The Origin of Stroma Influences the Biological Characteristics of Oral Squamous Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Gingival Stromal Cells and Periodontal Ligament Stromal Cells
2.2. OSCC Cell Lines
2.3. Tumor Morphology
2.4. Tumor Proliferation Assay in 2D Co-Culture (MTS Assay)
2.5. Tumor Proliferation Assay in 2D Co-Culture (Ki-67 Labeling Index)
2.6. Invasion Assay and Migration Assay
2.7. Double-Fluorescence Immunohistochemical Staining
2.8. Experimental Animals
2.9. Hematoxylin-Eosin Staining (HE Staining)
2.10. Cell Proliferation Assay (Ki-67 Labeling Index)
2.11. Tartrate-Resistant Acid Phosphatase Staining (TRAP Staining)
2.12. Microarray and Bioinformatics Analyses
2.13. Statistical Analysis
3. Results
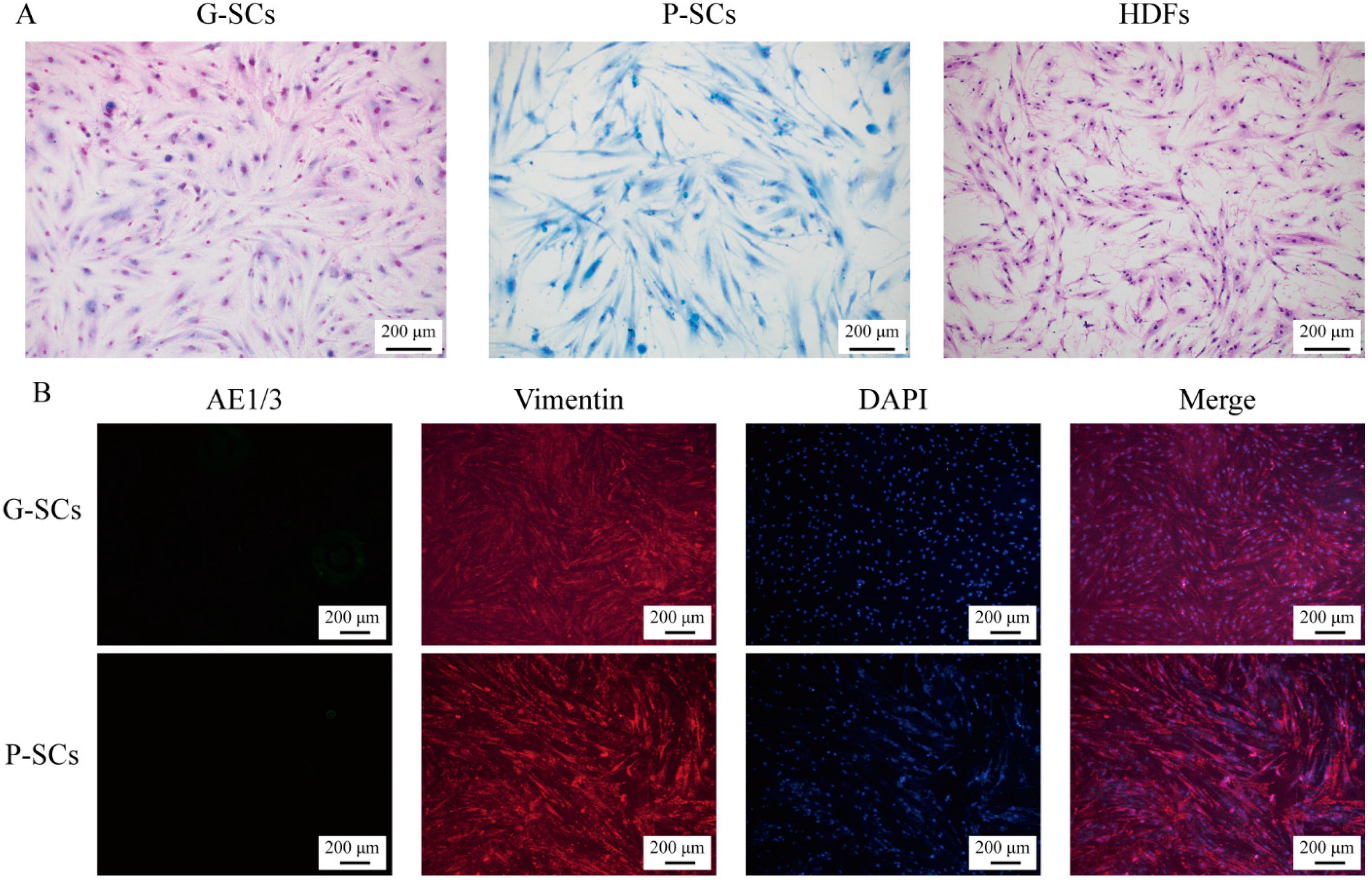
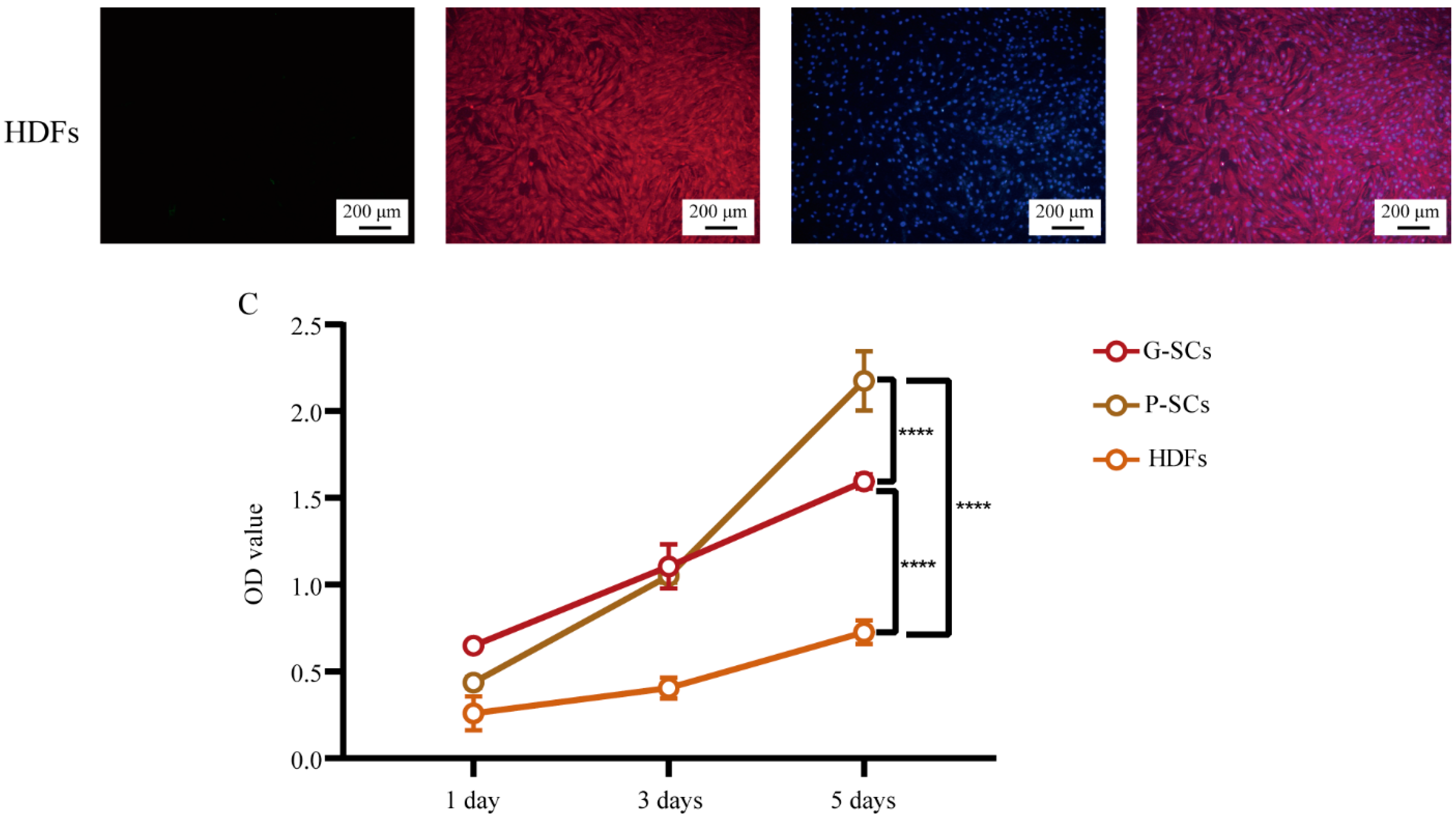
3.1. Morphology and Proliferation of Normal Stromal Cells (G-SCs, P-SCs, and HDFs)
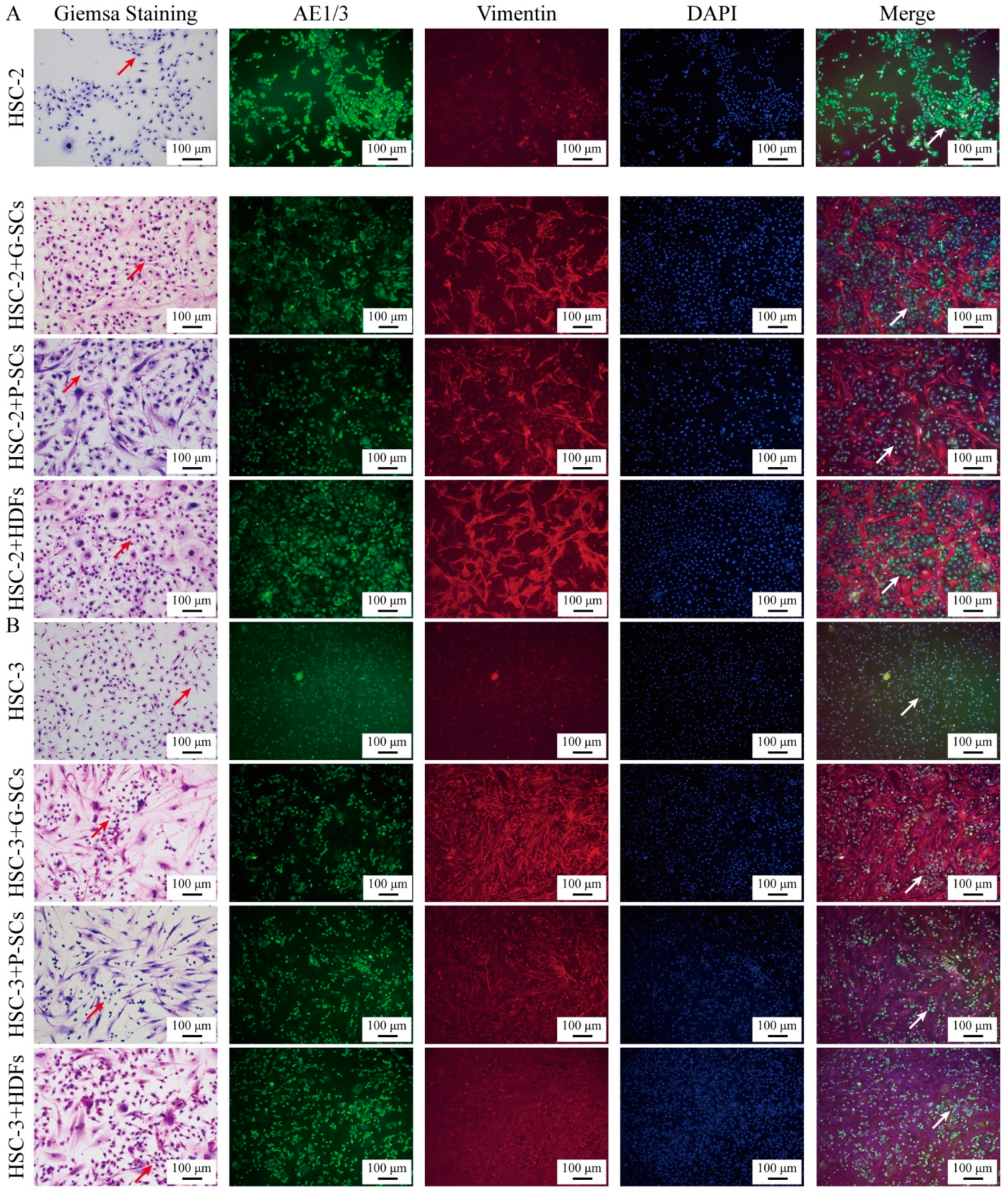
3.2. G-SCs and P-SCs Influenced the Differentiation of OSCC Cells In Vitro
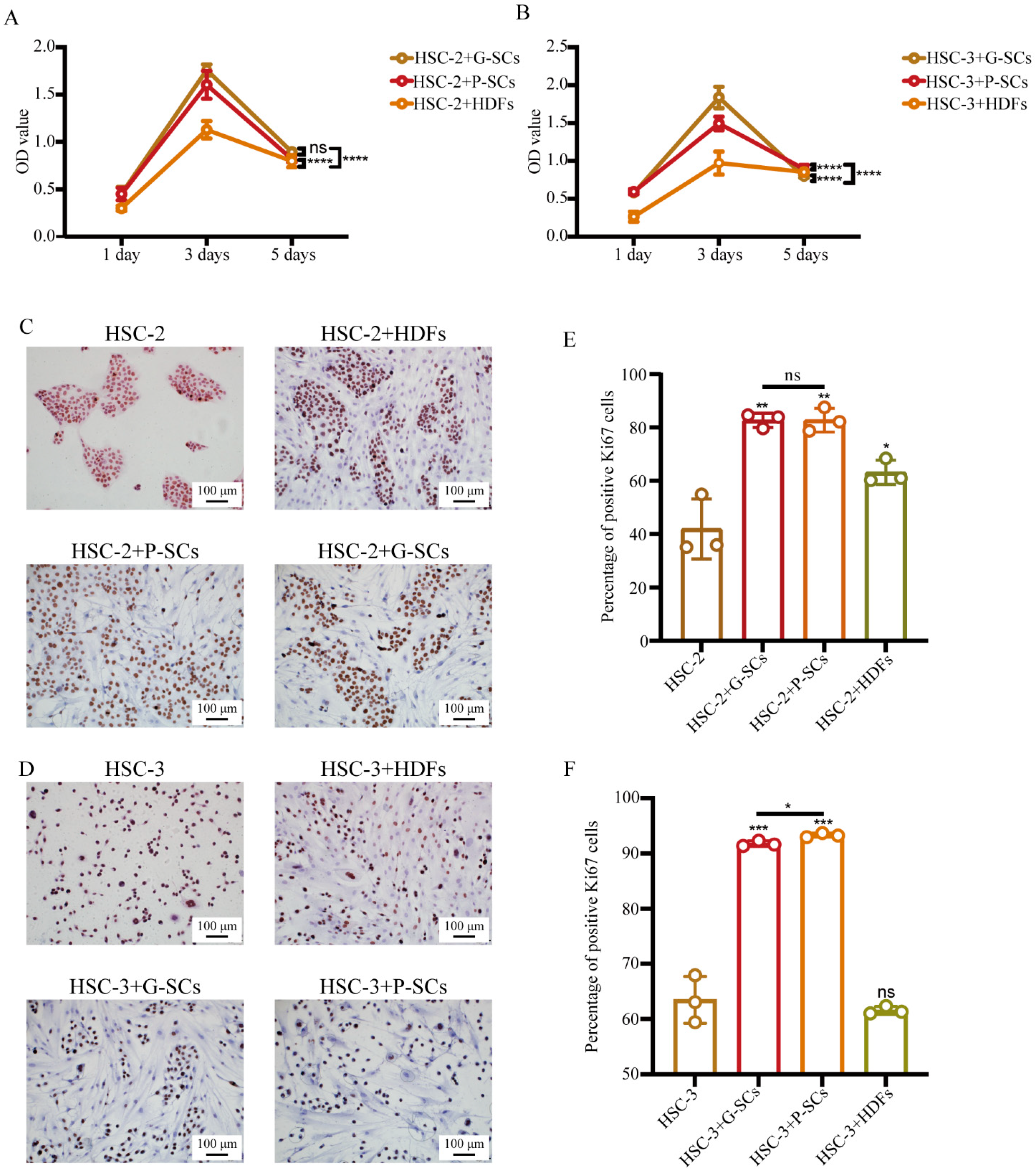
3.3. G-SCs and P-SCs Promoted the Proliferation of OSCC Cells In Vitro
3.4. G-SCs and P-SCs Promoted the Invasion of OSCC Cells In Vitro
3.5. G-SCs and P-SCs Promoted the Migration of OSCC Cells In Vitro
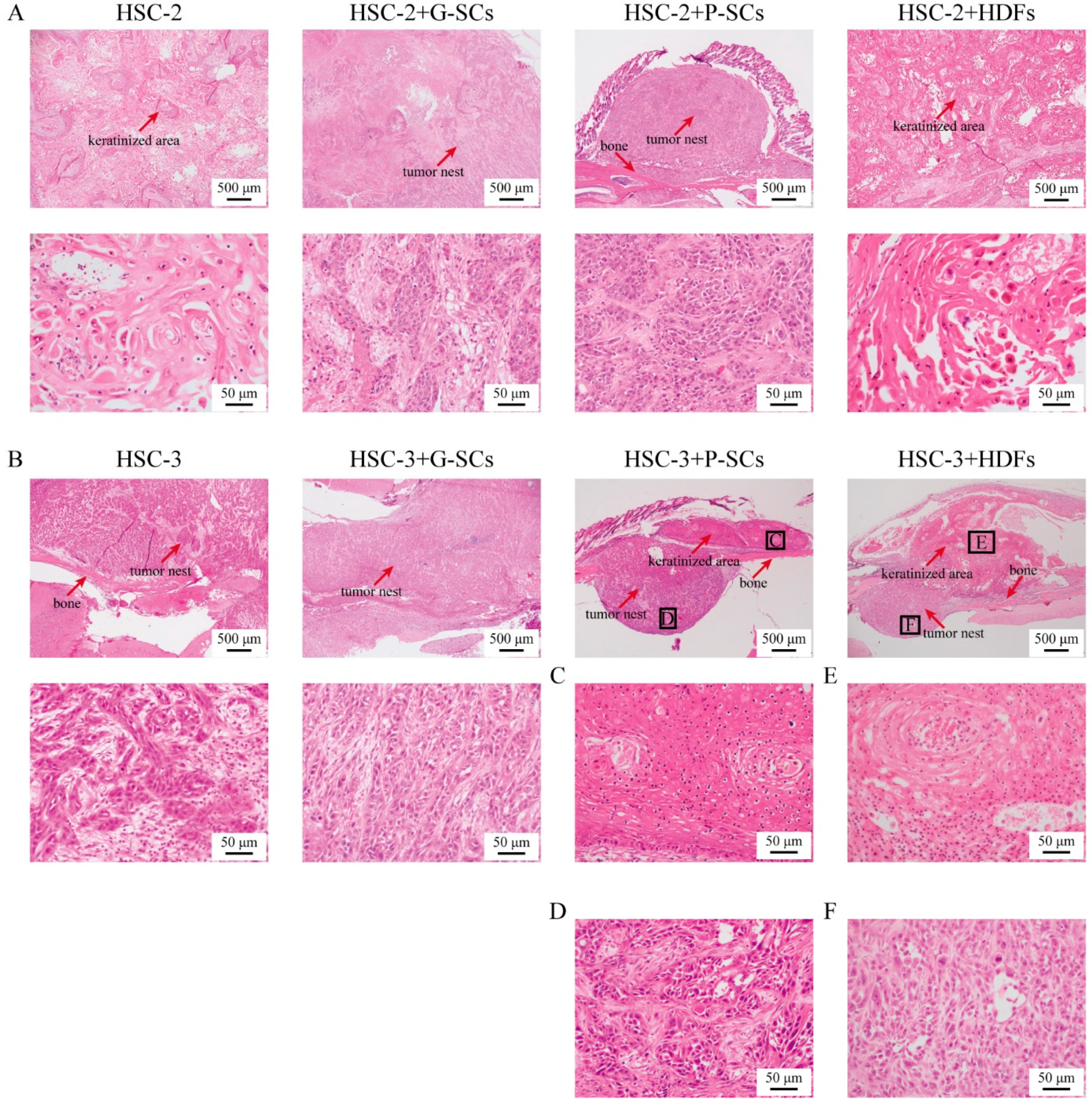
3.6. G-SCs and P-SCs Influence the Histological Differentiation of OSCC In Vivo
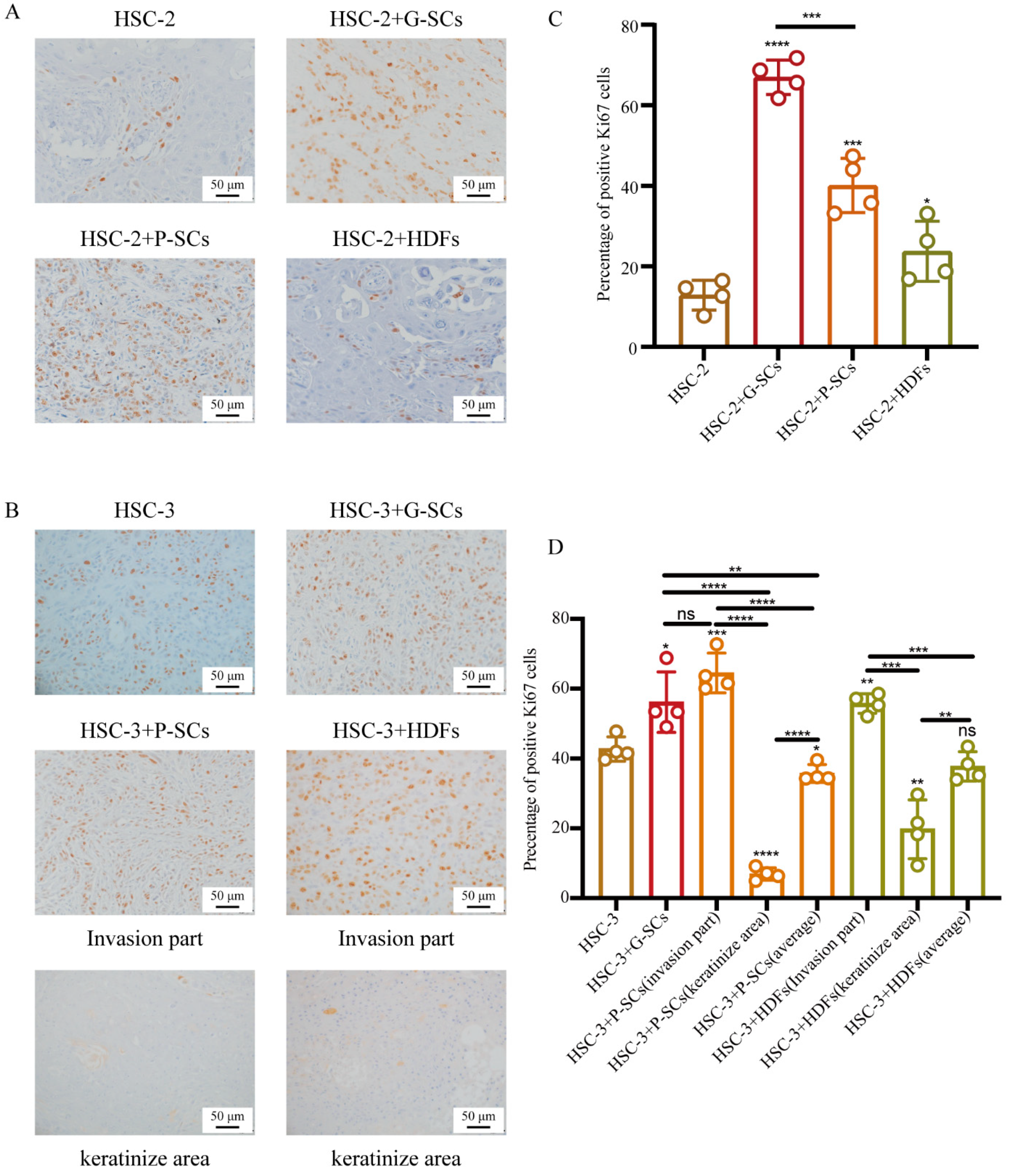
3.7. G-SCs and P-SCs Influenced the Proliferation of OSCC In Vivo
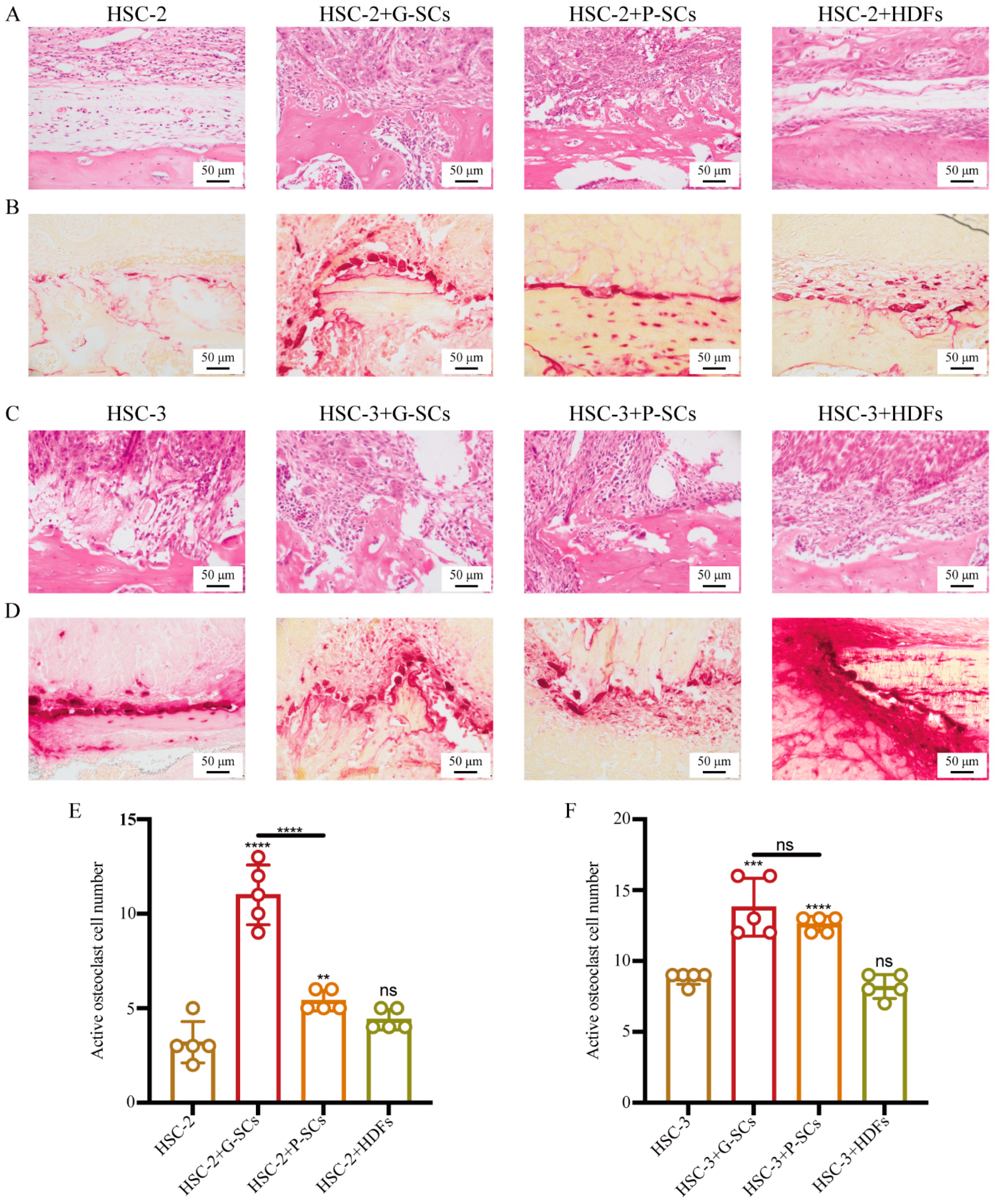
3.8. G-SCs and P-SCs Promoted Bone Invasion by OSCC In Vivo
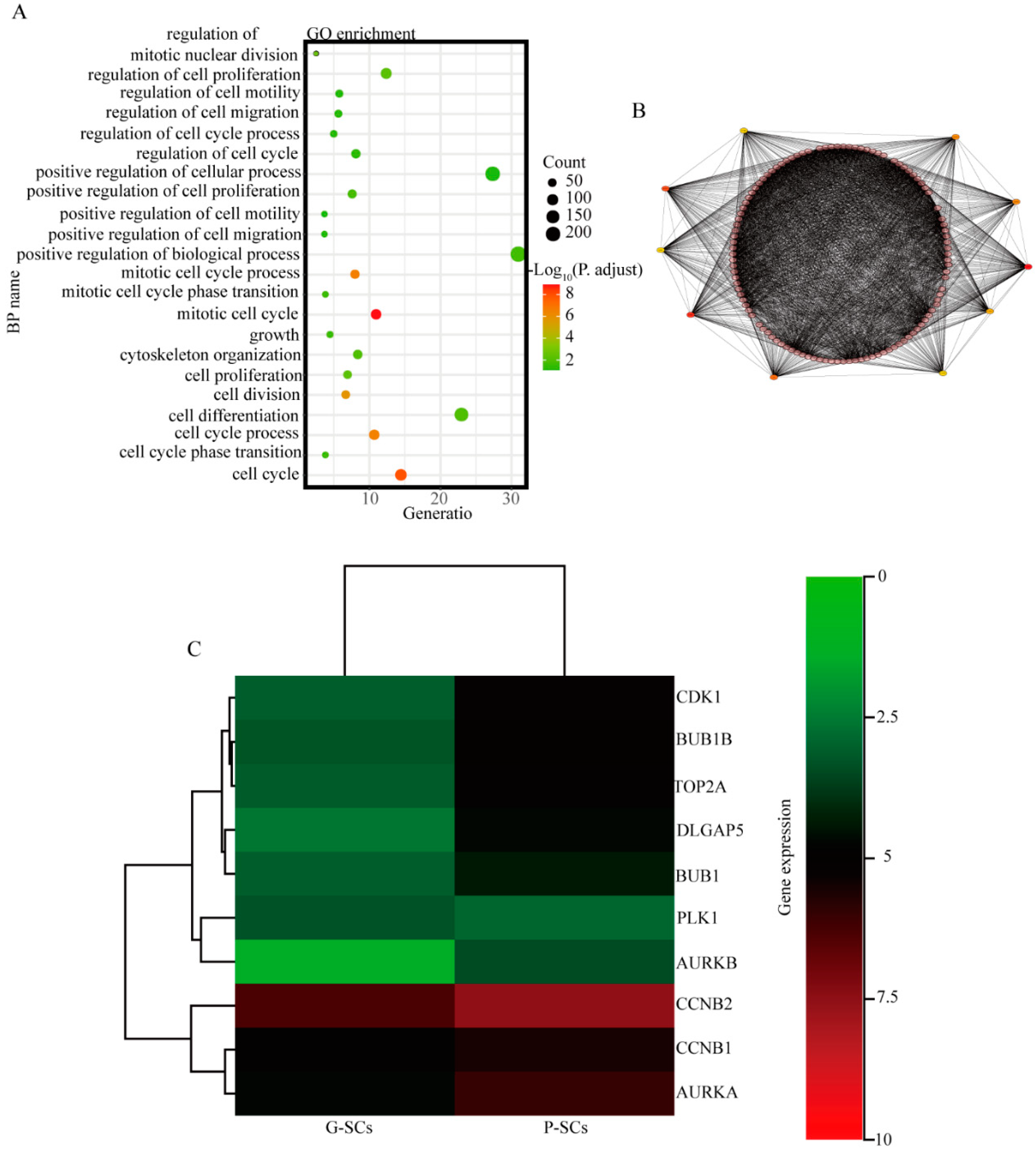
3.9. CDK1, BUB1B, TOP2A, DLGAP5, BUB1, and CCNB2 Have Great Potential to Cause the Different Effects of G-SCs and P-SCs, Hence Resulting in the Progression of OSCC
4. Discussion
4.1. Effect of G-SCs and P-SCs for OSCC Comparing to HDF
4.2. Effect of the Stromal Cells on the Proliferative, Invasive, and Migration Abilities of OSCC
4.3. Effect of the Stromal Cells on the Morphology and Differentiation Degree of OSCC
4.4. Microarray Analysis of Stromal Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef]
- Wang, W.Q.; Liu, L.; Xu, H.X.; Luo, G.P.; Chen, T.; Wu, C.T.; Xu, Y.F.; Xu, J.; Liu, C.; Zhang, B.; et al. Intratumoral α-SMA enhances the prognostic potency of CD34 associated with maintenance of microvessel integrity in hepatocellular carcinoma and pancreatic cancer. PLoS ONE 2013, 8, e71189. [Google Scholar]
- Chen, Y.; Zou, L.; Zhang, Y.; Chen, Y.; Xing, P.; Yang, W.; Li, F.; Ji, X.; Liu, F.; Lu, X. Transforming growth factor-β1 and α-smooth muscle actin in stromal fibroblasts are associated with a poor prognosis in patients with clinical stage I-IIIA nonsmall cell lung cancer after curative resection. Tumour Biol. 2014, 35, 6707–6713. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Noma, K.; Ohara, T.; Kashima, H.; Katsura, Y.; Sato, H.; Komoto, S.; Katsube, R.; Ninomiya, T.; Tazawa, H.; et al. Cancer-associated fibroblasts affect intratumoral CD8+ and FoxP3+ T cells via IL6 in the tumor microenvironment. Clin. Cancer Res. 2018, 24, 4820–4833. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Neto, H.H.; Gleber-Netto, F.O.; de Sousa, S.F.; França, C.M.; Aguiar, M.C.; Silva, T.A.; Batista, A.C. A comparative study of microvessel density in squamous cell carcinoma of the oral cavity and lip. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Zhou, H.; Liu, X.; He, Y.; Tang, Y.; Zhu, G.; Zheng, M.; Yang, J. Effect of local hyperthermia on lymphangiogenic factors VEGF-C and -D in a nude mouse xenograft model of tongue squamous cell carcinoma. Oral Oncol. 2010, 46, 111–115. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [Green Version]
- Krutchkoff, D.J.; Chen, J.K.; Eisenberg, E.; Katz, R.V. Oral cancer: A survey of 566 cases from the University of Connecticut Oral Pathology Biopsy Service, 1975–1986. Oral Surg. Oral Med. Oral Pathol. 1990, 70, 192–198. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Montero, P.H.; Migliacci, J.C.; Shah, J.P.; Wong, R.J.; Ganly, I.; Patel, S.G. Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol. 2019, 90, 115–121. [Google Scholar] [CrossRef]
- Takabatake, K.; Kawai, H.; Omori, H.; Qiusheng, S.; Oo, M.W.; Sukegawa, S.; Nakano, K.; Tsujigiwa, H.; Nagatsuka, H. Impact of the stroma on the biological characteristics of the parenchyma in oral squamous cell carcinoma. Int. J. Mol. Sci. 2020, 21, 7714. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Ostman, A.; Augsten, M. Cancer-associated fibroblasts and tumor growth—Bystanders turning into key players. Curr. Opin. Genet. Dev. 2009, 19, 67–73. [Google Scholar] [CrossRef]
- Jing, H.; Torphy, R.; Steiger, K.; Hongo, H.; Ritchie, A.J.; Kriegsmann, M.; Horst, D.; Umtsu, S.E.; Joseph, N.M.; McGregor, K.; et al. Pancreatic ductal adenocarcinoma progression is restrained by stromal matrix. J. Clin. Investig. 2020, 130, 4704–4709. [Google Scholar] [CrossRef]
- Kwa, R.E.; Campana, K.; Moy, R.L. Biology of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 1992, 26, 1–26. [Google Scholar] [CrossRef]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Brantsch, K.D.; Meisner, C.; Schönfisch, B.; Trilling, B.; Wehner-Caroli, J.; Röcken, M.; Breuninger, H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: A prospective study. Lancet Oncol. 2008, 9, 713–720. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Gil, Z.; Amit, M.; Yen, T.C.; Liao, C.T.; Chaturvedi, P.; Agarwal, J.P.; Kowalski, L.P.; Köhler, H.F.; Kreppel, M.; et al. International consortium for outcome research (ICOR) in head and neck cancer. Depth of invasion alone as an indication for postoperative radiotherapy in small oral squamous cell carcinomas: An international collaborative study. Head Neck 2019, 41, 1935–1942. [Google Scholar] [CrossRef]
- Pantel, K.; Cote, R.J.; Fodstad, O. Detection and clinical importance of micrometastatic disease. J. Natl. Cancer Inst. 1999, 91, 1113–1124. [Google Scholar] [CrossRef]
- Fidler, I.J.; Yano, S.; Zhang, R.D.; Fujimaki, T.; Bucana, C.D. The seed and soil hypothesis: Vascularisation and brain metastases. Lancet Oncol. 2002, 3, 53–57. [Google Scholar] [CrossRef]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papac, R.J. Distant metastases from head and neck cancer. Cancer 1984, 53, 342–345. [Google Scholar] [CrossRef]
- Probert, J.C.; Thompson, R.W.; Bagshaw, M.A. Patterns of spread of distant metastases in head and neck cancer. Cancer 1974, 33, 127–133. [Google Scholar] [CrossRef]
- Coffman, L.G.; Pearson, A.T.; Frisbie, L.G.; Freeman, Z.; Christie, E.; Bowtell, D.D.; Buckanovich, R.J. Ovarian carcinoma-associated mesenchymal stem cells arise from tissue-specific normal stroma. Stem Cells 2019, 37, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Spaeth, E.L.; Dembinski, J.L.; Sasser, A.K.; Watson, K.; Klopp, A.; Hall, B.; Andreeff, M.; Marini, F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE 2009, 4, e4992. [Google Scholar] [CrossRef] [Green Version]
- Guan, J.; Chen, J. Mesenchymal stem cells in the tumor microenvironment. Biomed. Rep. 2013, 1, 517–521. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Sun, R.; Origuchi, M.; Kanehira, M.; Takahata, T.; Itoh, J.; Umezawa, A.; Kijima, H.; Fukuda, S.; Saijo, Y. Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol. Med. 2011, 17, 579–587. [Google Scholar] [CrossRef]
- Han, Z.; Tian, Z.; Lv, G.; Zhang, L.; Jiang, G.; Sun, K.; Wang, C.; Bu, X.; Li, R.; Shi, Y.; et al. Immunosuppressive effect of bone marrow-derived mesenchymal stem cells in inflammatory microenvironment favours the growth of B16 melanoma cells. J. Cell. Mol. Med. 2011, 15, 2343–2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halpern, J.L.; Kilbarger, A.; Lynch, C.C. Mesenchymal stem cells promote mammary cancer cell migration in vitro via the CXCR2 receptor. Cancer Lett. 2011, 308, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Ma, J.; Fan, Y.; Wang, Z.; Tian, R.; Ji, W.; Zhang, F.; Niu, R. TGF-β transactivates EGFR and facilitates breast cancer migration and invasion through canonical Smad3 and ERK/Sp1 signaling pathways. Mol. Oncol. 2018, 12, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.L.; Leung, C.S.; Wong, K.K.; Samimi, G.; Thompson, M.S.; Liu, J.; Zaid, T.M.; Ghosh, S.; Birrer, M.J.; Mok, S.C. TGF-β modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res. 2013, 73, 5016–5028. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Liu, J.; Xue, H.; Li, C.; Liu, Q.; Zhou, Y.; Wang, T.; Wang, H.; Qian, H.; Wen, T. A TGF-β-MTA1-SOX4-EZH2 signaling axis drives epithelial-mesenchymal transition in tumor metastasis. Oncogene 2020, 39, 2125–2139. [Google Scholar] [CrossRef] [PubMed]
- Strand, D.W.; Liang, Y.Y.; Yang, F.; Barron, D.A.; Ressler, S.J.; Schauer, I.G.; Feng, X.H.; Rowley, D.R. TGF-β induction of FGF-2 expression in stromal cells requires integrated smad3 and MAPK pathways. Am. J. Clin. Exp. Urol. 2014, 2, 239–248. [Google Scholar]
- Akatsu, Y.; Takahashi, N.; Yoshimatsu, Y.; Kimuro, S.; Muramatsu, T.; Katsura, A.; Maishi, N.; Suzuki, H.I.; Inazawa, J.; Hida, K.; et al. Fibroblast growth factor signals regulate transforming growth factor-β-induced endothelial-to-myofibroblast transition of tumor endothelial cells via Elk1. Mol. Oncol. 2019, 13, 1706–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikpegbu, E.; Basta, L.; Clements, D.N.; Fleming, R.; Vincent, T.L.; Buttle, D.J.; Pitsillides, A.A.; Staines, K.A.; Farquharson, C. FGF-2 promotes osteocyte differentiation through increased E11/podoplanin expression. J. Cell. Physiol. 2018, 233, 5334–5347. [Google Scholar] [CrossRef] [Green Version]
- Tsurushima, H.; Marushima, A.; Suzuki, K.; Oyane, A.; Sogo, Y.; Nakamura, K.; Matsumura, A.; Ito, A. Enhanced bone formation using hydroxyapatite ceramic coated with fibroblast growth factor-2. Acta Biomater. 2010, 6, 2751–2759. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, P.C.; Jin, Y.T.; Chang, C.W.; Huang, C.C.; Liao, S.C.; Yuan, K. Elastin in oral connective tissue modulates the keratinization of overlying epithelium. J. Clin. Periodontol. 2010, 37, 705–711. [Google Scholar] [CrossRef]
- Nanci, A. The Periodontium. In Ten Cate’s Oral Histology: Development, Structure, and Function; Nanci, A., Wurm-Cutter, E., Eds.; Elsevier: St. Louis, MO, USA, 2018; pp. 193–217. [Google Scholar]
- Green, H.; Easley, K.; Iuchi, S. Marker succession during the development of keratinocytes from cultured human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2003, 100, 15625–15630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, M.; Kiuru, M.; Cairo, M.S.; Christiano, A.M. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, 8797–8802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanovski, S.; Li, H.; Haase, H.R.; Bartold, P.M. Expression of bone associated macromolecules by gingival and periodontal ligament fibroblasts. J. Periodontal Res. 2001, 36, 131–141. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef]
- Garg, P.; Mazur, M.M.; Buck, A.C.; Wandtke, M.E.; Liu, J.; Ebraheim, N.A. Prospective review of mesenchymal stem cells differentiation into osteoblasts. Orthop. Surg. 2017, 9, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Chen, Y.H.; Li, L.Y.; Lang, J.; Yeh, S.P.; Shi, B.; Yang, C.C.; Yang, J.Y.; Lin, C.Y.; Lai, C.C.; et al. CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat. Cell Biol. 2011, 13, 87–94. [Google Scholar] [CrossRef]
- Datta, N.S.; Pettway, G.J.; Chen, C.; Koh, A.J.; McCauley, L.K. Cyclin D1 as a target for the proliferative effects of PTH and PTHrP in early osteoblastic cells. J. Bone Miner. Res. 2007, 22, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.H.; Mu, L.J.; Wang, M.Y.; Zeng, J.; Long, Q.Z.; Guan, B.; Wang, W.; Jiang, Y.M.; Bai, X.J.; Du, Y.F. BUB1B promotes proliferation of prostate cancer via transcriptional regulation of MELK. Anticancer Agents Med. Chem. 2020, 20, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhang, S.; Wang, P.; Wang, H.; Sha, B.; Peng, H.; Ju, Z.; Rao, J.; Lu, L. BUB1B promotes hepatocellular carcinoma progression via activation of the mTORC1 signaling pathway. Cancer Med. 2020, 9, 8159–8172. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, H.; Yi, S.; Gu, L.; Zhou, M. Mutual regulation of MDM4 and TOP2A in cancer cell proliferation. Mol. Oncol. 2019, 13, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Xu, J.; Zhao, J.; Bai, J.H. Proliferation and invasion of colon cancer cells are suppressed by knockdown of TOP2A. J. Cell. Biochem. 2018, 119, 7256–7263. [Google Scholar] [CrossRef]
- Zheng, R.; Shi, Z.; Li, W.; Yu, J.; Wang, Y.; Zhou, Q. Identification and prognostic value of DLGAP5 in endometrial cancer. PeerJ 2020, 8, e10433. [Google Scholar] [CrossRef]
- Branchi, V.; García, S.A.; Radhakrishnan, P.; Győrffy, B.; Hissa, B.; Schneider, M.; Reißfelder, C.; Schölch, S. Prognostic value of DLGAP5 in colorectal cancer. Int. J. Colorectal Dis. 2019, 34, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pan, Y.; Fu, H.; Zhang, J. Nucleolar and spindle associated protein 1 (NUSAP1) inhibits cell proliferation and enhances susceptibility to epirubicin in invasive breast cancer cells by regulating Cyclin D Kinase (CDK1) and DLGAP5 expression. Med. Sci. Monit. 2018, 24, 8553–8564. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Zhu, L.; Sun, J.; Li, N.; Dong, B.; Yang, Y.; Chen, L. High expression of CDK1 and BUB1 predicts poor prognosis of pancreatic ductal adenocarcinoma. Gene 2019, 701, 15–22. [Google Scholar] [CrossRef]
- Fujibayashi, Y.; Isa, R.; Nishiyama, D.; Sakamoto-Inada, N.; Kawasumi, N.; Yamaguchi, J.; Kuwahara-Ota, S.; Matsumura-Kimoto, Y.; Tsukamoto, T.; Chinen, Y.; et al. Aberrant BUB1 overexpression promotes mitotic segregation errors and chromosomal instability in multiple myeloma. Cancers 2020, 12, 2206. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, H.; Wu, D.; Zhang, D.; Zhang, Z. miR-335–5p regulates cell cycle and metastasis in lung adenocarcinoma by targeting CCNB2. OncoTargets Ther. 2020, 13, 6255–6263. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Jiao, K.; Zhao, C.; Liu, H.; Meng, Q.; Wang, Z.; Feng, C.; Li, Y. Effect of miR-205 on proliferation and migration of thyroid cancer cells by targeting CCNB2 and the mechanism. Oncol. Lett. 2020, 19, 2568–2574. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omori, H.; Shan, Q.; Takabatake, K.; Nakano, K.; Kawai, H.; Sukegawa, S.; Tsujigiwa, H.; Nagatsuka, H. The Origin of Stroma Influences the Biological Characteristics of Oral Squamous Cell Carcinoma. Cancers 2021, 13, 3491. https://doi.org/10.3390/cancers13143491
Omori H, Shan Q, Takabatake K, Nakano K, Kawai H, Sukegawa S, Tsujigiwa H, Nagatsuka H. The Origin of Stroma Influences the Biological Characteristics of Oral Squamous Cell Carcinoma. Cancers. 2021; 13(14):3491. https://doi.org/10.3390/cancers13143491
Chicago/Turabian StyleOmori, Haruka, Qiusheng Shan, Kiyofumi Takabatake, Keisuke Nakano, Hotaka Kawai, Shintaro Sukegawa, Hidetsugu Tsujigiwa, and Hitoshi Nagatsuka. 2021. "The Origin of Stroma Influences the Biological Characteristics of Oral Squamous Cell Carcinoma" Cancers 13, no. 14: 3491. https://doi.org/10.3390/cancers13143491
APA StyleOmori, H., Shan, Q., Takabatake, K., Nakano, K., Kawai, H., Sukegawa, S., Tsujigiwa, H., & Nagatsuka, H. (2021). The Origin of Stroma Influences the Biological Characteristics of Oral Squamous Cell Carcinoma. Cancers, 13(14), 3491. https://doi.org/10.3390/cancers13143491





