Cellulitis Is Associated with Severe Breast Cancer-Related Lymphedema: An Observational Study of Tissue Composition
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
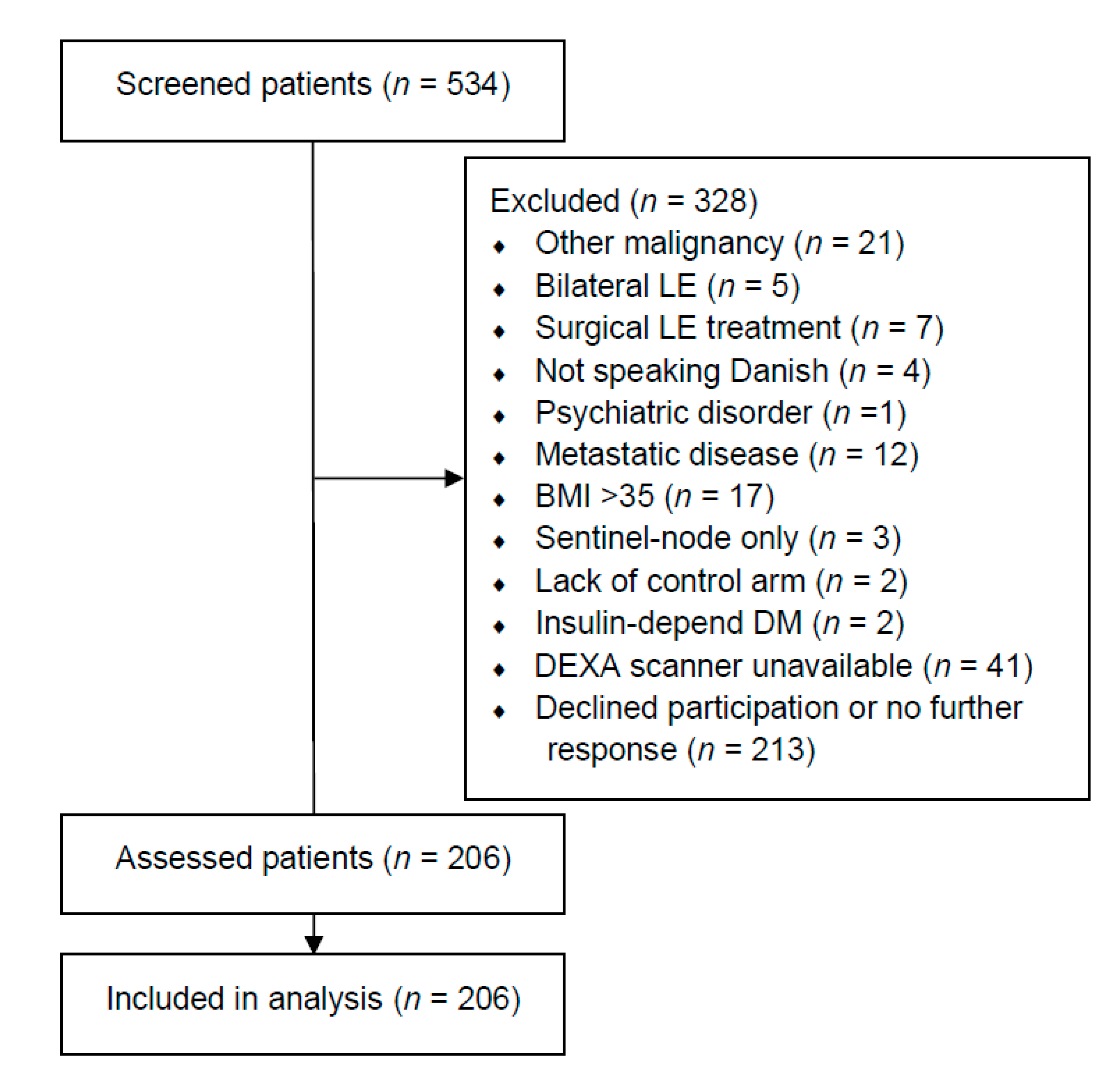
2.2. Participants
- Previous treatment for loco-regional breast cancer with axillary lymph node dissection
- Cancer-free for more than one year
- Body mass index ≤ 35
- American Society of Anesthesiology score of 1 or 2 [18]
- Able to give informed consent
- No history of other malignancy apart from breast cancer and non-melanoma skin cancer
- No insulin-dependent diabetes
- No known hepatitis, HIV, or syphilis infection
- No primary lymphedema or non-breast cancer-related lymphedema
2.3. Outcome Measurements
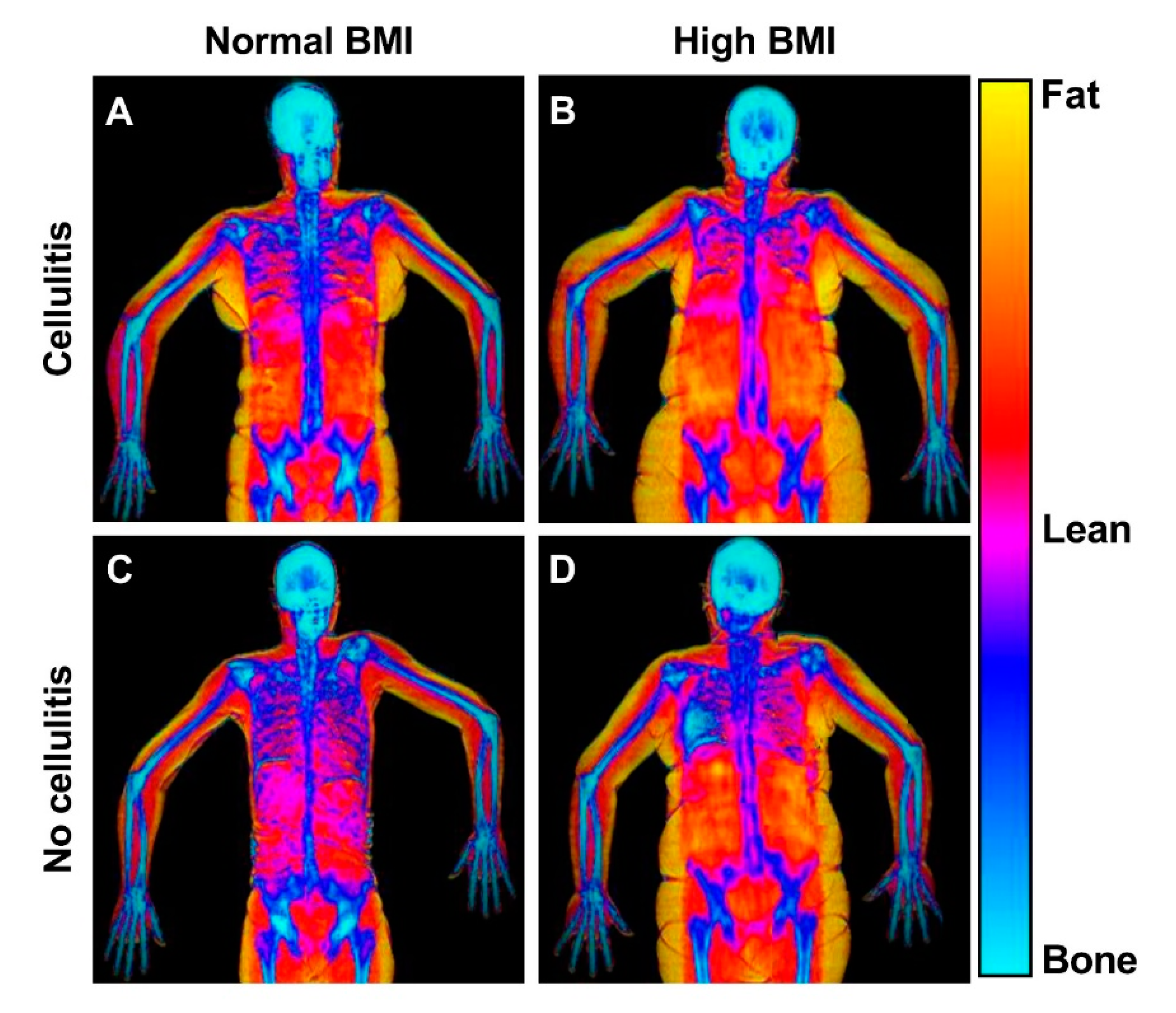
Dual-Energy X-ray Absorptiometry
2.4. Bioimpedance Spectroscopy
2.5. Indocyanine Green Lymphangiography
- Stage 0: Normal linear lymphatics and no dermal backflow
- Stage 1: Many patent lymphatics and minimal lymphatic dermal backflow
- Stage 2: Moderate number of patent lymphatics and segmental dermal backflow
- Stage 3: Few patent lymphatics and extensive dermal backflow
- Stage 4: Dermal backflow involving the hand
- Stage 5: No proximal uptake of ICG from the injection site
2.6. Clinical Examination
2.7. Danish Breast Cancer Registry
2.8. Outcome Variables
2.9. Statistical Methods
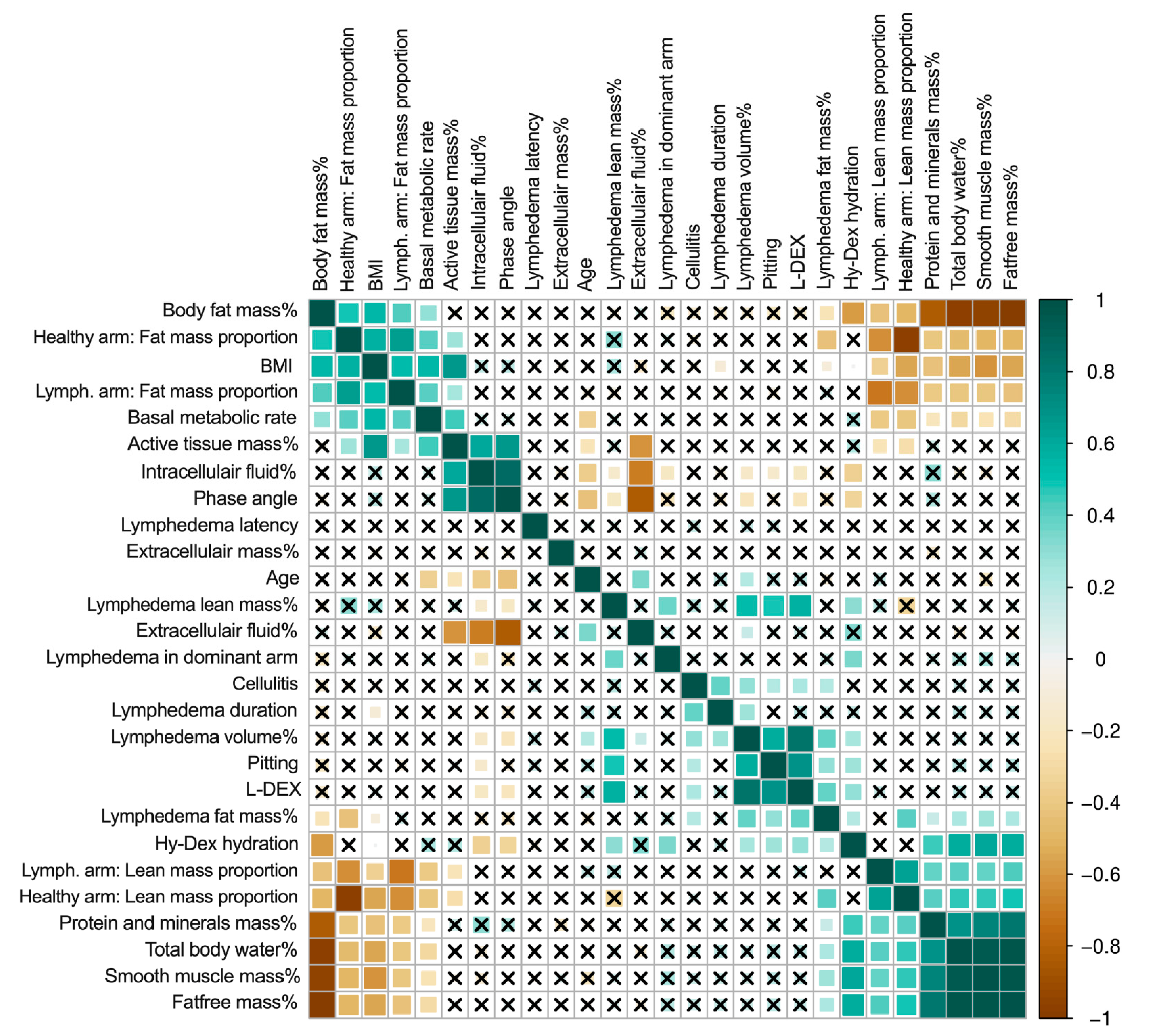
3. Results
3.1. Excess Arm Mass
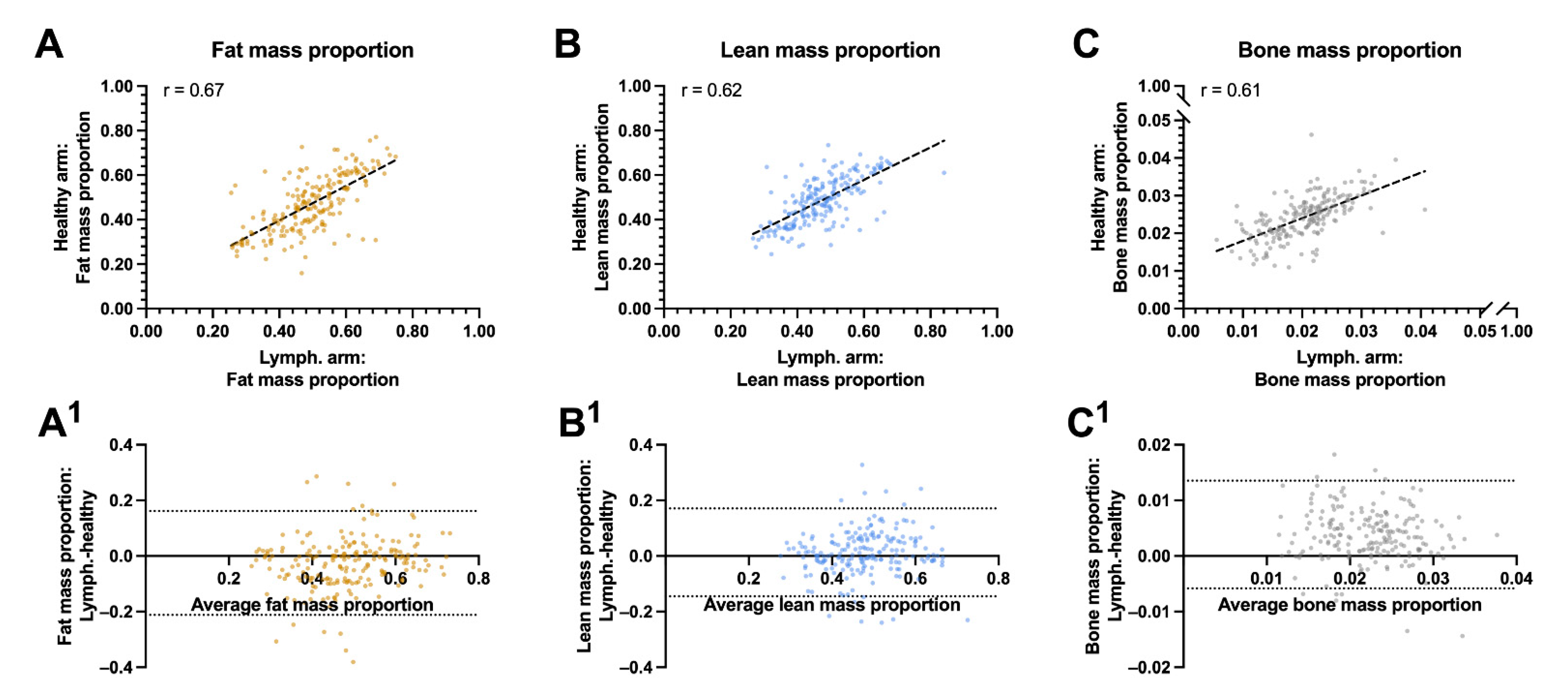
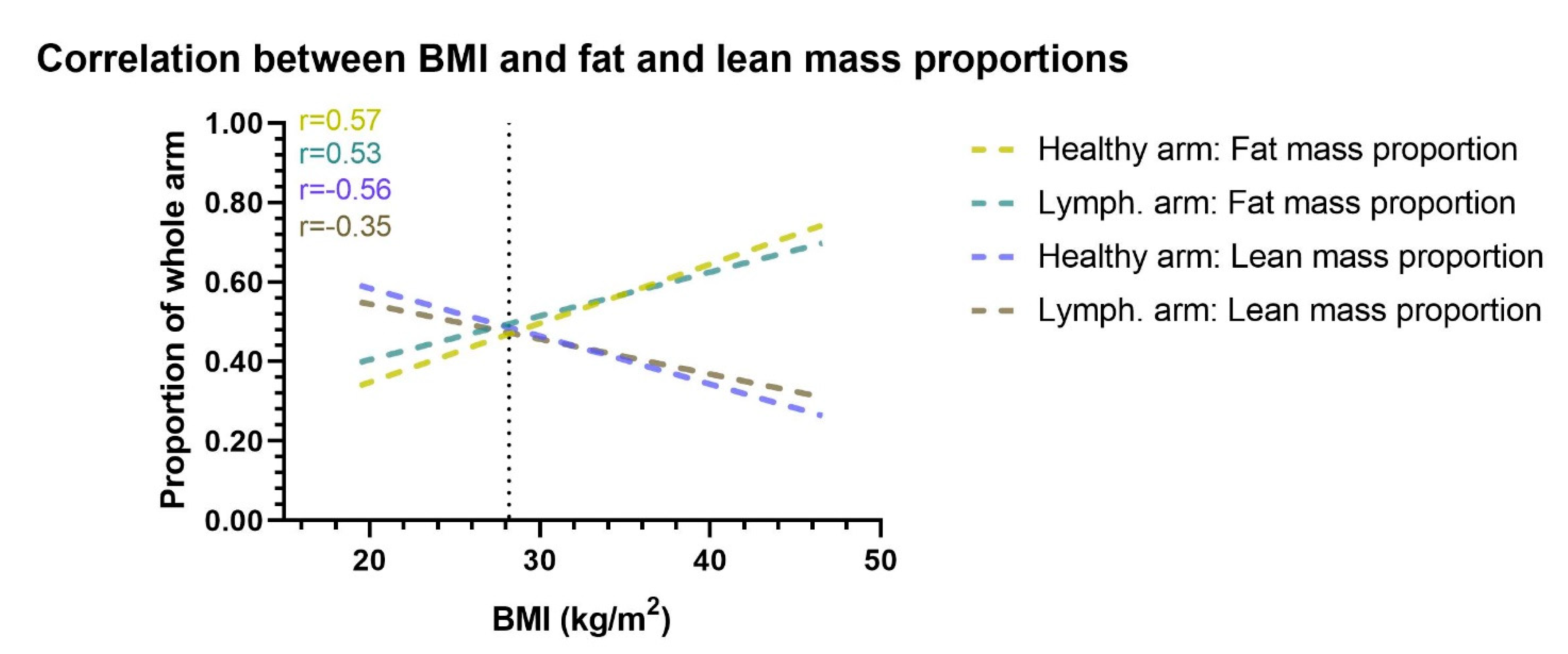
3.2. Fat and Lean Arm Mass Proportions
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Toyserkani, N.M.; Hansen, F.G.; Bygum, A.; Sørensen, J.A. The impact of lymphedema on health-related quality of life up to 10 years after breast cancer treatment. npj Breast Cancer 2021, 7, 70. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Toyserkani, N.M.; Hansen, F.C.G.; Thomsen, J.B.; Sørensen, J.A. Prospective Validation of Indocyanine Green Lymphangiography Staging of Breast Cancer-Related Lymphedema. Cancers 2021, 13, 1540. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.; Benchikhi, H.; Roujeau, J.C.; Bernard, P.; Vaillant, L.; Chosidow, O.; Sassolas, B.; Guillaume, J.-C.; Grob, J.-J.; Bastuji-Garin, S.; et al. Risk factors for erysipelas of the leg (cellulitis): Case-control study. BMJ 1999, 318, 1591–1594. [Google Scholar] [CrossRef] [Green Version]
- Markkula, S.P.; Leung, N.; Allen, V.B.; Furniss, D. Surgical interventions for the prevention or treatment of lymphoedema after breast cancer treatment. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Ezzo, J.; Manheimer, E.; McNeely, M.; Howell, D.; Weiss, R.; Johansson, I.K.; Bao, T.; Bily, L.; Tuppo, C.M.; Williams, A.F.; et al. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brorson, H.; Ohlin, K.; Olsson, G.; Karlsson, M.K. Breast Cancer-Related Chronic Arm Lymphedema Is Associated with Excess Adipose and Muscle Tissue. Lymphat. Res. Biol. 2009, 7, 3–10. [Google Scholar] [CrossRef]
- Dylke, E.S.; Ward, L.C.; Meerkin, J.D.; Nery, L.; Kilbreath, S.L. Tissue composition changes and secondary lymphedema. Lymphat. Res. Biol. 2013, 11, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Kim, J.; Seo, Y.J.; Song, K.J.; Gelvosa, M.N.; Kwon, J.G.; Pak, C.J.; Suh, H.P.; Hong, J.P.; Kim, H.J.; et al. Who Will Continuously Depend on Compression to Control Persistent or Progressive Breast Cancer-Related Lymphedema Despite 2 Years of Conservative Care? J. Clin. Med. 2020, 9, 3640. [Google Scholar] [CrossRef]
- Vignes, S.; Arrault, M.; Dupuy, A. Factors associated with increased breast cancer-related lymphedema volume. Acta Oncol. 2007, 46, 1138–1142. [Google Scholar] [CrossRef]
- He, L.; Qu, H.; Wu, Q.; Song, Y. Lymphedema in survivors of breast cancer (Review). Oncol Lett. 2020, 19, 2085–2096. [Google Scholar] [PubMed] [Green Version]
- Jørgensen, M.G.; Toyserkani, N.M.; Thomsen, J.B.; Sørensen, J.A. Prophylactic incisional negative pressure wound therapy shows promising results in prevention of wound complications following inguinal lymph node dissection for Melanoma: A retrospective case-control series. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 1178–1183. [Google Scholar] [CrossRef] [Green Version]
- Asdourian, M.S.; Skolny, M.N.; Brunelle, C.; Seward, C.E.; Salama, L.; Taghian, A.G. Precautions for breast cancer-related lymphoedema: Risk from air travel, ipsilateral arm blood pressure measurements, skin puncture, extreme temperatures, and cellulitis. Lancet Oncol. 2016, 17, e392–e405. [Google Scholar] [CrossRef]
- Dayan, J.H.; Wiser, I.; Verma, R.; Shen, J.; Talati, N.; Goldman, D.; Mehrara, B.J.; Smith, M.L.; Dayan, E.; Coriddi, M.; et al. Regional Patterns of Fluid and Fat Accumulation in Patients with Lower Extremity Lymphedema Using Magnetic Resonance Angiography. Plast. Reconstr. Surg. 2020, 145, 555–563. [Google Scholar] [CrossRef]
- Zeltzer, A.A.; Brussaard, C.; Koning, M.; De Baerdemaeker, R.; Hendrickx, B.; Hamdi, M.; De Mey, J. MR lymphography in patients with upper limb lymphedema: The GPS for feasibility and surgical planning for lympho-venous bypass. J. Surg. Oncol. 2018, 118, 407–415. [Google Scholar] [CrossRef] [PubMed]
- The diagnosis and treatment of peripheral lymphedema: 2020 consensus document of the international society of lymphology. Lymphology 2003, 36, 84–91.
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Doyle, D.J.; Goyal, A.; Bansal, P.; Garmon, E.H. American Society of Anesthesiologist Classification 2021. (accessed on March 2021).
- Newman, A.L.; Rosenthall, L.; Towers, A.; Hodgson, P.; A Shay, C.; Tidhar, D.; Vigano, A.; Kilgour, R.D. Determining the Precision of Dual Energy X-Ray Absorptiometry and Bioelectric Impedance Spectroscopy in the Assessment of Breast Cancer-Related Lymphedema. Lymphat. Res. Biol. 2013, 11, 104–109. [Google Scholar] [CrossRef]
- Gjorup, C.A.; Zerahn, B.; Juul, S.; Hendel, H.W.; Christensen, K.B.; Hölmich, L.R. Repeatability of Volume and Regional Body Composition Measurements of the Lower Limb Using Dual-energy X-ray Absorptiometry. J. Clin. Densitom. 2017, 20, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, L. Dual Energy X-ray Absorptiometry for Bone Mineral Density and Body Composition Assessment; IAEA Human Health Series Number 15; International Atomic Energy Agency: Vienna, Austria, 2010. [Google Scholar]
- Gjorup, C.A.; Hendel, H.W.; Klausen, T.W.; Zerahn, B.; Hölmich, L.R. Reference Values for Assessment of Unilateral Limb Lymphedema with Dual-Energy X-Ray Absorptiometry. Lymphat. Res. Biol. 2018, 16, 75–84. [Google Scholar] [CrossRef]
- Koelmeyer, L.A.; Ward, L.C.; Dean, C.; Boyages, J. Body Positional Effects on Bioimpedance Spectroscopy Measurements for Lymphedema Assessment of the Arm. Lymphat. Res. Biol. 2020, 18, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.W.; Suami, H.; Skoracki, R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast. Reconstr. Surg. 2013, 132, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Suami, H.; Hanasono, M.M.; Womack, V.A.; Wong, F.C.; Chang, E.I. Long-term outcomes of the minimally invasive free vascularized omental lymphatic flap for the treatment of lymphedema. J. Surg. Oncol. 2017, 115, 84–89. [Google Scholar] [CrossRef]
- De Vrieze, T.; Gebruers, N.; Nevelsteen, I.; Groef, A.D.; Tjalma, W.A.A.; Thomis, S.; Dams, L.; Van der Gucht, E.; Penen, F.; Devoogdt, N. Reliability of the MoistureMeterD Compact Device and the Pitting Test to Evaluate Local Tissue Water in Subjects with Breast Cancer-Related Lymphedema. Lymphat. Res. Biol. 2020, 18, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, P.; Ejlertsen, B.; Jensen, M.B.; Mouridsen, H. Danish breast cancer cooperative group. Clin. Epidemiol. 2016, 8, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Boyages, J.; Kastanias, K.; Koelmeyer, L.; Winch, C.; Lam, T.C.; Sherman, K.; Munnoch, D.A.; Brorson, H.; Ngo, Q.; Heydon-White, A.; et al. Liposuction for Advanced Lymphedema: A Multidisciplinary Approach for Complete Reduction of Arm and Leg Swelling. Ann. Surg. Oncol. 2015, 22, 1263–1270. [Google Scholar] [CrossRef] [Green Version]
- Beederman, M.; Garza, R.M.; Agarwal, S.; Chang, D.W. Outcomes for Physiologic Microsurgical Treatment of Secondary Lymphedema Involving the Extremity. Ann Surg. 2020. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Toyserkani, N.M.; Jensen, C.H.; Andersen, D.C.; Sheikh, S.P.; Sørensen, J.A. Adipose-derived regenerative cells and lipotransfer in alleviating breast cancer-related lymphedema: An open-label phase I trial with 4 years of follow-up. Stem Cells Transl. Med. 2021, 10, 844–854. [Google Scholar] [CrossRef]
- Czerniec, S.A.; Ward, L.C.; Meerkin, J.D.; Kilbreath, S.L. Assessment of Segmental Arm Soft Tissue Composition in Breast Cancer-Related Lymphedema: A Pilot Study Using Dual Energy X-ray Absorptiometry and Bioimpedance Spectroscopy. Lymphat. Res. Biol. 2015, 13, 33–39. [Google Scholar] [CrossRef]
- Ciudad, P.; Manrique, O.J.; Bustos, S.; Agko, M.; Huang, T.C.-T.; Vizcarra, L.; Nuñez, M.L.; Torto, F.L.; Forte, A.J. Single-stage VASER-assisted liposuction and lymphatico-venous anastomoses for the treatment of extremity lymphedema: A case series and systematic review of the literature. Gland Surg. 2020, 9, 545–557. [Google Scholar] [CrossRef]
- Di Taranto, G.; Bolletta, A.; Chen, S.-H.; Losco, L.; Elia, R.; Cigna, E.; Rubino, C.; Ribuffo, D.; Chen, H.-C. A prospective study on combined lymphedema surgery: Gastroepiploic vascularized lymph nodes transfer and lymphaticovenous anastomosis followed by suction lipectomy. Microsurgery 2021, 41, 34–43. [Google Scholar] [CrossRef]
- Masia, J.; Pons, G.; Nardulli, M. Combined Surgical Treatment in Breast Cancer-Related Lymphedema. J. Reconstr. Microsurg. 2015, 32, 016–027. [Google Scholar] [CrossRef] [Green Version]
- Borri, M.; Gordon, K.; Hughes, J.C.; Scurr, E.D.; Koh, D.-M.; Leach, M.O.; Mortimer, P.S.; Schmidt, M.A. Magnetic Resonance Imaging-Based Assessment of Breast Cancer-Related Lymphoedema Tissue Composition. Investig. Radiol. 2017, 52, 554–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seward, C.; Skolny, M.; Brunelle, C.; Asdourian, M.; Salama, L.; Taghian, A.G. A comprehensive review of bioimpedance spectroscopy as a diagnostic tool for the detection and measurement of breast cancer-related lymphedema. J. Surg. Oncol. 2016, 114, 537–542. [Google Scholar] [CrossRef]
- Pain, R.W. Body Fluid Compartments. Anaesth. Intensive Care 1977, 5, 284–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, L.L. Body Composition, Normal Electrolyte Concentrations, and the Maintenance of Normal Volume, Tonicity, and Acid-Base Metabolism. Pediatr. Clin. North Am. 1990, 37, 241–256. [Google Scholar] [CrossRef]
- Al-Niaimi, F.; Cox, N. Cellulitis and lymphoedema: A vicious cycle. J. Lymphoedema 2009, 4, 38–42. [Google Scholar]
- Jørgensen, M.G.; Toyserkani, N.M.; Thomsen, J.B.; Sørensen, J.A. Surgical-site infection following lymph node excision indicates susceptibility for lymphedema: A retrospective cohort study of malignant melanoma patients. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 590–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, D.; Meijer, E.F.J.; Blatter, C.; Liao, S.; Pereira, E.R.; Bouta, E.M.; Jung, K.; Chin, S.M.; Huang, P.; Munn, L.L.; et al. Methicillin-resistant Staphylococcus aureus causes sustained collecting lymphatic vessel dysfunction. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Soo, J.K.; Bicanic, T.A.; Heenan, S.; Mortimer, P.S. Lymphatic abnormalities demonstrated by lymphoscintigraphy after lower limb cellulitis. Br. J. Dermatol. 2008, 158, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Mihara, M.; Seki, Y.; Todokoro, T.; Iida, T.; Koshima, I. Comparison of indocyanine green lymphographic findings with the conditions of collecting lymphatic vessels of limbs in patients with lymphedema. Plast. Reconstr. Surg. 2013, 132, 1612–1618. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Toyserkani, N.M.; Hansen, C.R.; Hvidsten, S.; Baun, C.; Hejbøl, E.K.; Schrøder, H.D.; Sørensen, J.A. Quantification of Chronic Lymphedema in a Revised Mouse Model. Ann. Plast. Surg. 2018, 81, 594–603. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Toyserkani, N.M.; Sørensen, J.A. The effect of prophylactic lymphovenous anastomosis and shunts for preventing cancer-related lymphedema: A systematic review and meta-analysis. Microsurgery 2017, 38, 576–585. [Google Scholar] [CrossRef]
- Shaitelman, S.F.; Cromwell, K.D.; Rasmussen, J.C.; Stout, N.L.; Armer, J.M.; Lasinski, B.B.; Cormier, J.J. Recent progress in the treatment and prevention of cancer-related lymphedema. CA Cancer J. Clin. 2014, 65, 55–81. [Google Scholar] [CrossRef]
- Toyserkani, N.M.; Jørgensen, M.G.; Haugaard, K.; Sørensen, J.A. Seroma indicates increased risk of lymphedema following breast cancer treatment: A retrospective cohort study. Breast 2017, 32, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Fanizzi, A.; Pomarico, D.; Paradiso, A.; Bove, S.; Diotiaiuti, S.; Didonna, V.; Giotta, F.; La Forgia, D.; Latorre, A.; Pastena, M.; et al. Predicting of Sentinel Lymph Node Status in Breast Cancer Patients with Clinically Negative Nodes: A Validation Study. Cancers 2021, 13, 352. [Google Scholar] [CrossRef]
- Hasenoehrl, T.; Palma, S.; Ramazanova, D.; Kölbl, H.; Dorner, T.E.; Keilani, M.; Crevenna, R. Resistance exercise and breast cancer-related lymphedema-a systematic review update and meta-analysis. Support Care Cancer 2020, 28, 3593–3603. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Janda, M.; Cornish, B.; Battistutta, D.; Newman, B. Lymphedema After Breast Cancer: Incidence, Risk Factors, and Effect on Upper Body Function. J. Clin. Oncol. 2008, 26, 3536–3542. [Google Scholar] [CrossRef]
- Zaremski, J.L.; Zeppieri, G.; Tripp, B.L. Sport Specialization and Overuse Injuries in Adolescent Throwing Athletes: A Narrative Review. J. Athl. Train. 2019, 54, 1030–1039. [Google Scholar] [CrossRef] [Green Version]
- Greene, A.K.; Zurakowski, D.; Goss, J.A. Body Mass Index and Lymphedema Morbidity: Comparison of Obese versus Normal-Weight Patients. Plast. Reconstr. Surg. 2020, 146, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Duyur Cakıt, B.; Pervane Vural, S.; Ayhan, F.F. Complex Decongestive Therapy in Breast Cancer-Related Lymphedema: Does Obesity Affect the Outcome Negatively? Lymphat. Res. Biol. 2019, 17, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.A.; Shin, Y.B.; Shin, M.J.; Yun, R.Y.; Kim, K.Y.; Song, Y.S.; Bae, Y.; Lee, S.; Jung, Y.; Lee, S.H. An Assessment of the Relationship Between Abdominal Obesity and the Severity of Upper Extremity Lymphedema. Lymphat. Res. Biol. 2018, 16, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Ciudad, P.; Forte, A.J.; Huayllani, M.T.; Boczar, D.; Manrique, O.J.; Bustos, S.; Bustamante, A.; Chen, H.-C. Impact of body mass index on long-term surgical outcomes of vascularized lymph node transfer in lymphedema patients. Gland. Surg. 2020, 9, 603–613. [Google Scholar] [CrossRef]
| All patients (n = 206) | No Cellulitis (n = 137) | Cellulitis (n = 69) | p Value | ||
|---|---|---|---|---|---|
| Age (years) | Mean ± SD | 59.47 ± 10.05 | 58.87 ± 10.45 | 60.63 ± 9.17 | n.s |
| BMI (kg/m2) | Median (IQR) | 27.82 (7.13) | 28.55 (6.96) | 26.83 (7.48) | n.s |
| Breast cancer treatment variables | |||||
| Radiation therapy | N (%) | 193 (93.69%) | 128 (93.43%) | 63 (94.20%) | n.s |
| Chemotherapy | N (%) | 171 (83.41%) | 112 (82.35%) | 59 (85.51%) | n.s |
| Endocrine therapy | N (%) | 164 (80.79%) | 109 (81.34%) | 55 (79.71%) | n.s |
| Mastectomy | N (%) | 108 (52.43%) | 69 (50.36%) | 39 (56.52%) | n.s |
| Post-mastectomy reconstruction | N (%) | 48 (44.44%) | 29 (42.03%) | 19 (48.72%) | n.s |
| Abdominal free flap | N (%) | 21 (19.44%) | 13 (18.84%) | 8 (20.51%) | n.s |
| Pedicled back flap | N (%) | 16 (14.81%) | 13 (15.94%) | 5 (12.82%) | n.s |
| Implant | N (%) | 12 (11.11%) | 6 (8.70%) | 6 (15.38%) | n.s |
| Lymph nodes removed (No.) | Median (IQR) | 17 (7) | 17 (7) | 17 (7) | n.s |
| Clinical lymphedema characteristics | |||||
| Lymphedema latency (years) | Median (IQR) | 0.67 (1.43) | 0.66 (1.35) | 0.72 (1.79) | n.s |
| Lymphedema duration (years) | Median (IQR) | 4.40 (5.52) | 3.75 (4.42) | 7.24 (6.57) | <0.001 |
| Clinical pitting edema | N (%) | 113 (54.85%) | 65 (47.45%) | 48 (69.57%) | <0.05 |
| Dominant arm affected | N (%) | 98 (47.57%) | 61 (44.53%) | 37 (53.62%) | n.s |
| Compression sleeve | N (%) | 181 (87.86%) | 118 (86.13%) | 63 (91.30%) | n.s |
| Compression gauntlet | N (%) | 125 (60.68%) | 85 (62.04%) | 40 (57.97%) | n.s |
| Night compression | N (%) | 64 (31.07%) | 36 (26.28%) | 28 (40.58%) | <0.05 |
| Pneumatic compression device | N (%) | 40 (19.42%) | 20 (14.60%) | 20 (28.99%) | <0.05 |
| Dual Energy X-Ray Absorptiometry analysis | |||||
| Lymphedema excess volume (%) | Median (IQR) | 21.58 (29.03) | 14.36 (26.31) | 30.60 (27.82) | <0.001 |
| Lymphedema excess lean mass (%) | Median (IQR) | 13.57 (36.41) | 9.13 (30.48) | 21.30 (37.57) | <0.05 |
| Lymphedema excess fat mass (%) | Median (IQR) | 23.33 (39.55) | 20.51 (35.57) | 37.48 (50.53) | <0.05 |
| Lymphedema excess bone mass (%) | Median (IQR) | −2.00 (30.21) | −3.37 (28.86) | 2.03 (35.10) | n.s |
| Lymphedema arm: Fat mass proportion | Median (IQR) | 0.50 (0.15) | 0.50 (0.15) | 0.48 (0.12) | n.s |
| Healthy arm: Fat mass proportion | Median (IQR) | 0.47 (0.19) | 0.49 (0.19) | 0.45 (0.18) | n.s |
| Lymphedema arm: Lean mass proportion | Median (IQR) | 0.47 (0.12) | 0.46 (0.13) | 0.48 (0.11) | n.s |
| Healthy arm: Fat mass/arm ratio | Median (IQR) | 0.48 (0.16) | 0.47 (0.16) | 0.50 (0.15) | n.s |
| Bioimpedance spectroscopy analysis | |||||
| L-DEX | Median (IQR) | 21.90 (29.50) | 18.9 (28.50) | 30.2 (29.40) | <0.001 |
| Fat mass % | Median (IQR) | 32.52 (9.00) | 33.24 (9.07) | 31.04 (7.71) | n.s |
| Protein and minerals mass % | Median (IQR) | 18.07 (2.49) | 17.89 (2.42) | 18.40 (2.31) | n.s |
| Total body water % | Median (IQR) | 49.75 (6.80) | 49.34 (6.83) | 50.59 (5.87) | n.s |
| Intracellular fluid % | Median (IQR) | 54.64 (2.47) | 54.64 (2.53) | 54.67 (2.39) | n.s |
| Extracellular fluid % | Median (IQR) | 45.31 (2.43) | 45.32 (2.57) | 45.25 (2.34) | n.s |
| Fat-free mass % | Median (IQR) | 67.50 (9.19) | 66.97 (9.08) | 68.96 (8.05) | n.s |
| Active tissue mass % | Median (IQR) | 35.24 (5.18) | 35.03 (5.22) | 35.91 (5.00) | n.s |
| Extracellular mass % | Median (IQR) | 32.35 (5.07) | 32.08 (5.04) | 33.36 (4.71) | n.s |
| Smooth muscle mass % | Median (IQR) | 27.09 (5.22) | 26.76 (5.52) | 27.88 (4.46) | n.s |
| Basal metabolic rate (cals/day) | Median (IQR) | 1336.30 (235.25) | 1355.51 (226.50) | 1319.82 (256.00) | n.s |
| Phase angle (°) | Median (IQR) | 4.50 (0.90) | 4.50 (0.70) | 4.45 (0.90) | n.s |
| Hy-DEX hydration analysis | Median (IQR) | 13.95 (23.5) | 12.55 (19.4) | 16.5 (27.4) | n.s |
| Indocyanine green lymphangiography | |||||
| MD Anderson stage (median stage) | Median (IQR) | 3 (2) | 2 (2) | 3 (1) | <0.05 |
| Variables | Lymphedema Volume (%) | Lymphedema Fat Mass (%) | Lymphedema Lean Mass (%) | |||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-Value | Coefficient (95% CI) | p-Value | Coefficient (95% CI) | p-Value | |
| Age (per age) | −0.01 (−0.29; 0.26) | n.s | −0.45 (−1.14; 0.25) | n.s | 0.19 (−0.02; 0.59) | n.s |
| BMI (per point) | 1.44 (0.74; 2.13) | <0.001 | 0.69 (-1.12; 2.50) | n.s | 2.18 (1.21; 3.15) | <0.001 |
| Lymphedema duration (per year) | 0.18 (−0.51; 0.88) | n.s | 0.26 (−1.48; 2.00) | n.s | 0.31 (−0.67; 1.29) | n.s |
| Cellulitis (yes) | 12.35 (6.23; 18.47) | <0.001 | 20.74 (5.35; 36.12) | <0.001 | 9.95 (1.28; 18.62) | <0.05 |
| Bodily smooth muscle mass (per 1%) | 1.77 (−0.55; 3.00) | n.s | 2.99 (−0.07; 6.05) | n.s | 1.66 (−0.05; 3.38) | n.s |
| Bodily fat mass (per 1%) | −0.11 (−0.87; 0.65) | n.s | 0.16 (−1.72; 2.05) | n.s | 0.07 (−0.98; 1.12) | n.s |
| Lymphedema in dominant arm (yes) | 13.18 (7.62; 18.73) | <0.001 | 8.76 (−5.67; 23.18) | n.s | 17.36 (9.62; 25.10) | <0.001 |
| Variables | Lymph. Arm: Lean Mass Proportion | Healthy Arm: Lean Mass Proportion | Lymph. Arm: Fat Mass Proportion | Healthy Arm: Fat Mass Proportion | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-Value | Coefficient (95% CI) | p-Value | Coefficient (95% CI) | p-Value | Coefficient (95%CI) | p-Value | |
| Age (per age) | 0.01 (0.00; 0.01) | <0.05 | 0.00(−0.00; 0.01) | n.s | −0.01(−0.01; −0.00) | <0.05 | −0.00 (−0.01; 0.00) | n.s |
| BMI (per point) | −0.02 (−0.04; −0.01) | <0.001 | −0.04 (−0.05; −0.03) | <0.001 | 0.03 (0.02; 0.05) | <0.001 | 0.05 (0.03; 0.06) | <0.001 |
| Lymphedema duration (per year) | 0.00 (−0.02; 0.01) | n.s | −0.00 (−0.02; 0.01) | n.s | 0.00 (−0.01; 0.02) | n.s | 0.01 (−0.01; 0.02) | n.s |
| Cellulitis (yes) | −0.02 (−0.12; 0.09) | n.s | 0.03 (−0.07; 0.14) | n.s | 0.01 (−0.11; 0.13.) | n.s | −0.06 (−0.19; 0.07) | n.s |
| Bodily smooth muscle mass (per 1%) | 0.00 (−0.02; 2.10) | n.s | −0.00 (−0.02; 0.02) | n.s | 0.00 (−0.02; 0.03) | n.s | −0.00 (−0.03; 0.02) | n.s |
| Body fat mass (per 1%) | −0.02 (−0.03; -0.00) | <0.05 | −0.02 (−0.03; −0.00) | <0.05 | 0.02 (0.00; 0.03) | <0.05 | 0.02 (0.00; 0.03) | <0.05 |
| Lymphedema in dominant arm (yes) | 0.09 (−0.08;0.26) | n.s | 0.03 (−0.14; 0.20) | n.s | −0.11 (−0.29; 0.08) | n.s | −0.04 (−0.24; 0.17) | n.s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jørgensen, M.G.; Hermann, A.P.; Madsen, A.R.; Christensen, S.; Ingwersen, K.G.; Thomsen, J.B.; Sørensen, J.A. Cellulitis Is Associated with Severe Breast Cancer-Related Lymphedema: An Observational Study of Tissue Composition. Cancers 2021, 13, 3584. https://doi.org/10.3390/cancers13143584
Jørgensen MG, Hermann AP, Madsen AR, Christensen S, Ingwersen KG, Thomsen JB, Sørensen JA. Cellulitis Is Associated with Severe Breast Cancer-Related Lymphedema: An Observational Study of Tissue Composition. Cancers. 2021; 13(14):3584. https://doi.org/10.3390/cancers13143584
Chicago/Turabian StyleJørgensen, Mads Gustaf, Anne Pernille Hermann, Anette Riis Madsen, Steffanie Christensen, Kim Gordon Ingwersen, Jørn Bo Thomsen, and Jens Ahm Sørensen. 2021. "Cellulitis Is Associated with Severe Breast Cancer-Related Lymphedema: An Observational Study of Tissue Composition" Cancers 13, no. 14: 3584. https://doi.org/10.3390/cancers13143584
APA StyleJørgensen, M. G., Hermann, A. P., Madsen, A. R., Christensen, S., Ingwersen, K. G., Thomsen, J. B., & Sørensen, J. A. (2021). Cellulitis Is Associated with Severe Breast Cancer-Related Lymphedema: An Observational Study of Tissue Composition. Cancers, 13(14), 3584. https://doi.org/10.3390/cancers13143584






