Therapeutic Application of Brain-Specific Angiogenesis Inhibitor 1 for Cancer Therapy
Abstract
Simple Summary
Abstract
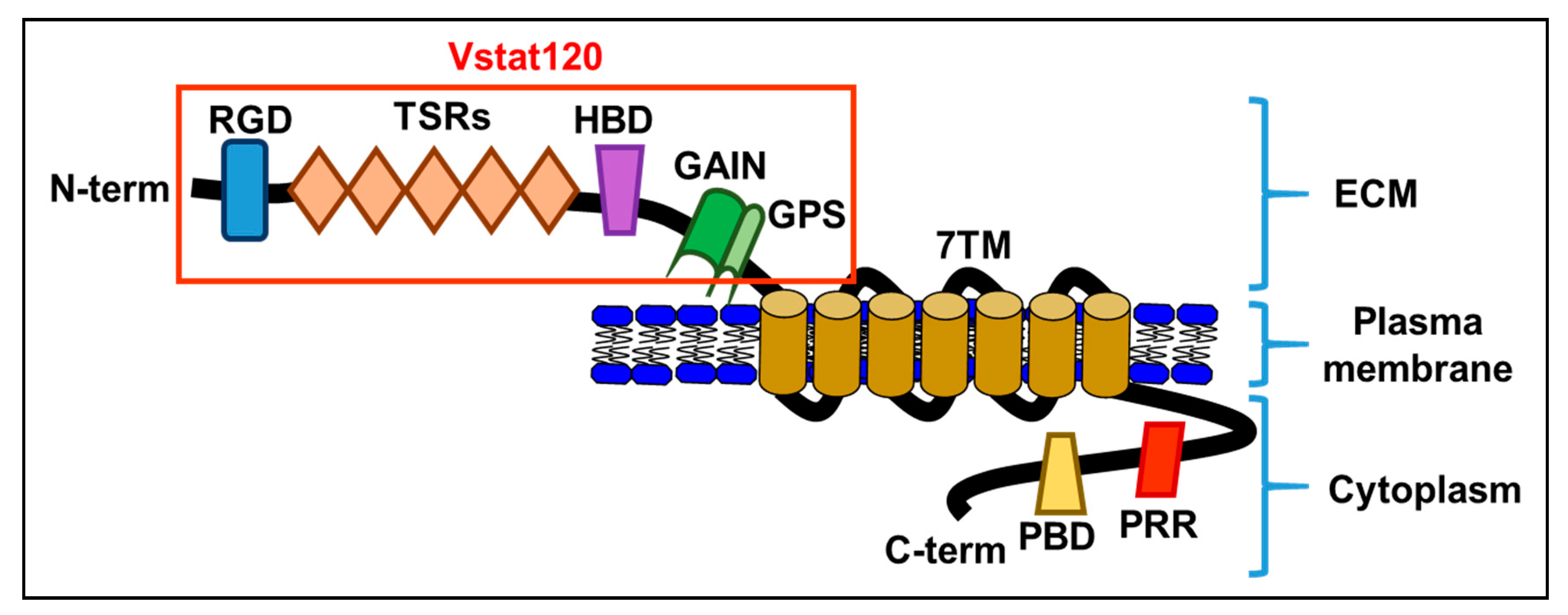
1. Structure of Brain-Specific Angiogenesis Inhibitor 1 (BAI1)
2. Functions of BAI1
2.1. Anti-Tumor and Anti-Angiogenic Activity
2.2. Engulfment
2.3. Myoblast Fusion
2.4. Synaptogenesis
3. Treatment Applications of BAI1
4. Application of Vstat120 in the Context of Oncolytic Virotherapy
5. Utilization of Vstat120 in Combination with Bevacizumab in Glioblastoma
6. Utilization of Oncolytic Virus Expressing Vstat120 in Other Tumor Types
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hatanaka, H.; Oshika, Y.; Abe, Y.; Yoshida, Y.; Hashimoto, T.; Handa, A.; Kijima, H.; Yamazaki, H.; Inoue, H.; Ueyama, Y.; et al. Vascularization is decreased in pulmonary adenocarcinoma expressing brain-specific angiogenesis inhibitor 1 (BAI1). Int. J. Mol. Med. 2000, 5, 181–183. [Google Scholar] [CrossRef]
- Kaur, B.; Brat, D.J.; Calkins, C.C.; Van Meir, E.G. Brain angiogenesis inhibitor 1 is differentially expressed in normal brain and glioblastoma independently of p53 expression. Am. J. Pathol. 2003, 162, 19–27. [Google Scholar] [CrossRef]
- Duda, D.G.; Sunamura, M.; Lozonschi, L.; Yokoyama, T.; Yatsuoka, T.; Motoi, F.; Horii, A.; Tani, K.; Asano, S.; Nakamura, Y.; et al. Overexpression of the p53-inducible brain-specific angiogenesis inhibitor 1 suppresses efficiently tumour angiogenesis. Br. J. Cancer 2002, 86, 490–496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kudo, S.; Konda, R.; Obara, W.; Kudo, D.; Tani, K.; Nakamura, Y.; Fujioka, T. Inhibition of tumor growth through suppression of angiogenesis by brain-specific angiogenesis inhibitor 1 gene transfer in murine renal cell carcinoma. Oncol. Rep. 2007, 18, 785–791. [Google Scholar] [CrossRef]
- Miyamoto, N.; Yamamoto, H.; Taniguchi, H.; Miyamoto, C.; Oki, M.; Adachi, Y.; Imai, K.; Shinomura, Y. Differential expression of angiogenesis-related genes in human gastric cancers with and those without high-frequency microsatellite instability. Cancer Lett. 2007, 254, 42–53. [Google Scholar] [CrossRef]
- Yoshida, Y.; Oshika, Y.; Fukushima, Y.; Tokunaga, T.; Hatanaka, H.; Kijima, H.; Yamazaki, H.; Ueyama, Y.; Tamaoki, N.; Miura, S.; et al. Expression of angiostatic factors in colorectal cancer. Int. J. Oncol. 1999, 15, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, Y.; Oshika, Y.; Tsuchida, T.; Tokunaga, T.; Hatanaka, H.; Kijima, H.; Yamazaki, H.; Ueyama, Y.; Tamaoki, N.; Nakamura, M. Brain-specific angiogenesis inhibitor 1 expression is inversely correlated with vascularity and distant metastasis of colorectal cancer. Int. J. Oncol. 1998, 13, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Koh, J.T.; Shin, B.A.; Ahn, K.Y.; Roh, J.H.; Kim, Y.J.; Kim, K.K. Comparative study of angiostatic and anti-invasive gene expressions as prognostic factors in gastric cancer. Int. J. Oncol. 2001, 18, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.-W.; Hu, H.-L.; Sun, Y.; Tang, Y.; Lei, M.-D.; Liu, L.-W.; Han, R.-F.; Wu, C.-L. Biological effects of eukaryotic recombinant plasmid pReceiver-M61-BAI-1 transfection on T24 cells and HUVECs. Mol. Med. Rep. 2016, 14, 1553–1559. [Google Scholar] [CrossRef][Green Version]
- de Fraipont, F.; Nicholson, A.C.; Feige, J.-J.; Van Meir, E.G. Thrombospondins and tumor angiogenesis. Trends Mol. Med. 2001, 7, 401–407. [Google Scholar] [CrossRef]
- Koh, J.T.; Kook, H.; Kee, H.J.; Seo, Y.-W.; Jeong, B.C.; Lee, J.H.; Kim, M.-Y.; Yoon, K.C.; Jung, S.; Kim, K.K. Extracellular fragment of brain-specific angiogenesis inhibitor 1 suppresses endothelial cell proliferation by blocking alphavbeta5 integrin. Exp. Cell Res. 2004, 294, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Ravichandran, K.S. Emerging roles of brain-specific angiogenesis inhibitor 1. Adv. Exp. Med. Biol. 2010, 706, 167–178. [Google Scholar] [PubMed]
- Langenhan, T.; Aust, G.; Hamann, J. Sticky signaling—Adhesion class G protein-coupled receptors take the stage. Sci. Signal 2013, 6, re3. [Google Scholar] [CrossRef] [PubMed]
- Perler, F.B.; Xu, M.-Q.; Paulus, H. Protein splicing and autoproteolysis mechanisms. Curr. Opin. Chem. Biol. 1997, 1, 292–299. [Google Scholar] [CrossRef]
- Krasnoperov, V.; Lu, Y.; Buryanovsky, L.; Neubert, T.A.; Ichtchenko, K.; Petrenko, A.G. Post-translational proteolytic processing of the calcium-independent receptor of alpha-latrotoxin (CIRL), a natural chimera of the cell adhesion protein and the G protein-coupled receptor. Role of the G protein-coupled receptor proteolysis site (GPS) motif. J. Biol. Chem. 2002, 277, 46518–46526. [Google Scholar] [CrossRef]
- Arac, D.; Boucard, A.A.; Bolliger, M.F.; Nguyen, J.; Soltis, S.M.; Südhof, T.C.; Brunger, A.T. A novel evolutionarily conserved domain of cell-adhesion GPCRs mediates autoproteolysis. EMBO J. 2012, 31, 1364–1378. [Google Scholar] [CrossRef]
- Arac, D.; Aust, G.; Calebiro, D.; Engel, F.B.; Formstone, C.; Goffinet, A.; Hamann, J.; Kittel, R.J.; Liebscher, I.; Lin, H.-H.; et al. Dissecting signaling and functions of adhesion G protein-coupled receptors. Ann. N. Y. Acad. Sci. 2012, 1276, 1–25. [Google Scholar] [CrossRef]
- Huang, J.; Chen, S.; Zhang, J.J.; Huang, X. Crystal structure of oligomeric beta1-adrenergic G protein-coupled receptors in ligand-free basal state. Nat. Struct. Mol. Biol. 2013, 20, 419–425. [Google Scholar] [CrossRef]
- Kaur, B.; Brat, D.J.; Devi, N.S.; Van Meir, E.G. Vasculostatin, a proteolytic fragment of brain angiogenesis inhibitor 1, is an antiangiogenic and antitumorigenic factor. Oncogene 2005, 24, 3632–3642. [Google Scholar] [CrossRef]
- Doolittle, R.F. The multiplicity of domains in proteins. Annu. Rev. Biochem. 1995, 64, 287–314. [Google Scholar] [CrossRef]
- Cork, S.M.; Van Meir, E.G. Emerging roles for the BAI1 protein family in the regulation of phagocytosis, synaptogenesis, neurovasculature, and tumor development. J. Mol. Med. 2011, 89, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Shiratsuchi, T.; Nishimori, H.; Inazawa, J.; Yoshikawa, H.; Taketani, Y.; Nakamura, Y.; Tokino, T. Identification of BAIAP2 (BAI-associated protein 2), a novel human homologue of hamster IRSp53, whose SH3 domain interacts with the cytoplasmic domain of BAI1. Cytogenet. Cell Genet. 1999, 84, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Khwaja, F.W.; Severson, E.A.; Matheny, S.L.; Brat, D.J.; Van Meir, E.G. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro. Oncol. 2005, 7, 134–153. [Google Scholar] [CrossRef] [PubMed]
- Gatson, N.N.; Chiocca, E.A.; Kaur, B. Anti-angiogenic gene therapy in the treatment of malignant gliomas. Neurosci. Lett. 2012, 527, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Da, R.; Wang, M.; Wang, T.; Qi, L.; Jiang, H.; Chen, W.; Li, Q. Expression of brain-specific angiogenesis inhibitor 1 is inversely correlated with pathological grade, angiogenesis and peritumoral brain edema in human astrocytomas. Oncol. Lett. 2013, 5, 1513–1518. [Google Scholar] [CrossRef]
- Nishimori, H.; Shiratsuchi, T.; Urano, T.; Kimura, Y.; Kiyono, K.; Tatsumi, K.; Yoshida, S.; Ono, M.; Kuwano, M.; Nakamura, Y.; et al. A novel brain-specific p53-target gene, BAI1, containing thrombospondin type 1 repeats inhibits experimental angiogenesis. Oncogene 1997, 15, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Hu, H.; Sun, Y.; Tang, Y.; Lei, M.; Liu, L.; Han, R.; Wu, C. Expression of brainspecific angiogenesis inhibitor1 and association with p53, microvessel density and vascular endothelial growth factor in the tissue of human bladder transitional cell carcinoma. Mol. Med. Rep. 2015, 12, 4522–4529. [Google Scholar] [CrossRef][Green Version]
- Izutsu, T.; Konda, R.; Sugimura, J.; Iwasaki, K.; Fujioka, T. Brain-specific angiogenesis inhibitor 1 is a putative factor for inhibition of neovascular formation in renal cell carcinoma. J. Urol. 2011, 185, 2353–2358. [Google Scholar] [CrossRef]
- Zhu, D.; Osuka, S.; Zhang, Z.; Reichert, Z.R.; Yang, L.; Kanemura, Y.; Jiang, Y.; You, S.; Zhang, H.; Devi, N.S.; et al. BAI1 Suppresses Medulloblastoma Formation by Protecting p53 from Mdm2-Mediated Degradation. Cancer Cell 2018, 33, 1004–1016.e5. [Google Scholar] [CrossRef]
- Zhu, D.; Hunter, S.B.; Vertino, P.M.; Van Meir, E.G. Overexpression of MBD2 in glioblastoma maintains epigenetic silencing and inhibits the antiangiogenic function of the tumor suppressor gene BAI1. Cancer Res. 2011, 71, 5859–5870. [Google Scholar] [CrossRef]
- Zohrabian, V.M.; Nandu, H.; Gulati, N.; Khitrov, G.; Zhao, C.; Mohan, A.; Demattia, J.; Braun, A.; Das, K.; Murali, R.; et al. Gene expression profiling of metastatic brain cancer. Oncol. Rep. 2007, 18, 321–328. [Google Scholar] [CrossRef]
- Meisen, W.H.; Dubin, S.; Sizemore, S.T.; Mathsyaraja, H.; Thies, K.; Lehman, N.L.; Boyer, P.; Jaime-Ramirez, A.C.; Elder, J.B.; Powell, K.; et al. Changes in BAI1 and nestin expression are prognostic indicators for survival and metastases in breast cancer and provide opportunities for dual targeted therapies. Mol. Cancer Ther. 2015, 14, 307–314. [Google Scholar] [CrossRef]
- Liu, L.; Chai, L.; Ran, J.; Yang, Y.; Zhang, L. BAI1 acts as a tumor suppressor in lung cancer A549 cells by inducing metabolic reprogramming via the SCD1/HMGCR module. Carcinogenesis 2020, 41, 1724–1734. [Google Scholar] [CrossRef]
- Kaur, B.; Cork, S.M.; Sandberg, E.M.; Devi, N.S.; Zhang, Z.; Klenotic, P.A.; Febbraio, M.; Shim, H.; Mao, H.; Tucker-Burden, C.; et al. Vasculostatin inhibits intracranial glioma growth and negatively regulates in vivo angiogenesis through a CD36-dependent mechanism. Cancer Res. 2009, 69, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.C.; Ahn, K.Y.; Lee, J.H.; Chun, B.J.; Park, S.W.; Seo, M.S.; Park, Y.-G.; Kim, K.K. Lipid-mediated delivery of brain-specific angiogenesis inhibitor 1 gene reduces corneal neovascularization in an in vivo rabbit model. Gene Ther. 2005, 12, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.R.; Paavola, K.J.; Schaefer, S.A.; Kaur, B.; Van Meir, E.G.; Hall, R.A. Brain-specific angiogenesis inhibitor-1 signaling, regulation, and enrichment in the postsynaptic density. J. Biol. Chem. 2013, 288, 22248–22256. [Google Scholar] [CrossRef] [PubMed]
- Cork, S.M.; Kaur, B.; Devi, N.S.; Cooper, L.; Saltz, J.H.; Sandberg, E.M.; Kaluz, S.; Van Meir, E.G. A proprotein convertase/MMP-14 proteolytic cascade releases a novel 40 kDa vasculostatin from tumor suppressor BAI1. Oncogene 2012, 31, 5144–5152. [Google Scholar] [CrossRef]
- Tolsma, S.S.; Volpert, O.; Good, D.J.; Frazier, W.A.; Polverini, P.J.; Bouck, N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J. Cell Biol. 1993, 122, 497–511. [Google Scholar] [CrossRef]
- Swerlick, R.A.; Lee, K.H.; Wick, T.; Lawley, T.J. Human dermal microvascular endothelial but not human umbilical vein endothelial cells express CD36 in vivo and in vitro. J. Immunol. 1992, 148, 78–83. [Google Scholar]
- Saravanan, S.; Vimalraj, S.; Pavani, K.; Nikarika, R.; Sumantran, V.N.; Ramesh, N. Intussusceptive angiogenesis as a key therapeutic target for cancer therapy. Life Sci. 2020, 252, 117670. [Google Scholar] [CrossRef]
- Mazaheri, F.; Breus, O.; Durdu, S.; Haas, P.; Wittbrodt, J.; Gilmour, D.; Peri, F. Distinct roles for BAI1 and TIM-4 in the engulfment of dying neurons by microglia. Nat. Commun. 2014, 5, 4046. [Google Scholar] [CrossRef]
- Sokolowski, J.D.; Nobles, S.L.; Heffron, D.S.; Park, D.; Ravichandran, K.S.; Mandell, J.W. Brain-specific angiogenesis inhibitor-1 expression in astrocytes and neurons: Implications for its dual function as an apoptotic engulfment receptor. Brain Behav. Immun. 2011, 25, 915–921. [Google Scholar] [CrossRef]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992, 148, 2207–2216. [Google Scholar]
- Park, D.; Tosello-Trampont, A.-C.; Elliott, M.; Lu, M.; Haney, L.B.; Ma, Z.; Klibanov, A.L.; Mandell, J.W.; Ravichandran, K. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 2007, 450, 430–434. [Google Scholar] [CrossRef]
- Juncadella, I.J.; Kadl, A.; Sharma, A.K.; Shim, Y.M.; Hochreiter-Hufford, A.; Borish, L.; Ravichandran, K.S. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature 2013, 493, 547–551. [Google Scholar] [CrossRef]
- Hochreiter-Hufford, A.E.; Lee, C.S.; Kinchen, J.; Sokolowski, J.D.; Arandjelovic, S.; Call, J.; Klibanov, A.L.; Yan, Z.; Mandell, J.W.; Ravichandran, K. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature 2013, 497, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Shiratsuchi, T.; Futamura, M.; Oda, K.; Nishimori, H.; Nakamura, Y.; Tokino, T. Cloning and characterization of BAI-associated protein 1: A PDZ domain-containing protein that interacts with BAI1. Biochem. Biophys. Res. Commun. 1998, 247, 597–604. [Google Scholar] [CrossRef]
- Shiratsuchi, T.; Oda, K.; Nishimori, H.; Suzuki, M.; Takahashi, E.; Tokino, T.; Nakamura, Y. Cloning and characterization of BAP3 (BAI-associated protein 3), a C2 domain-containing protein that interacts with BAI1. Biochem. Biophys. Res. Commun. 1998, 251, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kanemura, Y.; Fujikawa, H.; Nakano, A.; Ikemoto, H.; Ozaki, I.; Matsumoto, T.; Tamura, K.; Yokota, M.; Arita, N. Brain-specific angiogenesis inhibitor 1 (BAI1) is expressed in human cerebral neuronal cells. Neurosci. Res. 2002, 43, 69–74. [Google Scholar] [CrossRef]
- Duman, J.G.; Tzeng, C.P.; Tu, Y.; Munjal, T.; Schwechter, B.; Ho, T.S.; Tolias, K.F. The adhesion-GPCR BAI1 regulates synaptogenesis by controlling the recruitment of the Par3/Tiam1 polarity complex to synaptic sites. J. Neurosci. 2013, 33, 6964–6978. [Google Scholar] [CrossRef]
- Kang, X.; Xiao, X.; Harata, M.; Bai, Y.; Nakazaki, Y.; Soda, Y.; Kurita, R.; Tanaka, T.; Komine, F.; Izawa, K.; et al. Antiangiogenic activity of BAI1 in vivo: Implications for gene therapy of human glioblastomas. Cancer Gene Ther. 2006, 13, 385–392. [Google Scholar] [CrossRef]
- Baylin, S.B.; Jones, P.A. A decade of exploring the cancer epigenome—Biological and translational implications. Nat. Rev. Cancer 2011, 11, 726–734. [Google Scholar] [CrossRef]
- Bird, A.P.; Wolffe, A.P. Methylation-induced repression—Belts, braces, and chromatin. Cell 1999, 99, 451–454. [Google Scholar] [CrossRef]
- Xiao, X.-R.; Kang, X.-X.; Zhao, J.-Z. [Therapeutic effect of brain-specific angiogenesis inhibitor 1 on glioblastoma: An animal experiment]. Zhonghua Yi Xue Za Zhi 2006, 86, 1342–1346. [Google Scholar]
- Tomita, Y.; Kurozumi, K.; Yoo, J.Y.; Fujii, K.; Ichikawa, T.; Matsumoto, Y.; Uneda, A.; Hattori, Y.; Shimizu, T.; Otani, Y.; et al. Oncolytic Herpes Virus Armed with Vasculostatin in Combination with Bevacizumab Abrogates Glioma Invasion via the CCN1 and AKT Signaling Pathways. Mol. Cancer Ther. 2019, 18, 1418–1429. [Google Scholar] [CrossRef]
- Bolyard, C.; Yoo, J.Y.; Wang, P.-Y.; Saini, U.; Rath, K.S.; Cripe, T.P.; Zhang, J.; Selvendiran, K.; Kaur, B. Doxorubicin synergizes with 34.5ENVE to enhance antitumor efficacy against metastatic ovarian cancer. Clin. Cancer Res. 2014, 20, 6479–6494. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Khosla, M.; Otani, Y.; Yeh, M.; Park, F.; Shimizu, T.; Kang, J.M.; Bolyard, C.; Yu, J.-G.; Banasavadi-Siddegowda, Y.K.; et al. Enhancing Antitumor Efficacy of Heavily Vascularized Tumors by RAMBO Virus through Decreased Tumor Endothelial Cell Activation. Cancers 2020, 12, 1040. [Google Scholar] [CrossRef]
- Abe, T.; Terada, K.; Wakimoto, H.; Inoue, R.; Tyminski, E.; Bookstein, R.; Basilion, J.P.; Chiocca, E.A. PTEN decreases in vivo vascularization of experimental gliomas in spite of proangiogenic stimuli. Cancer Res. 2003, 63, 2300–2305. [Google Scholar] [PubMed]
- Hardcastle, J.; Kurozumi, K.; Dmitrieva, N.; Sayers, M.P.; Ahmad, S.; Waterman, P.; Weissleder, R.; Chiocca, E.A.; Kaur, B. Enhanced antitumor efficacy of vasculostatin (Vstat120) expressing oncolytic HSV-1. Mol. Ther. 2010, 18, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Haseley, A.; Bratasz, A.; Chiocca, E.A.; Zhang, J.; Powell, K.; Kaur, B. Antitumor efficacy of 34.5ENVE: A transcriptionally retargeted and “Vstat120”-expressing oncolytic virus. Mol. Ther. 2012, 20, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Bolyard, C.; Meisen, W.H.; Banasavadi-Siddegowda, Y.; Hardcastle, J.; Yoo, J.Y.; Wohleb, E.S.; Wojton, J.; Yu, J.-G.; Dubin, S.; Khosla, M.; et al. BAI1 Orchestrates Macrophage Inflammatory Response to HSV Infection-Implications for Oncolytic Viral Therapy. Clin. Cancer Res. 2017, 23, 1809–1819. [Google Scholar] [CrossRef]
- Lee, T.J.; Nair, M.; Banasavadi-Siddegowda, Y.; Liu, J.; Nallanagulagari, T.; Jaime-Ramirez, A.C.; Guo, J.Y.; Quadri, H.; Zhang, J.; Bockhorst, K.H.; et al. Enhancing Therapeutic Efficacy of Oncolytic Herpes Simplex Virus-1 with Integrin beta1 Blocking Antibody OS2966. Mol. Cancer Ther. 2019, 18, 1127–1136. [Google Scholar] [CrossRef]
- Jahangiri, A.; Aghi, M.K.; Carbonell, W.S. beta1 integrin: Critical path to antiangiogenic therapy resistance and beyond. Cancer Res. 2014, 74, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, W.S.; DeLay, M.; Jahangiri, A.; Park, C.C.; Aghi, M.K. beta1 integrin targeting potentiates antiangiogenic therapy and inhibits the growth of bevacizumab-resistant glioblastoma. Cancer Res. 2013, 73, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Yu, J.-G.; Kaka, A.; Pan, Q.; Kumar, P.; Kumar, B.; Zhang, J.; Mazar, A.; Teknos, T.N.; Kaur, B.; et al. ATN-224 enhances antitumor efficacy of oncolytic herpes virus against both local and metastatic head and neck squamous cell carcinoma. Mol. Ther. Oncolytics 2015, 2, 15008. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Briehl, M.M.; Mazar, A.P.; Batinic-Haberle, I.; Reboucas, J.S.; Glinsmann-Gibson, B.; Rimsza, L.M.; Tome, M.E. The copper chelator ATN-224 induces peroxynitrite-dependent cell death in hematological malignancies. Free Radic. Biol. Med. 2013, 60, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Benencia, F.; Courreges, M.C.; Conejo-García, J.R.; Buckanovich, R.J.; Zhang, L.; Carroll, R.H.; Morgan, M.A.; Coukos, G. Oncolytic HSV exerts direct antiangiogenic activity in ovarian carcinoma. Hum. Gene Ther. 2005, 16, 765–778. [Google Scholar] [CrossRef]
- Hong, B.; Chapa, V.; Saini, U.; Modgil, P.; Cohn, D.E.; He, G.; Siddik, Z.H.; Sood, A.K.; Yan, Y.; Selvendiran, K.; et al. Oncolytic HSV Therapy Modulates Vesicular Trafficking Inducing Cisplatin Sensitivity and Antitumor Immunity. Clin. Cancer Res. 2021, 27, 542–553. [Google Scholar] [CrossRef]
| References | Cancer Type | Sample | n | Results | Correlation with Disease? |
|---|---|---|---|---|---|
| [1] | Pulmonary Adenocarcinoma | Human Pulmonary Adenocarcinoma Tissue Samples | 48 | BAI1 protein expression found in 38/48 samples, with vascularity inversely proportional to BAI1 express | BAI expression is inversely correlated with |
| [2] | Glioblastoma | Human Glioblastoma Cell Lines | 37 | BAI1 protein expression in 13/37 of cell lines | BAI1 expression is inversely correlated |
| [5] | Gastric Cancer | Human Gastric Cancer Tissue Samples | 200 | No significant difference in BAI1 expression in either normal tissues or cancers | BAI1 expression is not correlated |
| [6] | Colorectal Cancer | Tissues from colorectal cancers and extraneoplastic colon mucosa | 102 (62 colorectal cancers and 40 extraneoplastic colon mucosa) | BAI1 protein expression was slightly decreased in the cancer tissue, suggesting specific types of angiogenic factors have a protective roles against cancer | BAI expression is inversely correlated |
| [7] | Colorectal Cancer | Human Colorectal Cancer Cell Lines | 49 | BAI1 protein expression is significantly reduced, and its expression is inversely correlated to vascular invasion and metastasis | BAI1 expression is inversely correlated |
| [8] | Gastric Cancer | Human Gastric Cancer Tissue Samples | 32 | BAI1 protein expression decreased in cancer tissue and metastatic lymph node tissue relative to extraneoplastic mucosa and non-metastatic lymph node tissue | BAI1 expression inversely correlated |
| [25] | Astrocytoma | Human Tissue Samples | 101 (90 human brain astrocytoma specimens, 11 normal human brain tissue specimens) | BAI1 protein expression decreased as tumor grade increased | BAI1 expression is inversely correlated |
| [27] | bladder transitional cell carcinoma (BTCC) | human BTCC biopsy specimens | 131 | BAI1 protein expression is negatively correlated with BTCC angiogenesis, and its expression is associated with reduced p53 mutations. | BAI expression is inversely correlated |
| [28] | Renal Cell Carcinoma | Human Tissue Samples | 57 (32 localized carcinomas, 15 advanced carcinomas, 10 normal kidney tissue specimens) | BAI1 protein expression in 31/32 localized carcinomas, and 9/15 in advanced carcinomas | BAI1 expression is inversely correlated |
| [30] | Glioblastoma | Human Glioblostoma Tissue Samples | 424 | Consistent and dramatic reduction in the expression of BAI1 | BAI expression is inversely correlated |
| [31] | brain metastasis | Tissues from brain metastasis of primary adenocarcinoma of the lung | 2 | Decreased BAI1 expression is involved in the periphery-to-brain metastasis | BAI expression is inversely correlated |
| [33] | Lung Cancer | Human Lung Cancer Cell Lines | 103 (primary lung tumor tissues) | BAI1 functions as a tumor suppressor by inducing metabolic reprogramming in lung cancer | BAI expression is inversely correlated |
| Model | Therapeutic | Experimental Read Out | Results/Outcome | References |
|---|---|---|---|---|
| Human pancreatic adenocarcinoma cell lines in vitro and in vivo | Transfection of BAI1 gene into pancreatic adenocarcinoma cell lines by means of adenoviruses | Tumor growth | No difference in tumor growth between treated and untreated cells in vitro Diminished tumor growth (p < 0.05) | [3] |
| Mouse Renal Cell Carcinoma (Renca) cell line xenografted into BALB/c mice | Transfection of Renca cells with BAI1 | Tumor Size and Tumor Blood Flow | Decreased tumor size and blood flow (p < 0.01) | [4] |
| Human glioblastoma cells xenografted into mice | Induced expression of vasculostatin in glioblastoma cells | Vascular Channel Length | Vascular channel length is decreased (p < 0.03) | [19] |
| Human Glioblastoma Cells | Transfection of glioma cells with BAI1 | Tumor cell migration | Decreased tumor cell migration (p < 0.001) | [30] |
| Human Lung Cancer | Stably BAI1 overexpression in lung cancer cells | Tumor cell migration, colony formation, tumor cell growth in vitro and in vivo | Decreased tumor cell migration, colony formation, and tumor growth (p < 0.001) | [33] |
| Human intracranial glioma cells xenografted into mice | Transfection of glioma cell lines with Vasculostatin 120 expression vectors | Survival in mice and Vascular density of gliomas | Increased survival time (p < 0.05) Decreased blood vessel density (p < 0.05) | [34] |
| Corneal neovascularization in an in-vivo rabbit model | subconjunctival injection of the BAI1-ECR gene mixed with nonliposomal lipid | Neovascularized area | Less neovascularized area (p < 0.05) | [35] |
| Human glioblastoma cells xenografted into SCID mice | In vivo transduction of transplanted tumors by adenoviral vector encoding BAI1 (AdBAI1) | Neovascularization post transplant and Tumor Growth | Angiogenesis completely inhibited and Tumor growth inhibited (p < 0.05) | [51] |
| Human glioblastoma cells xenografted into in-vivo mouse models | Recombinant adenovirus carrying human BAI1 cDNA | Survival in mice | Increased survival (p < 0.05) | [54] |
| Human intracranial glioma cells implanted into athymic nude mice | Combination treatment of Vasculostatin expressing virus and Bevacizumab | Survival in mice | Increased survival time (p < 0.05) | [55] |
| Human ovarian cancer cells implanted into athymic nude mice | Combination treatment of Vasculostatin expressing virus and Doxorubicin | Survival in mice | Increased survival time (p < 0.001) | [56] |
| Human soft tissue sarcoma and glioblastoma cells implanted into athymic nude mice | Vstat120-expressing RAMBO virus decreases tumor endothelial cell activation, inreasing anti-tumor efficacy | Tumor growth | Increased anti-tumor efficacy (p < 0.001) | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, M.; Bolyard, C.; Lee, T.J.; Kaur, B.; Yoo, J.Y. Therapeutic Application of Brain-Specific Angiogenesis Inhibitor 1 for Cancer Therapy. Cancers 2021, 13, 3562. https://doi.org/10.3390/cancers13143562
Nair M, Bolyard C, Lee TJ, Kaur B, Yoo JY. Therapeutic Application of Brain-Specific Angiogenesis Inhibitor 1 for Cancer Therapy. Cancers. 2021; 13(14):3562. https://doi.org/10.3390/cancers13143562
Chicago/Turabian StyleNair, Mitra, Chelsea Bolyard, Tae Jin Lee, Balveen Kaur, and Ji Young Yoo. 2021. "Therapeutic Application of Brain-Specific Angiogenesis Inhibitor 1 for Cancer Therapy" Cancers 13, no. 14: 3562. https://doi.org/10.3390/cancers13143562
APA StyleNair, M., Bolyard, C., Lee, T. J., Kaur, B., & Yoo, J. Y. (2021). Therapeutic Application of Brain-Specific Angiogenesis Inhibitor 1 for Cancer Therapy. Cancers, 13(14), 3562. https://doi.org/10.3390/cancers13143562







