March 2016; Kessel, D.H., Hasan, T., Eds.; SPIE: Washington, DC, USA, 2016; Volume 9694, p. 969417. [Google Scholar]
- Klimenko, V.V.; Knyazev, N.A.; Moiseenko, F.V.; Rusanov, A.A.; Bogdanov, A.A.; Dubina, M.V. Pulse mode of laser photodynamic treatment induced cell apoptosis. Photodiagn. Photodyn. Ther. 2016, 13, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, D.S.; Shirmanova, M.V.; Dudenkova, V.V.; Subochev, P.V.; Turchin, I.V.; Zagaynova, E.V.; Lukyanov, S.A.; Shakhov, B.E.; Kamensky, V.A. Photobleaching and phototoxicity of KillerRed in tumor spheroids induced by continuous wave and pulsed laser illumination. J. Biophotonics 2015, 8, 952–960. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Umebayashi, Y.; Nishisaka, T. Comparison of phototoxicity mechanism between pulsed and continuous wave irradiation in photodynamic therapy. J. Photochem. Photobiol. B Biol. 1999, 53, 53–59. [Google Scholar] [CrossRef]
- Strasswimmer, J.; Grande, D.J. Do pulsed lasers produce an effective photodynamic therapy response? Lasers Surg. Med. 2006, 38, 22–25. [Google Scholar] [CrossRef]
- Alexiades-Armenakas, M.R.; Geronemus, R.G. Laser-mediated photodynamic therapy of actinic keratoses. Arch. Dermatol. 2003, 139, 1313–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexiades-Armenakas, M. Laser-mediated photodynamic therapy. Clin. Dermatol. 2006, 24, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Park, J.; Lee, K.; Yoon, J.; Kim, K.; Lee, S.; Park, Y. Recent advances in wavefront shaping techniques for biomedical applications. Curr. Appl. Phys. 2015, 15, 632–641. [Google Scholar] [CrossRef] [Green Version]
- Bender, N.; Yamilov, A.; Yllmaz, H.; Cao, H. Fluctuations and Correlations of Transmission Eigenchannels in Diffusive Media. Phys. Rev. Lett. 2020, 125, 165901. [Google Scholar] [CrossRef] [PubMed]
- Vellekoop, I.M.; Mosk, A.P. Phase control algorithms for focusing light through turbid media. Opt. Commun. 2008, 281, 3071–3080. [Google Scholar] [CrossRef] [Green Version]
- Maire, C.; Vignion-Dewalle, A.S.; Cartier, H.; Mordon, S. Artificial white light photodynamic therapy for actinic keratosis: A study of 38 patients in private office practice. J. Eur. Acad. Dermatol. Venereol. 2020. [Google Scholar] [CrossRef]
- Marra, K.; LaRochelle, E.P.; Chapman, M.S.; Hoopes, P.J.; Lukovits, K.; Maytin, E.V.; Hasan, T.; Pogue, B.W. Comparison of Blue and White Lamp Light with Sunlight for Daylight-Mediated, 5-ALA Photodynamic Therapy, in vivo. Photochem. Photobiol. 2018, 94, 1049–1057. [Google Scholar] [CrossRef]
- Lerche, C.M.; Heerfordt, I.M.; Heydenreich, J.; Wulf, H.C. Alternatives to outdoor daylight illumination for photodynamic therapy—Use of greenhouses and artificial light sources. Int. J. Mol. Sci. 2016, 17, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mordon, S.; Thécua, E.; Ziane, L.; Lecomte, F.; Deleporte, P.; Baert, G.; Vignion-Dewalle, A. Light emitting fabrics for photodynamic therapy: Technology, experimental and clinical applications. Transl. Biophotonics 2020, 2, e202000005. [Google Scholar] [CrossRef]
- O’Mahoney, P.; Haigh, N.; Wood, K.; Brown, C.T.A.; Ibbotson, S.; Eadie, E. A novel light source with tuneable uniformity of light distribution for artificial daylight photodynamic therapy. Photodiagn. Photodyn. Ther. 2018, 23, 144–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochrane, C.; Mordon, S.R.; Lesage, J.C.; Koncar, V. New design of textile light diffusers for photodynamic therapy. Mater. Sci. Eng. C 2013, 33, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Tylcz, J.B.; Vicentini, C.; Mordon, S. Light emitting textiles for a photodynamic therapy. In Smart Textiles and Their Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 71–87. ISBN 9780081005835. [Google Scholar]
- Masuda, H.; Kimura, M.; Nishioka, A.; Kato, H.; Morita, A. Dual wavelength 5-aminolevulinic acid photodynamic therapy using a novel flexible light-emitting diode unit. J. Dermatol. Sci. 2019, 93, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.; Xie, Z.; Mousavi, M.; Bendsoe, N.; Brydegaard, M.; Axelsson, J.; Andersson-Engels, S. Design and validation of a fiber optic point probe instrument for therapy guidance and monitoring. J. Biomed. Opt. 2014, 19, 071408. [Google Scholar] [CrossRef] [Green Version]
- Rendon, A.; Weersink, R.; Lilge, L. Towards conformal light delivery using tailored cylindrical diffusers: Attainable light dose distributions. Phys. Med. Biol. 2006, 51, 5967–5975. [Google Scholar] [CrossRef]
- Stock, K.; Stegmayer, T.; Graser, R.; Förster, W.; Hibst, R. Comparison of different focusing fiber tips for improved oral diode laser surgery. Lasers Surg. Med. 2012, 44, 815–823. [Google Scholar] [CrossRef]
- Mikolajewska, P.; Donnelly, R.F.; Garland, M.J.; Morrow, D.I.J.; Singh, T.R.R.; Iani, V.; Moan, J.; Juzeniene, A. Microneedle pre-treatment of human skin improves 5-aminolevulininc acid (ALA)- and 5-aminolevulinic acid methyl ester (MAL)-induced PpIX production for topical photodynamic therapy without increase in pain or erythema. Pharm. Res. 2010, 27, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Dolganova, I.N.; Shikunova, I.A.; Katyba, G.M.; Zotov, A.K.; Mukhina, E.E.; Shchedrina, M.A.; Tuchin, V.V.; Zaytsev, K.I.; Kurlov, V.N. Optimization of sapphire capillary needles for interstitial and percutaneous laser medicine. J. Biomed. Opt. 2019, 24, 1. [Google Scholar] [CrossRef]
- Dolganova, I.N.; Shikunova, I.A.; Zotov, A.K.; Shchedrina, M.A.; Reshetov, I.V.; Zaytsev, K.I.; Tuchin, V.V.; Kurlov, V.N. Microfocusing sapphire capillary needle for laser surgery and therapy: Fabrication and characterization. J. Biophotonics 2020, 13, e202000164. [Google Scholar] [CrossRef] [PubMed]
- Jäger, H.R.; Taylor, M.N.; Theodossy, T.; Hopper, C. MR Imaging-Guided Interstitial Photodynamic Laser Therapy for Advanced Head and Neck Tumors. Am. J. Neuroradiol. 2005, 26, 1193–1200. [Google Scholar] [PubMed]
- Johansson, A.; Faber, F.; Kniebühler, G.; Stepp, H.; Sroka, R.; Egensperger, R.; Beyer, W.; Kreth, F.-W. Protoporphyrin IX Fluorescence and Photobleaching During Interstitial Photodynamic Therapy of Malignant Gliomas for Early Treatment Prognosis. Lasers Surg. Med. 2013, 45, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.; Bartusik-Aebisher, D.; Osuchowski, F.; Aebisher, D. Photodynamic therapy for prostate cancer—A narrative review. Photodiagn. Photodyn. Ther. 2021, 33, 102158. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, K.; Zhu, T.C. A light blanket for intraoperative photodynamic therapy. In Photodynamic Therapy: Back to the Future; SPIE: Washington, DC, USA, 2009; Volume 7380, p. 73801W. [Google Scholar]
- Chamberlain, S.; Bellnier, D.; Yendamuri, S.; Lindenmann, J.; Demmy, T.; Nwogu, C.; Ramer, M.; Tworek, L.; Oakley, E.; Mallory, M.; et al. An Optical Surface Applicator for Intraoperative Photodynamic Therapy. Lasers Surg. Med. 2020, 52, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Protti, S.; Albini, A.; Viswanathan, R.; Greer, A. Targeting Photochemical Scalpels or Lancets in the Photodynamic Therapy Field-The Photochemist’s Role. Photochem. Photobiol. 2017, 93, 1139–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mordon, S. New optical sources for interstitial and metronomic photodynamic therapy. Photodiagn. Photodyn. Ther. 2018, 23, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Swartling, J.; Höglund, O.V.; Hansson, K.; Södersten, F.; Axelsson, J.; Lagerstedt, A.-S. Online dosimetry for temoporfin-mediated interstitial photodynamic therapy using the canine prostate as model. J. Biomed. Opt. 2016, 21, 028002. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, J.; Betz, V.; Lilge, L. Treatment plan evaluation for interstitial photodynamic therapy in a mouse model by Monte Carlo simulation with FullMonte. Front. Phys. 2015, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.K.-H.; Zhu, T.C. Reconstruction of in-vivo optical properties for human prostate using interstitial diffuse optical tomography. Opt. Express 2009, 17, 11665. [Google Scholar] [CrossRef] [Green Version]
- Chinna Ayya Swamy, P.; Sivaraman, G.; Priyanka, R.N.; Raja, S.O.; Ponnuvel, K.; Shanmugpriya, J.; Gulyani, A. Near Infrared (NIR) absorbing dyes as promising photosensitizer for photodynamic therapy. Coord. Chem. Rev. 2020, 411, 213233. [Google Scholar] [CrossRef]
- Bolze, F.; Jenni, S.; Sour, A.; Heitz, V. Molecular photosensitisers for two-photon photodynamic therapy. Chem. Commun. 2017, 53, 12857–12877. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Zhao, C.; Baev, A.; Yong, K.-T.; Wen, S.; Prasad, P.N. Molecular nonlinear optics: Recent advances and applications. Adv. Opt. Photonics 2016, 8, 328. [Google Scholar] [CrossRef]
- Lenz, P. In vivo excitation of photosensitizers by infrared light. Photochem. Photobiol. 1995, 62, 333–338. [Google Scholar] [CrossRef]
- Collins, H.A.; Khurana, M.; Moriyama, E.H.; Mariampillai, A.; Dahlstedt, E.; Balaz, M.; Kuimova, M.K.; Drobizhev, M.; Yang, V.X.D.; Phillips, D.; et al. Blood-vessel closure using photosensitizers engineered for two-photon excitation. Nat. Photonics 2008, 2, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Kachynski, A.V.; Pliss, A.; Kuzmin, A.N.; Ohulchanskyy, T.Y.; Baev, A.; Qu, J.; Prasad, P.N. Photodynamic therapy by in situ nonlinear photon conversion. Nat. Photonics 2014, 8, 455–461. [Google Scholar] [CrossRef]
- Qiu, H.; Tan, M.; Ohulchanskyy, T.Y.; Lovell, J.F.; Chen, G. Recent progress in upconversion photodynamic therapy. Nanomaterials 2018, 8, 344. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.X.; Chen, H.J.; Li, F.; Wang, W.N.; Li, D.D.; Yang, X.Z.; Miao, Z.H.; Zha, Z.B.; Lu, Y.; Qian, H.S. Controlled synthesis of upconverting nanoparticles/CuS yolk-shell nanoparticles for: In vitro synergistic photothermal and photodynamic therapy of cancer cells. J. Mater. Chem. B 2017, 5, 9487–9496. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, S.; Wang, Y. Photosensitizer coated upconversion nanoparticles for triggering reactive oxygen species under 980 nm near-infrared excitation. J. Mater. Chem. B 2019, 7, 7306–7313. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Chen, Y. Energy-Converting Nanomedicine. Small 2019, 15, 1805339. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wang, H.; Pan, H.; Liang, C.; Ji, W.; Zhao, L.; Chen, H.; Gong, X.; Wu, X.; Chang, J. Near-Infrared Light Triggered Upconversion Optogenetic Nanosystem for Cancer Therapy. ACS Nano 2017, 11, 11898–11907. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xu, S.; Wang, N.; Jiang, H.; Huang, Y.; Jin, X.; Xue, B.; Zhang, C.; Zhu, X. Prodrug-embedded angiogenic vessel-targeting nanoparticle: A positive feedback amplifier in hypoxia-induced chemo-photo therapy. Biomaterials 2017, 144, 188–198. [Google Scholar] [CrossRef]
- Le, X.T.; Youn, Y.S. Emerging NIR light-responsive delivery systems based on lanthanide-doped upconverting nanoparticles. Arch. Pharm. Res. 2020, 43, 134–152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, L.; Li, S.; Jiang, X.F.; Jiang, C.; Zhou, N.; Gao, N.; Xu, Q.H. Gold nanorod-enhanced two-photon excitation fluorescence of conjugated oligomers for two-photon imaging guided photodynamic therapy. J. Mater. Chem. C 2019, 7, 14693–14700. [Google Scholar] [CrossRef]
- Yaghini, E.; Seifalian, A.M.; MacRobert, A.J. Quantum dots and their potential biomedical applications in photosensitization for photodynamic therapy. Nanomedicine 2009, 4, 353–363. [Google Scholar] [CrossRef]
- Fowley, C.; Nomikou, N.; McHale, A.P.; McCaughan, B.; Callan, J.F. Extending the tissue penetration capability of conventional photosensitisers: A carbon quantum dot-protoporphyrin IX conjugate for use in two-photon excited photodynamic therapy. Chem. Commun. 2013, 49, 8934–8936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.P.; Cheng, S.H.; Chen, N.T.; Lo, L.W. Intra/inter-particle energy transfer of luminescence nanocrystals for biomedical applications. J. Nanomater. 2012, 2012, 16. [Google Scholar] [CrossRef]
- Edelhoch, H.; Brand, L.; Wilchek, M. Fluorescence Studies with Tryptophyl Peptides. Biochemistry 1967, 6, 547–559. [Google Scholar] [CrossRef]
- Vekshin, N. Energy Transfer in Macromolecules; SPIE: Washington, DC, USA, 1996; ISBN 9780819420817. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 0387312781. [Google Scholar]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, X.; Bu, W. Upconversion-based photodynamic cancer therapy. Coord. Chem. Rev. 2019, 379, 82–98. [Google Scholar] [CrossRef]
- Shen, J.; Chen, G.; Vu, A.M.; Fan, W.; Bilsel, O.S.; Chang, C.C.; Han, G. Engineering the upconversion nanoparticle excitation wavelength: Cascade sensitization of tri-doped upconversion colloidal nanoparticles at 800 nm. Adv. Opt. Mater. 2013, 1, 644–650. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, Y.; Deng, K.; Chen, Y.; Li, X.; Deng, X.; Cheng, Z.; Lian, H.; Li, C.; Lin, J. UV-emitting upconversion-based TiO2 photosensitizing nanoplatform: Near-infrared light mediated in vivo photodynamic therapy via mitochondria-involved apoptosis pathway. ACS Nano 2015, 9, 2584–2599. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Pan, Y.; Tian, Y.; Wang, X.; Ren, W.; Wang, S.; Lu, G.; Wu, A. Doxorubicin-loaded NaYF4: Yb/Tm-TiO2 inorganic photosensitizers for NIR-triggered photodynamic therapy and enhanced chemotherapy in drug-resistant breast cancers. Biomaterials 2015, 57, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Muhammad Idris, N.; Li, Z.; Huang, K.; Soo, K.C.; Zhang, Y. Titania coated upconversion nanoparticles for near-infrared light triggered photodynamic therapy. ACS Nano 2015, 9, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, L.; Pan, Y.; Luo, S.; Ren, W.; Gong, A.; Ma, X.; Liang, H.; Lu, G.; Wu, A. Inorganic photosensitizer coupled Gd-based upconversion luminescent nanocomposites for invivo magnetic resonance imaging and near-infrared-responsive photodynamic therapy in cancers. Biomaterials 2015, 44, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.Q.; Rengaramchandran, A.; Selvan, S.T.; Paulmurugan, R.; Zhang, Y. Core-Shell upconversion nanoparticle—Semiconductor heterostructures for photodynamic therapy. Sci. Rep. 2015, 5, 8252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Steelant, W.; Kumar, M.; Scholfield, M. Versatile photosensitizers for photodynamic therapy at infrared excitation. J. Am. Chem. Soc. 2007, 129, 4526–4527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Liu, X.; Zeng, Q.; Zhang, Y.; Tu, L.; Liu, T.; Kong, X.; Wang, Y.; Cao, F.; Lambrechts, S.A.G.; et al. Covalently assembled NIR nanoplatform for simultaneous fluorescence imaging and photodynamic therapy of cancer cells. ACS Nano 2012, 6, 4054–4062. [Google Scholar] [CrossRef]
- Borodziuk, A.; Kowalik, P.; Duda, M.; Wojciechowski, T.; Minikayev, R.; Kalinowska, D.; Klepka, M.; Sobczak, K.; Kłopotowski, Ł.; Sikora, B. Unmodified Rose Bengal photosensitizer conjugated with NaYF4:Yb,Er upconverting nanoparticles for efficient photodynamic therapy. Nanotechnology 2020, 31, 465101. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Qian, H.; Idris, N.M.; Zhang, Y. Singlet oxygen-induced apoptosis of cancer cells using upconversion fluorescent nanoparticles as a carrier of photosensitizer. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 486–495. [Google Scholar] [CrossRef]
- Li, S.; Cui, S.; Yin, D.; Zhu, Q.; Ma, Y.; Qian, Z.; Gu, Y. Dual antibacterial activities of a chitosan-modified upconversion photodynamic therapy system against drug-resistant bacteria in deep tissue. Nanoscale 2017, 9, 3912–3924. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tao, H.; Cheng, L.; Liu, Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials 2011, 32, 6145–6154. [Google Scholar] [CrossRef]
- Zhou, A.; Wei, Y.; Wu, B.; Chen, Q.; Xing, D. Pyropheophorbide A and c(RGDyK) comodified chitosan-wrapped upconversion nanoparticle for targeted near-infrared photodynamic therapy. Mol. Pharm. 2012, 9, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.I.; Kim, H.M.; Kim, J.H.; Moon, K.C.; Yoo, B.; Lee, K.T.; Lee, N.; Choi, Y.; Park, W.; Ling, D.; et al. Theranostic Probe Based on Lanthanide-Doped Nanoparticles for Simultaneous In Vivo Dual-Modal Imaging and Photodynamic Therapy. Adv. Mater. 2012, 24, 5755–5761. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Chen, H.; Zhu, H.; Tian, J.; Chi, X.; Qian, Z.; Achilefu, S.; Gu, Y. Amphiphilic chitosan modified upconversion nanoparticles for in vivo photodynamic therapy induced by near-infrared light. J. Mater. Chem. 2012, 22, 4861–4873. [Google Scholar] [CrossRef]
- Lim, M.E.; Lee, Y.L.; Zhang, Y.; Chu, J.J.H. Photodynamic inactivation of viruses using upconversion nanoparticles. Biomaterials 2012, 33, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.F.; Zhou, J.C.; Xiao, J.W.; Wang, Y.F.; Sun, L.D.; Yan, C.H. Triple-functional core-shell structured upconversion luminescent nanoparticles covalently grafted with photosensitizer for luminescent, magnetic resonance imaging and photodynamic therapy in vitro. Nanoscale 2012, 4, 4611–4623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med. 2012, 18, 1580–1585. [Google Scholar] [CrossRef]
- Shan, J.; Budijono, S.J.; Hu, G.; Yao, N.; Kang, Y.; Ju, Y.; Prud’homme, R.K. Pegylated Composite Nanoparticles Containing Upconverting Phosphors and meso-Tetraphenyl porphine (TPP) for Photodynamic Therapy. Adv. Funct. Mater. 2011, 21, 2488–2495. [Google Scholar] [CrossRef]
- Liang, L.; Lu, Y.; Zhang, R.; Care, A.; Ortega, T.A.; Deyev, S.M.; Qian, Y.; Zvyagin, A.V. Deep-penetrating photodynamic therapy with KillerRed mediated by upconversion nanoparticles. Acta Biomater. 2017, 51, 461–470. [Google Scholar] [CrossRef]
- Mironova, K.E.; Khochenkov, D.A.; Generalova, A.N.; Rocheva, V.V.; Sholina, N.V.; Nechaev, A.V.; Semchishen, V.A.; Deyev, S.M.; Zvyagin, A.V.; Khaydukov, E.V. Ultraviolet phototoxicity of upconversion nanoparticles illuminated with near-infrared light. Nanoscale 2017, 9, 14921–14928. [Google Scholar] [CrossRef] [PubMed]
- Khaydukov, E.V.; Mironova, K.E.; Semchishen, V.A.; Generalova, A.N.; Nechaev, A.V.; Khochenkov, D.A.; Stepanova, E.V.; Lebedev, O.I.; Zvyagin, A.V.; Deyev, S.M.; et al. Riboflavin photoactivation by upconversion nanoparticles for cancer treatment. Sci. Rep. 2016, 6, 35103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Yang, D.; Yang, P.; Lv, R.; Li, C.; Zhong, C.; He, F.; Gai, S.; Lin, J. A Single 808 nm Near-Infrared Light-Mediated Multiple Imaging and Photodynamic Therapy Based on Titania Coupled Upconversion Nanoparticles. Chem. Mater. 2015, 27, 7957–7968. [Google Scholar] [CrossRef]
- Wang, D.; Xue, B.; Kong, X.; Tu, L.; Liu, X.; Zhang, Y.; Chang, Y.; Luo, Y.; Zhao, H.; Zhang, H. 808 nm driven Nd3+-sensitized upconversion nanostructures for photodynamic therapy and simultaneous fluorescence imaging. Nanoscale 2015, 7, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Lv, R.; Yang, D.; Yang, P.; Xu, J.; He, F.; Gai, S.; Li, C.; Dai, Y.; Yang, G.; Lin, J. Integration of Upconversion Nanoparticles and Ultrathin Black Phosphorus for Efficient Photodynamic Theranostics under 808 nm Near-Infrared Light Irradiation. Chem. Mater. 2016, 28, 4724–4734. [Google Scholar] [CrossRef]
- Ai, F.; Ju, Q.; Zhang, X.; Chen, X.; Wang, F.; Zhu, G. A core-shell-shell nanoplatform upconverting near-infrared light at 808 nm for luminescence imaging and photodynamic therapy of cancer. Sci. Rep. 2015, 5, 10785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cline, B.; Delahunty, I.; Xie, J. Nanoparticles to mediate X-ray-induced photodynamic therapy and Cherenkov radiation photodynamic therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1541. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J. Nanosci. Nanotechnol. 2006, 6, 1159–1166. [Google Scholar] [CrossRef]
- Chen, X.; Song, J.; Chen, X.; Yang, H. X-ray-activated nanosystems for theranostic applications. Chem. Soc. Rev. 2019, 48, 3073–3101. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhou, Z.; Pratx, G.; Chen, X.; Chen, H. Nanoscintillator-mediated X-ray induced photodynamic therapy for deep-seated tumors: From concept to biomedical applications. Theranostics 2020, 10, 1296–1318. [Google Scholar] [CrossRef]
- Ren, X.D.; Hao, X.Y.; Li, H.C.; Ke, M.R.; Zheng, B.Y.; Huang, J.D. Progress in the development of nanosensitizers for X-ray-induced photodynamic therapy. Drug Discov. Today 2018, 23, 1791–1800. [Google Scholar] [CrossRef]
- Larue, L.; Ben Mihoub, A.; Youssef, Z.; Colombeau, L.; Acherar, S.; André, J.C.; Arnoux, P.; Baros, F.; Vermandel, M.; Frochot, C. Using X-rays in photodynamic therapy: An overview. Photochem. Photobiol. Sci. 2018, 17, 1612–1650. [Google Scholar] [CrossRef]
- Ma, L.; Zou, X.; Bui, B.; Chen, W.; Song, K.H.; Solberg, T. X-ray excited ZnS:Cu,Co afterglow nanoparticles for photodynamic activation. Appl. Phys. Lett. 2014, 105, 013702. [Google Scholar] [CrossRef]
- Daouk, J.; Dhaini, B.; Petit, J.; Frochot, C.; Barberi-Heyob, M.; Schohn, H. Can Cerenkov Light Really Induce an Effective Photodynamic Therapy? Radiation 2020, 1, 2. [Google Scholar] [CrossRef]
- Sudheendra, L.; Das, G.K.; Li, C.; Stark, D.; Cena, J.; Cherry, S.; Kennedy, I.M. NaGdF4:Eu3+ nanoparticles for enhanced X-ray excited optical imaging. Chem. Mater. 2014, 26, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Clement, S.; Deng, W.; Camilleri, E.; Wilson, B.C.; Goldys, E.M. X-ray induced singlet oxygen generation by nanoparticle-photosensitizer conjugates for photodynamic therapy: Determination of singlet oxygen quantum yield. Sci. Rep. 2016, 6, 19954. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, G.D.; Chuang, Y.J.; Zhen, Z.; Chen, X.; Biddinger, P.; Hao, Z.; Liu, F.; Shen, B.; Pan, Z.; et al. Nanoscintillator-Mediated X-ray Inducible Photodynamic Therapy for in Vivo Cancer Treatment. Nano Lett. 2015, 15, 2249–2256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, X.; Yao, M.; Ma, L.; Hossu, M.; Han, X.; Juzenas, P.; Chen, W. X-ray-induced nanoparticle-based photodynamic therapy of cancer. Nanomedicine 2014, 9, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Hu, J.; Elmenoufy, A.H.; Yang, X. Highly Efficient FRET System Capable of Deep Photodynamic Therapy Established on X-ray Excited Mesoporous LaF3:Tb Scintillating Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 12261–12269. [Google Scholar] [CrossRef]
- Elmenoufy, A.H.; Tang, Y.; Hu, J.; Xu, H.; Yang, X. A novel deep photodynamic therapy modality combined with CT imaging established via X-ray stimulated silica-modified lanthanide scintillating nanoparticles. Chem. Commun. 2015, 51, 12247–12250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbri, F.; Rossi, F.; Attolini, G.; Salviati, G.; Dierre, B.; Sekiguchi, T.; Fukata, N. Luminescence properties of SiC/SiO2 core-shell nanowires with different radial structure. Mater. Lett. 2012, 71, 137–140. [Google Scholar] [CrossRef]
- Chen, H.; Wang, F.; Moore, T.L.; Qi, B.; Sulejmanovic, D.; Hwu, S.J.; Mefford, O.T.; Alexis, F.; Anker, J.N. Bright X-ray and up-conversion nanophosphors annealed using encapsulated sintering agents for bioimaging applications. J. Mater. Chem. B 2017, 5, 5412–5424. [Google Scholar] [CrossRef] [PubMed]
- Bulin, A.L.; Truillet, C.; Chouikrat, R.; Lux, F.; Frochot, C.; Amans, D.; Ledoux, G.; Tillement, O.; Perriat, P.; Barberi-Heyob, M.; et al. X-ray-induced singlet oxygen activation with nanoscintillator-coupled porphyrins. J. Phys. Chem. C 2013, 117, 21583–21589. [Google Scholar] [CrossRef]
- Kaščáková, S.; Giuliani, A.; Lacerda, S.; Pallier, A.; Mercère, P.; Tóth, É.; Réfrégiers, M. X-ray-induced radiophotodynamic therapy (RPDT) using lanthanide micelles: Beyond depth limitations. Nano Res. 2015, 8, 2373–2379. [Google Scholar] [CrossRef] [Green Version]
- Lan, G.; Ni, K.; Xu, R.; Lu, K.; Lin, Z.; Chan, C.; Lin, W. Nanoscale Metal–Organic Layers for Deeply Penetrating X-ray-Induced Photodynamic Therapy. Angew. Chem. 2017, 56, 12102–12106. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zou, X.; Chen, W. A new x-ray activated nanoparticle photosensitizer for cancer treatment. J. Biomed. Nanotechnol. 2014, 10, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, K.; Bu, W.; Ni, D.; Liu, Y.; Feng, J.; Shi, J. Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence. Angew. Chem. Int. Ed. 2015, 54, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, X.; Wang, G.D.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J.; Shen, B. LiGa5O8:Cr-based theranostic nanoparticles for imaging-guided X-ray induced photodynamic therapy of deep-seated tumors. Mater. Horiz. 2017, 4, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Lin, S.L.; Chang, C.A. Lanthanide-Doped Core-Shell-Shell Nanocomposite for Dual Photodynamic Therapy and Luminescence Imaging by a Single X-ray Excitation Source. ACS Appl. Mater. Interfaces 2018, 10, 7859–7870. [Google Scholar] [CrossRef]
- Sengar, P.; Juárez, P.; Verdugo-Meza, A.; Arellano, D.L.; Jain, A.; Chauhan, K.; Hirata, G.A.; Fournier, P.G.J. Development of a functionalized UV-emitting nanocomposite for the treatment of cancer using indirect photodynamic therapy. J. Nanobiotechnol. 2018, 16, 1–19. [Google Scholar] [CrossRef]
- Ran, C.; Zhang, Z.; Hooker, J.; Moore, A. In vivo photoactivation without “light”: Use of cherenkov radiation to overcome the penetration limit of light. Mol. Imaging Biol. 2012, 14, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Robertson, R.; Germanos, M.S.; Li, C.; Mitchell, G.S.; Cherry, S.R.; Silva, M.D. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys. Med. Biol. 2009, 54, 355–365. [Google Scholar] [CrossRef]
- Kotagiri, N.; Sudlow, G.P.; Akers, W.J.; Achilefu, S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nanotechnol. 2015, 10, 370–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, D.; Liu, H.; Xu, Y.; Han, Y.; Xu, M.; Zhang, Z.; Liu, Z. Activating TiO2 Nanoparticles: Gallium-68 Serves as a High-Yield Photon Emitter for Cerenkov-Induced Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 5278–5286. [Google Scholar] [CrossRef] [PubMed]
- Hartl, B.A.; Hirschberg, H.; Marcu, L.; Cherry, S.R. Activating photodynamic therapy in vitro with Cerenkov radiation generated from yttrium-90. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratx, G.; Kapp, D.S. Is Cherenkov luminescence bright enough for photodynamic therapy? Nat. Nanotechnol. 2018, 13, 354. [Google Scholar] [CrossRef]
- Glaser, A.K.; Zhang, R.; Andreozzi, J.M.; Gladstone, D.J.; Pogue, B.W. Cherenkov radiation fluence estimates in tissue for molecular imaging and therapy applications. Phys. Med. Biol. 2015, 60, 6701–6718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, B.W.; Busch, T.M.; Snyder, J.W. Fluence rate as a modulator of PDT mechanisms. Lasers Surg. Med. 2006, 38, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Administration of Radioactive Substances Advisory Committee, Notes for Guidance: Good Clinical Practice in Nuclear Medicine. Available online: https://www.gov.uk/government/publications/arsac-notes-for-guidance (accessed on 6 June 2021).
- Lahham, A.; Issa, A.; Masri, H.A.L. Patient radiation dose from chest X-ray examinations in the West Bank-Palestine. Radiat. Prot. Dosim. 2018, 178, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bindman, R.; Lipson, J.; Marcus, R.; Kim, K.P.; Mahesh, M.; Gould, R.; Berrington de González, A.; Miglioretti, D.L. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch. Intern. Med. 2009, 169, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- American College of Radiology, Radiation Dose to Adults from Common Imaging Examinations. Available online: https://www.acr.org/-/media/ACR/Files/Radiology-Safety/Radiation-Safety/Dose-Reference-Card.pdf (accessed on 6 June 2021).
- Packer, S. Tumor detection with radiopharmaceuticals. Semin. Nucl. Med. 1984, 14, 21–30. [Google Scholar] [CrossRef]
- Magalhães, C.M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Chemiluminescence and Bioluminescence as an Excitation Source in the Photodynamic Therapy of Cancer: A Critical Review. ChemPhysChem 2016, 17, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Laptev, R.; Nisnevitch, M.; Siboni, G.; Malik, Z.; Firer, M.A. Intracellular chemiluminescence activates targeted photodynamic destruction of leukaemic cells. Br. J. Cancer 2006, 95, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Chong, H.; Wang, B.; Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Chemical molecule-induced light-activated system for anticancer and antifungal activities. J. Am. Chem. Soc. 2012, 134, 13184–13187. [Google Scholar] [CrossRef]
- Davis, R.W.; Snyder, E.; Miller, J.; Carter, S.; Houser, C.; Klampatsa, A.; Albelda, S.M.; Cengel, K.A.; Busch, T.M. Luminol Chemiluminescence Reports Photodynamic Therapy-Generated Neutrophil Activity in vivo and Serves as a Biomarker of Therapeutic Efficacy. Photochem. Photobiol. 2019, 95, 430–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Song, X.; Wang, Q.; Hu, W.; Shi, W.; Tang, Y.; Wu, Z.; Fan, Q.; Huang, W. Chemiluminescent organic nanophotosensitizer for a penetration depth independent photodynamic therapy. RSC Adv. 2020, 10, 11861–11864. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Pang, L.; Ma, C.; Tu, Q.; Zhang, R.; Saeed, E.; Mahmoud, A.E.; Wang, J. Small Molecule-Initiated Light-Activated Semiconducting Polymer Dots: An Integrated Nanoplatform for Targeted Photodynamic Therapy and Imaging of Cancer Cells. Anal. Chem. 2014, 86, 3092–3099. [Google Scholar] [CrossRef]
- Jiang, L.; Bai, H.; Liu, L.; Lv, F.; Ren, X.; Wang, S. Luminescent, Oxygen-Supplying, Hemoglobin-Linked Conjugated Polymer Nanoparticles for Photodynamic Therapy. Angew. Chem. Int. Ed. 2019, 58, 10660–10665. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, C.; Wei, X.; Ding, S.; Liu, C.; Tian, F.; Li, F. Self-Illuminating Photodynamic Therapy with Enhanced Therapeutic Effect by Optimization of the Chemiluminescence Resonance Energy Transfer Step to the Photosensitizer. Bioconjug. Chem. 2019, 31, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.; Da Silva, L.P.; Da Silva, J.C.G.E. Advances in the knowledge of light emission by firefly luciferin and oxyluciferin. J. Photochem. Photobiol. B Biol. 2012, 117, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Pinto da Silva, L.; Esteves da Silva, J.C.G. Firefly Chemiluminescence and Bioluminescence: Efficient Generation of Excited States. ChemPhysChem 2012, 13, 2257–2262. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.P.; Esteves da Silva, J.C.G. Computational studies of the luciferase light-emitting product: Oxyluciferin. J. Chem. Theory Comput. 2011, 7, 809–817. [Google Scholar] [CrossRef]
- Theodossiou, T.; Hothersall, J.S.; Woods, E.A.; Okkenhaug, K.; Jacobson, J.; MacRobert, A.J. Firefly luciferin-activated rose bengal: In vitro photodynamic therapy by intracellular chemiluminescence in transgenic NIH 3T3 cells. Cancer Res. 2003, 63, 1818–1821. [Google Scholar] [PubMed]
- Schipper, M.L.; Patel, M.R.; Gambhir, S.S. Evaluation of Firefly Luciferase Bioluminescence Mediated Photodynamic Toxicity in Cancer Cells. Mol. Imaging Biol. 2006, 8, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, C.; Liu, C.; Ding, S.; Tian, F.; Li, F. Bioluminescence-initiated photodynamic therapy bridged on high-luminescent carbon dots-conjugated protoporphyrin IX. J. Mater. Sci. 2019, 54, 3383–3391. [Google Scholar] [CrossRef]
- Stepanyuk, G.A.; Liu, Z.J.; Markova, S.S.; Frank, L.A.; Lee, J.; Vysotski, E.S.; Wang, B.C. Crystal structure of coelenterazine-binding protein from Renilla muelleri at 1.7 Å: Why it is not a calcium-regulated photoprotein. Photochem. Photobiol. Sci. 2008, 7, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Chen, C.W.; Yu, H.P.; Lin, Y.F.; Lai, P.S. Bioluminescence resonance energy transfer using luciferase-immobilized quantum dots for self-illuminated photodynamic therapy. Biomaterials 2013, 34, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Kim, S.; Choi, J.W.; Choi, S.Y.; Lee, S.H.; Kim, H.; Hahn, S.K.; Koh, G.Y.; Yun, S.H. Bioluminescence-activated deep-tissue photodynamic therapy of cancer. Theranostics 2015, 5, 805–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proshkina, G.M.; Shramova, E.I.; Shilova, O.N.; Ryabova, A.V.; Deyev, S.M. Phototoxicity of flavoprotein miniSOG induced by bioluminescence resonance energy transfer in genetically encoded system NanoLuc-miniSOG is comparable with its LED-excited phototoxicity. J. Photochem. Photobiol. B Biol. 2018, 188, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Park, S.; Kim, Y.K.Y.P.; Moon, M.; Park, J.; Lee, K.J.; Lee, S.; Kim, Y.K.Y.P. Self-luminescent photodynamic therapy using breast cancer targeted proteins. Sci. Adv. 2020, 6, 3009–3020. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Lu, N.; Xu, C.; Liu, Y.; Lin, J.; Wang, S.; Shen, Z.; Yang, Z.; Qu, J.; Wang, T.; et al. Enhanced Afterglow Performance of Persistent Luminescence Implants for Efficient Repeatable Photodynamic Therapy. ACS Nano 2017, 11, 5864–5872. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, P.; Zhao, M.; Liu, L.; Zhou, L.; Li, B.; Albaqami, F.H.; El-Toni, A.M.; Li, X.; Xie, Y.; et al. Near-infrared rechargeable “optical battery” implant for irradiation-free photodynamic therapy. Biomaterials 2018, 163, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Teh, D.B.L.; Bansal, A.; Chai, C.; Toh, T.B.; Tucker, R.A.J.; Gammad, G.G.L.; Yeo, Y.; Lei, Z.; Zheng, X.; Yang, F.; et al. A Flexi-PEGDA Upconversion Implant for Wireless Brain Photodynamic Therapy. Adv. Mater. 2020, 32, 2001459. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Yang, F.; Xi, T.; Zhang, Y.; Ho, J.S. In vivo wireless photonic photodynamic therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 1469–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamagishi, K.; Kirino, I.; Takahashi, I.; Amano, H.; Takeoka, S.; Morimoto, Y.; Fujie, T. Tissue-adhesive wirelessly powered optoelectronic device for metronomic photodynamic cancer therapy. Nat. Biomed. Eng. 2019, 3, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kirino, I.; Fujita, K.; Sakanoue, K.; Sugita, R.; Yamagishi, K.; Takeoka, S.; Fujie, T.; Uemoto, S.; Morimoto, Y. Metronomic photodynamic therapy using an implantable LED device and orally administered 5-aminolevulinic acid. Sci. Rep. 2020, 10, 22017. [Google Scholar] [CrossRef]
- Kim, A.; Zhou, J.; Samaddar, S.; Song, S.H.; Elzey, B.D.; Thompson, D.H.; Ziaie, B. An Implantable Ultrasonically-Powered Micro-Light-Source (µLight) for Photodynamic Therapy. Sci. Rep. 2019, 9, 1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisland, S.K.; Lilge, L.; Lin, A.; Rusnov, R.; Wilson, B.C. Metronomic Photodynamic Therapy as a New Paradigm for Photodynamic Therapy: Rationale and Preclinical Evaluation of Technical Feasibility for Treating Malignant Brain Tumors. Photochem. Photobiol. 2004, 80, 22–30. [Google Scholar] [CrossRef]
- Morrison, S.A.; Hill, S.L.; Rogers, G.S.; Graham, R.A. Efficacy and safety of continuous low-irradiance photodynamic therapy in the treatment of chest wall progression of breast cancer. J. Surg. Res. 2014, 192, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.W.; Lin, L.T.; Chen, P.H.; Ho, M.H.; Huang, W.T.; Lee, Y.J.; Chiou, S.H.; Hsieh, Y.S.; Dong, C.Y.; Wang, H.W. Low-fluence rate, long duration photodynamic therapy in glioma mouse model using organic light emitting diode (OLED). Photodiagn. Photodyn. Ther. 2015, 12, 504–510. [Google Scholar] [CrossRef] [PubMed]
- de Vijlder, H.C.; Sterenborg, H.J.C.M.; Martino Neumann, H.A.; Robinson, D.J.; de Haas, E.R.M. Light fractionation significantly improves the response of superficial basal cell carcinoma to aminolaevulinic acid photodynamic therapy: Five-year follow-up of a randomized, prospective trial. Acta Derm. Venereol. 2012, 92, 641–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laser Institute of America. American National Standard for Safe Use of Lasers; Laser Institute of America: Orlando, FL, USA, 2000. [Google Scholar]
- Ho, J.S.; Yeh, A.J.; Kim, S.; Poon, A.S.Y. Wireless Powering for Miniature Implantable Systems; Springer: New York, NY, USA, 2014; ISBN 9781461481515. [Google Scholar]
- Ozeri, S.; Shmilovitz, D. Ultrasonic transcutaneous energy transfer for powering implanted devices. Ultrasonics 2010, 50, 556–566. [Google Scholar] [CrossRef] [PubMed]
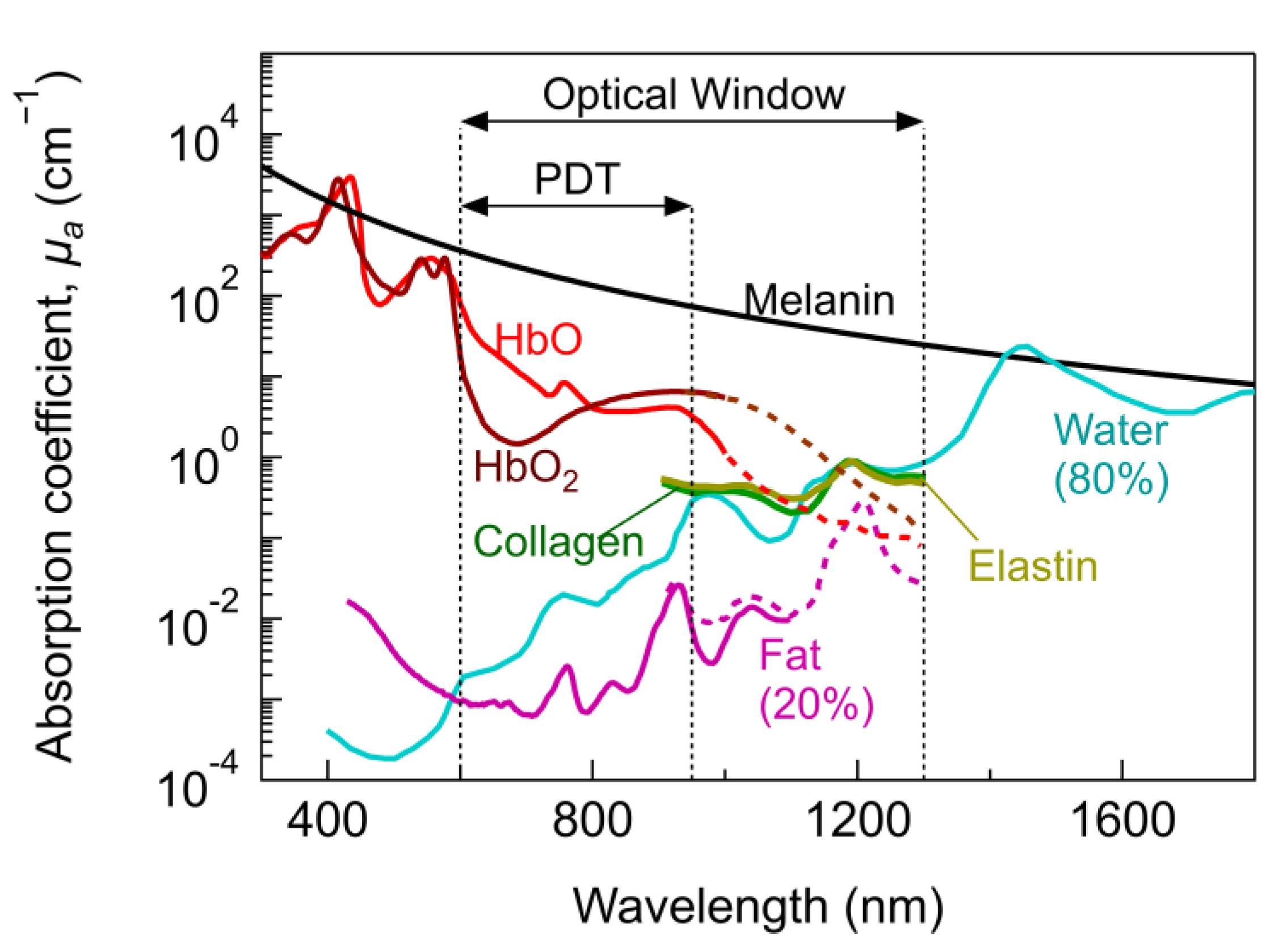


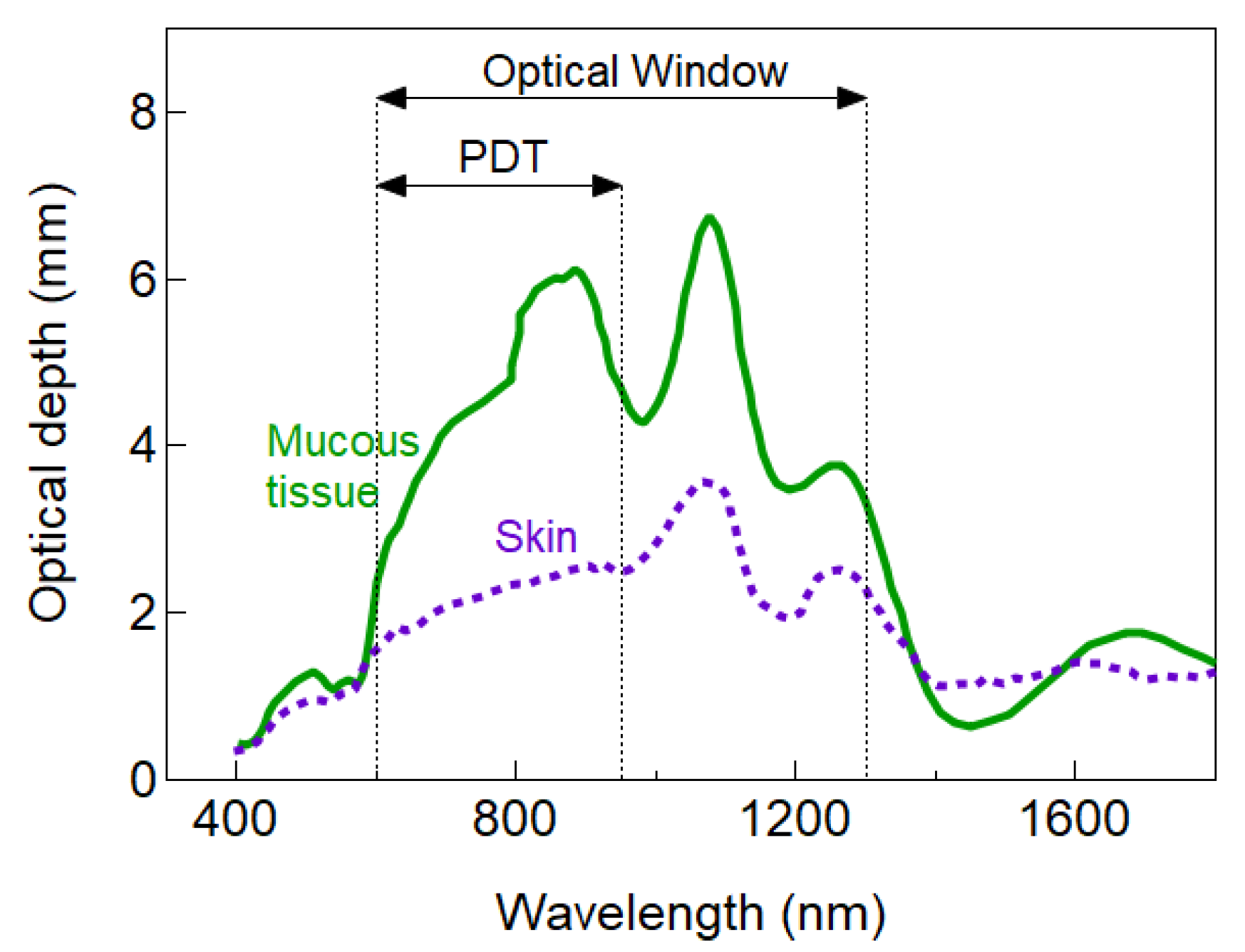
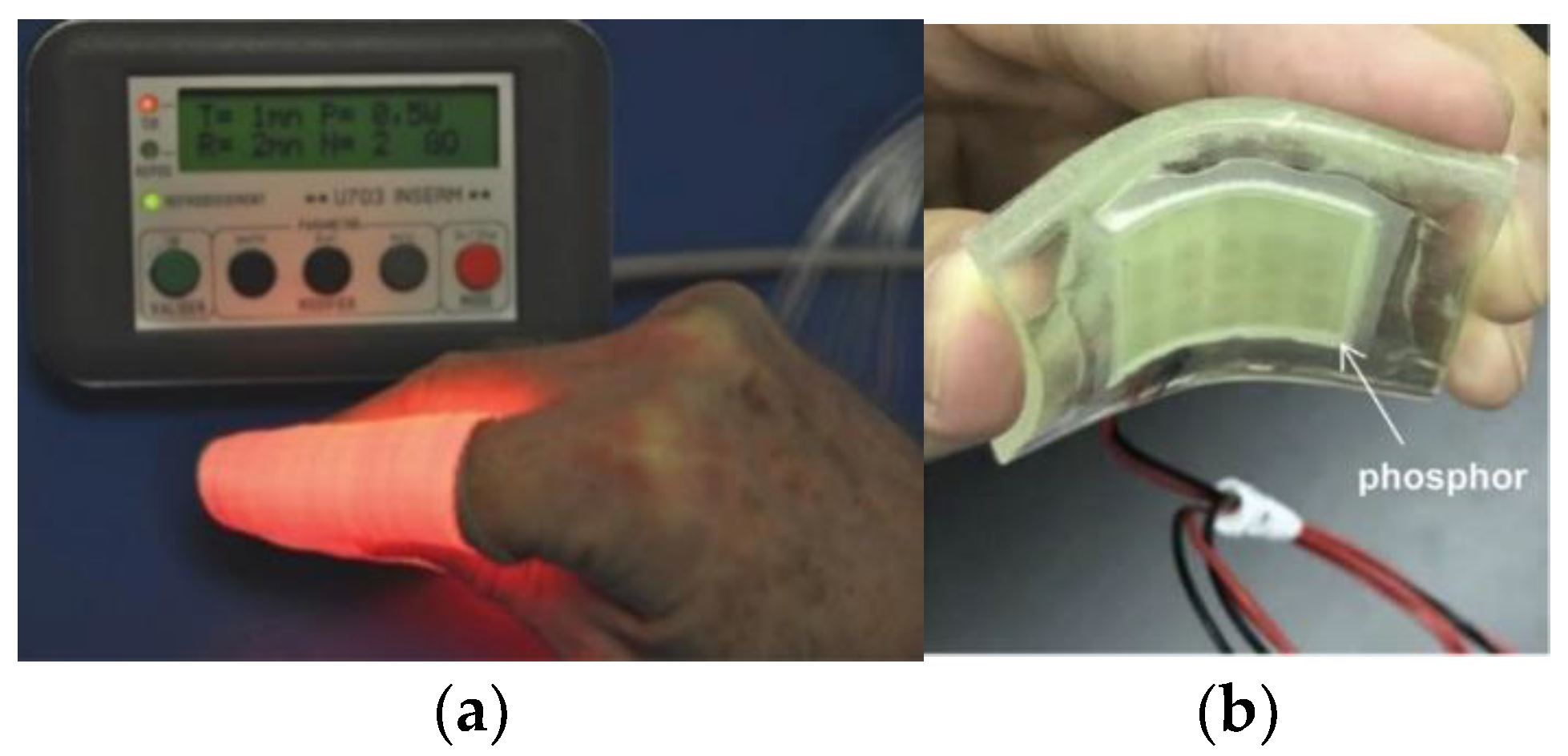
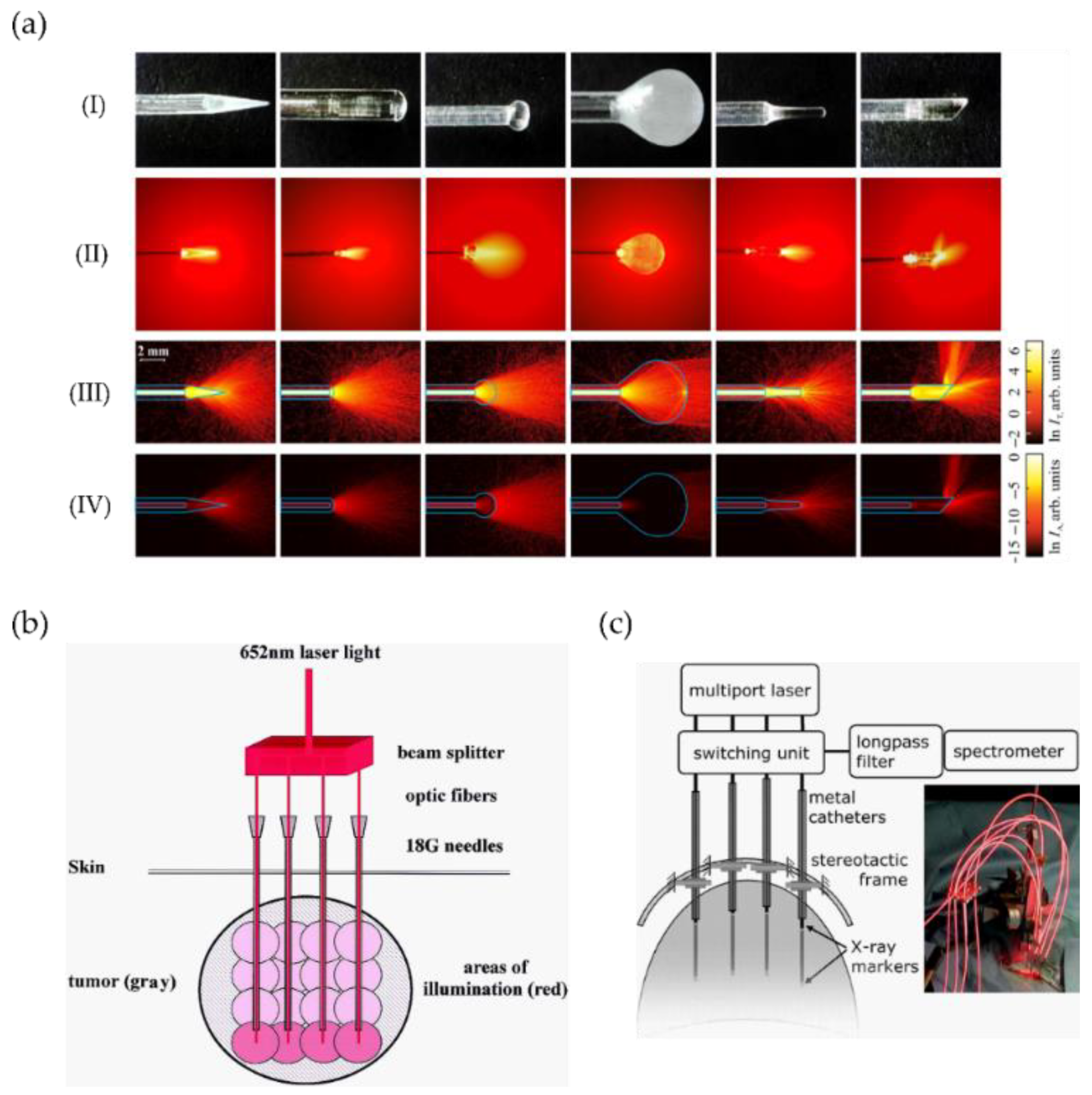




| Light Source | Main Benefits | Limitations |
|---|---|---|
| Laser (coherent) | <0.1 nm spectral bandwidth High power Efficient coupling to optical fibres Uniform irradiance can be easily achieved Adaptive emission (VCSEL, Edge-emitting laser) Faster modulation than LEDs Possibility for ultra-short pulses (fs-regime) | Expensive High maintenance Bulkier than an LED Less choice of wavelengths |
| LED (non-coherent) | Low cost Small Adaptive emission (SLED, ELED) Used for whole-body or point treatment LEDs can fit down biopsy channels permitting deep-seated PDT | 5–10 nm spectral bandwidth (FWHM) Large beam divergence Thermal effects for I-PDT (low electro-optical conversion efficiency) |
| Lamp (non-coherent) | Low cost Simple design Wide illumination field Multi-wavelength irradiance | UV and NIR radiation (optical filtering is needed) Large beam divergence High coupling losses with light guides |
| Pulsed Light Source | Parameters | CW Light Source | Conclusions | |
|---|---|---|---|---|
| [102,103] | Long-pulsed dye laser |
| NA |
|
| [101] | Pulsed Dye Laser Broadband flashlamp filtered pulsed light |
|
|
|
| [94] | Nd:YAG laser pumped optical parametric oscillator (OPO) |
|
|
|
| [100] | Nd:YAG laser-pumped OPO |
|
|
|
| [95] | Nd:YAG + OPO system |
|
|
|
| [96] | Ti:sapphire + optical parametric amplifier |
|
|
|
| [99] | Diode pumped solid state yellow laser |
|
|
|
| [98] | Semiconductor laser |
|
|
|
| Excitation (NIR) | Emission (nm) | Ref. | Laser Diodes (max. Power) |
|---|---|---|---|
| 980 nm | 345, 360, 450, 475 | [154,155,156,157] | L980P010 (10 mW) |
| 450, 475 | [158] | LP980-SF15 (15 mW) | |
| 540 | [159,160] | L980P030 (30 mW) | |
| 520, 545, 660 | [161] | L980P100A (100 mW) | |
| 409, 541, 656 | [162] | L980P200 (200 mW) | |
| 660 | [162,163,164,165,166,167,168,169] | C3-980-0500-S50 (500 mW) | |
| 540, 660 | [170,171,172] | WSLD-980-001-2 (1 W) | |
| 975 nm | 340, 360, 445, 475 620 660 | [173] [174] | 0975L-14A-NI-PT-NF (70 mW) |
| RLTMDL-975-100 (100 mW) | |||
| RLTMDL-975R-300 (300 mW) | |||
| PL980P330J (330 mW) | |||
| RLTMDL-975-500 (500 mW) | |||
| RLTMDL-975-1W (1 W) | |||
| 808 nm | 345, 360, 450, 475 350, 450 540 540, 660 543, 654 660 | [175] [156] [176] [175] [177] [178] | L808P010 (10 mW) |
| L808P030 (30 mW) | |||
| DBR808PN (42 mW) | |||
| LP808-SA60 (60 mW) | |||
| M9-808-0150 (150 mW) | |||
| L808P200 (200 mW) | |||
| FPL808S (250 mW) | |||
| LD808-SE500g (500 mW) | |||
| L808P1000MM (1 W) |
| Excitation (X-ray Dose) | Emission (nm) | X-ray Scintillator (Size) | Ref. |
|---|---|---|---|
| 6 MeV, 30 keV 1–6 Gy | 340 | CeF3 (9 nm) | [188] |
| 50 keV 1–10 Gy | 520 | SrAl2O4:Eu2+ (407 nm) | [189] |
| 90 keV, 3 Gy | 520 | LaF3:Ce3+ (2 µm) | [190] |
| 75 keV | 544 | LaF3:Tb (40 nm) | [191] |
| 75 keV | 540 | LaF3:Tb silica coated (45 nm) | [192] |
| 6 MeV, 0.4–2 Gy | 545 | SiC/SiOx core/shell nanowires (40 nm) | [193] |
| 80 keV | 540 | LaF3:Tb (25 nm) | [194] |
| 44 keV, 11 Gy | 540 | Tb2O3 coated polysiloxane (10 nm) | [195] |
| 15 keV | 595 | GdEuC12 (4.6 nm) | [196] |
| 225 keV, 2 Gy | 500 | HfnMOL (1.2 nm) | [197] |
| 120 keV, 2 Gy | 510 | ZnS:Cu,co (4 nm) | [198] |
| 220 keV, 8 Gy | 305 | LiYF4:Ce (35 nm) | [199] |
| 50 keV, 5Gy | 720 | LiGa5O8:Cr (100 nm) | [200] |
| 160 keV, 5 Gy | 543 | NaLuF4:Gd,Eu (25 nm) | [201] |
| 1.48 keV | 300–450 | Y2.99Pr0.01Al5O12@SiO2 (75 nm) | [202] |
| Light Source | Emission | Implant Size | Encapsulation | External Source (Activation/Charging) | PS |
|---|---|---|---|---|---|
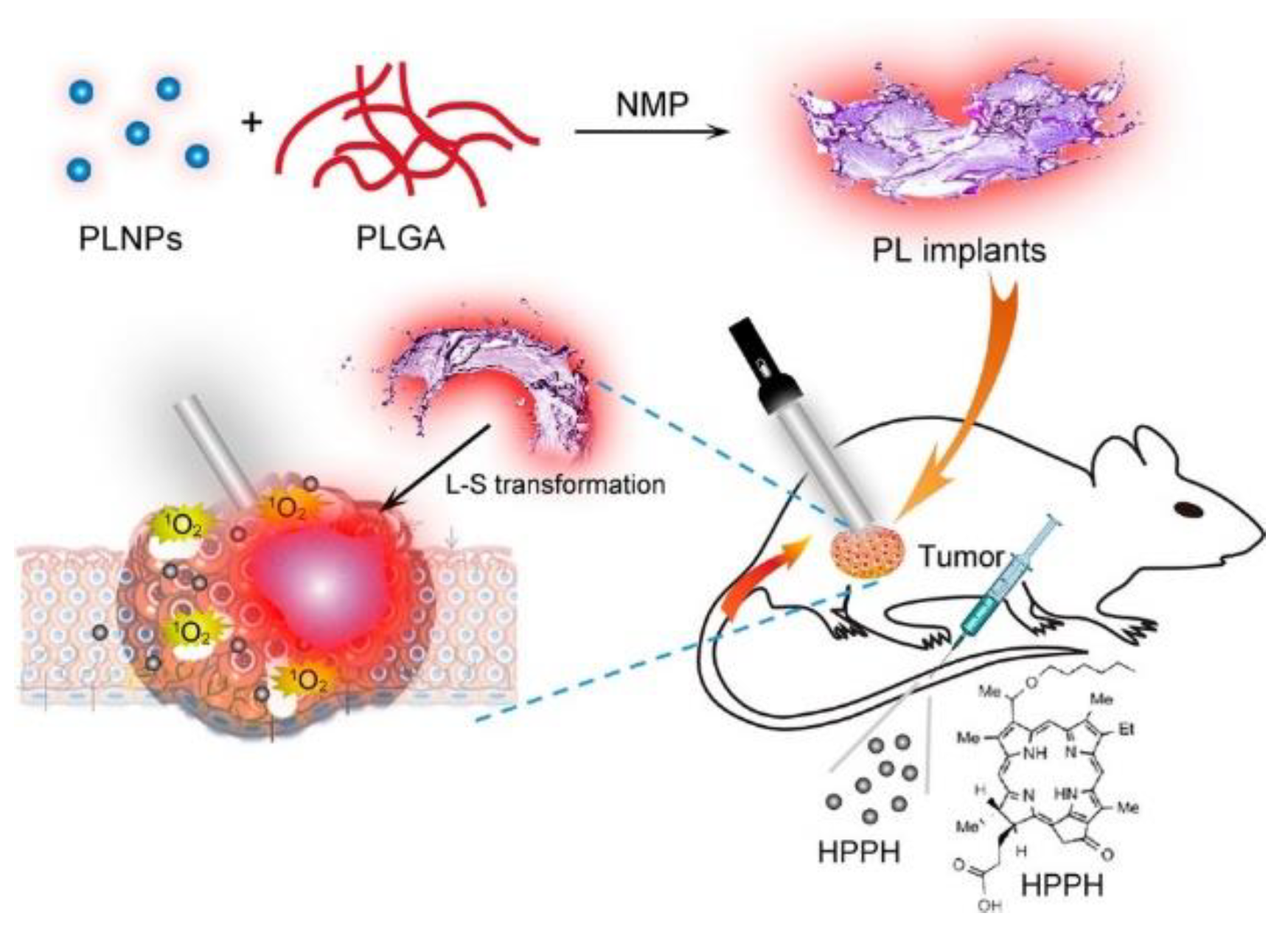
| (ZGC) PLNPs [236] | 695 nm Hours of emission | - | PLGA/NMP oleosol | LED: 400–750 nm for 2 or 5 min | HPPH, Photoclor Abs. peaks: 385 nm, 666 nm Emission: 670 nm |
| GPM (+PS) [237] | 520 nm >2 h emission | Various shapes and sizes (mm-range) | PDMS | Laser: 980 nm 2 W/cm2 for 5 s | Rose Bengal Abs. peak: 559 nm Emission: 571.5 nm |
| UCNPs [238] | 635 nm | 3 cm | PEGDA+FEP | Laser: 980 nm 1109 mW/cm2 for 5–10 min | 5-ALA Abs. Peaks: 405 nm, 630 nm |
| LEDs [239] | 660 and 440 nm | 15 mm3 | medical-grade silicone | RF (1 and 1.5 GHz) for 30 min | Ce6 Abs. Peaks: 400 nm, 663 nm Emission at 667 nm |
| LEDs [240] | 630, 530, 460 nm | LED chip: 7.0 × 11 × 0.8 mm3 +PDMS+PDA: ~650 nm-thick | PMDS+PDA (adhesive) | Near-field communication (13.56 MHz) for 10 days | Photofrin-saline & 5-ALA |
| LEDs [242] | 640, 470 nm | 2 × 2 × 2 mm3 2 × 4 × 2 mm3 +surface mounted LEDs | 5 μm of parylene-C | Ultrasonic 185 mW/cm2 at 720 kHz for 30 min | Verteporfin Abs. peak: 415, 580, 680 nm Emission: 690 nm |
| Deep PDT Modality | Source | Main Potential and Benefits | Main Challenges and Limitations |
|---|---|---|---|
| NIR radiation | TPA (ps-fs lasers) |
|
|
| NIR radiation | TPA, CARS, FWM, SHG. (ps-fs lasers) |
|
|
| NIR radiation | UCNPs |
|
|
| Ionising radiation | X-rays |
|
|
| Ionising radiation | Cherenkov |
|
|
| CRET, BRET | FireFly Renilla |
|
|
| Implants | - |
|
|
| Implants (NIR) | PLNPs |
|
|
| Implants (NIR) | GPM (+PS+upconversion materials) |
|
|
| Implants (NIR) | UCNPs |
|
|
| Implants (RF-NFC) | LEDs |
|
|
| Implants (US) | LEDs |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers 2021, 13, 3484. https://doi.org/10.3390/cancers13143484
Algorri JF, Ochoa M, Roldán-Varona P, Rodríguez-Cobo L, López-Higuera JM. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers. 2021; 13(14):3484. https://doi.org/10.3390/cancers13143484
Chicago/Turabian StyleAlgorri, José Francisco, Mario Ochoa, Pablo Roldán-Varona, Luís Rodríguez-Cobo, and José Miguel López-Higuera. 2021. "Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review" Cancers 13, no. 14: 3484. https://doi.org/10.3390/cancers13143484
APA StyleAlgorri, J. F., Ochoa, M., Roldán-Varona, P., Rodríguez-Cobo, L., & López-Higuera, J. M. (2021). Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers, 13(14), 3484. https://doi.org/10.3390/cancers13143484







