Repurposing DPP-4 Inhibitors for Colorectal Cancer: A Retrospective and Single Center Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
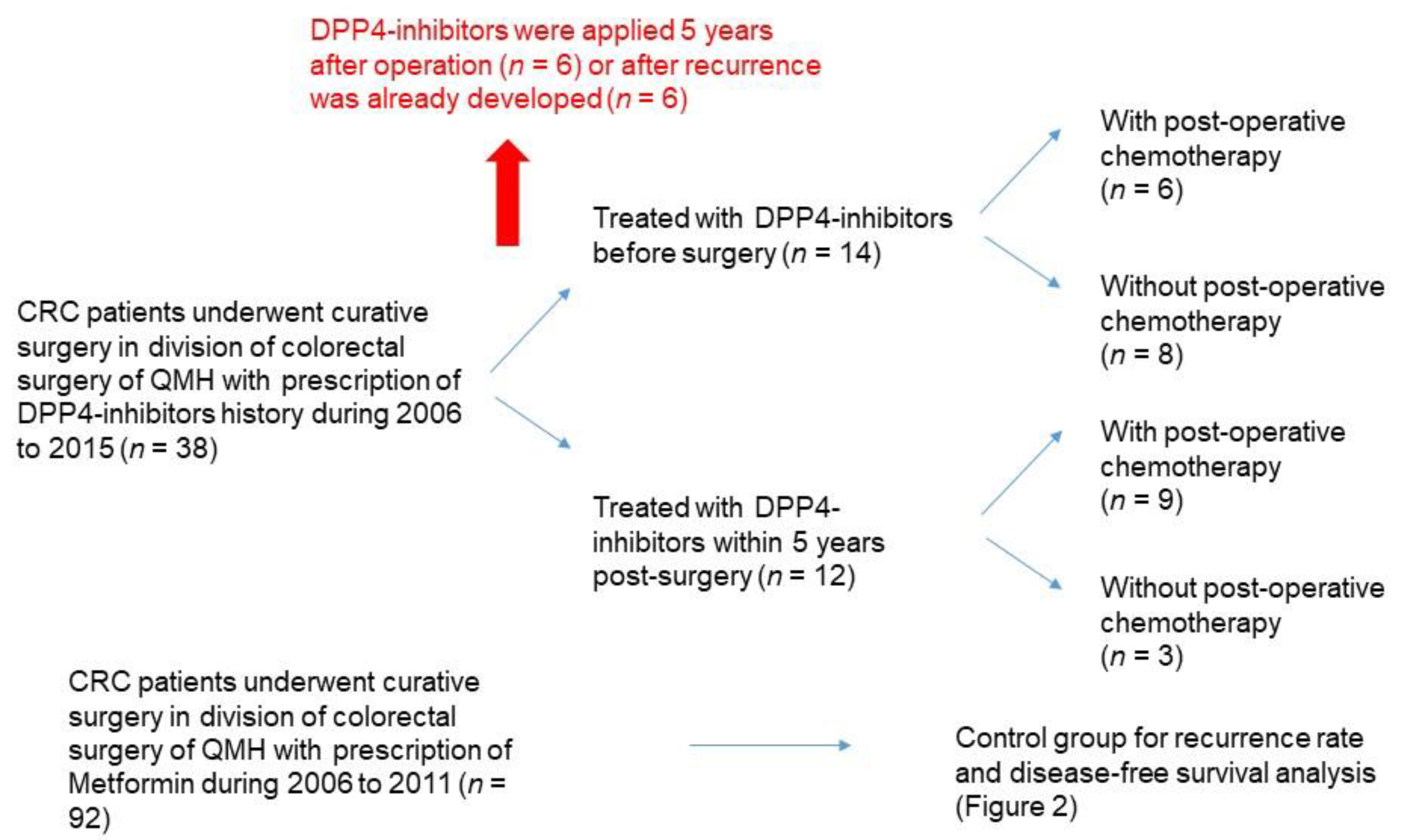
2.1. Subjects
2.2. Data Retrieval from Hong Kong Clinical Data Analysis and Reporting System (CDARS)
2.3. Statistical Analysis
3. Results
3.1. DPP4-Inhibitors Improve Prognosis of Post-Operative CRC Patients
3.2. DPP4-Inhibitors Alter the Immune Cell Profile of CRC Patients which Associates with a Better Prognosis
3.3. Immune Cell Profile of CRC Patients is a Potential Biomarker for Response to DPP4-Inhibitor Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Webb, E.S.; Liu, P.; Baleeiro, R.; Lemoine, N.R.; Yuan, M.; Wang, Y.H. Immune checkpoint inhibitors in cancer therapy. J. Biomed. Res. 2018, 32, 317–326. [Google Scholar] [CrossRef]
- Kamal, Y.; Schmit, S.L.; Frost, H.R.; Amos, C.I. The tumor microenvironment of colorectal cancer metastases: Opportunities in cancer immunotherapy. Immunotherapy 2020. [Google Scholar] [CrossRef]
- Makrilakis, K. The Role of DPP-4 Inhibitors in the Treatment Algorithm of Type 2 Diabetes Mellitus: When to Select, What to Expect. Int. J. Environ. Res. Public Health 2019, 16, 2720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Chen, J.; Yuan, Y.; Zou, Z.; Lai, X.; Rahmani, D.M.; Wang, F.; Xi, Y.; Huang, Q.; Bu, S. Dipeptidyl peptidase-4 inhibitors and cancer risk in patients with type 2 diabetes: A meta-analysis of randomized clinical trials. Sci. Rep. 2017, 7, 8273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, L.C.; Rados, D.V.; Barkan, S.S.; Leitao, C.B.; Gross, J.L. Dipeptidyl peptidase-4 inhibitors, pancreatic cancer and acute pancreatitis: A meta-analysis with trial sequential analysis. Sci. Rep. 2018, 8, 782. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.J.; Kim, D.J.; Shin, S. Incident cancer risk in dipeptidyl peptidase-4 inhibitor-treated patients with type 2 diabetes mellitus. Cancer Manag. Res. 2019, 11, 7427–7438. [Google Scholar] [CrossRef] [Green Version]
- Havre, P.A.; Abe, M.; Urasaki, Y.; Ohnuma, K.; Morimoto, C.; Dang, N.H. The role of CD26/dipeptidyl peptidase IV in cancer. Front. Biosci. J. Virtual Libr. 2008, 13, 1634–1645. [Google Scholar] [CrossRef]
- Pro, B.; Dang, N.H. CD26/dipeptidyl peptidase IV and its role in cancer. Histol. Histopathol. 2004, 19, 1345–1351. [Google Scholar] [CrossRef]
- Beckenkamp, A.; Davies, S.; Willig, J.B.; Buffon, A. DPPIV/CD26: A tumor suppressor or a marker of malignancy? Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 7059–7073. [Google Scholar] [CrossRef]
- Pang, R.; Law, W.L.; Chu, A.C.; Poon, J.T.; Lam, C.S.; Chow, A.K.; Ng, L.; Cheung, L.W.; Lan, X.R.; Lan, H.Y.; et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell 2010, 6, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.S.; Cheung, A.H.; Wong, S.K.; Wan, T.M.; Ng, L.; Chow, A.K.; Cheng, N.S.; Pak, R.C.; Li, H.S.; Man, J.H.; et al. Prognostic significance of CD26 in patients with colorectal cancer. PLoS ONE 2014, 9, e98582. [Google Scholar] [CrossRef] [Green Version]
- Grillet, F.; Bayet, E.; Villeronce, O.; Zappia, L.; Lagerqvist, E.L.; Lunke, S.; Charafe-Jauffret, E.; Pham, K.; Molck, C.; Rolland, N.; et al. Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut 2017, 66, 1802–1810. [Google Scholar] [CrossRef] [Green Version]
- Larrinaga, G.; Perez, I.; Sanz, B.; Beitia, M.; Errarte, P.; Fernandez, A.; Blanco, L.; Etxezarraga, M.C.; Gil, J.; Lopez, J.I. Dipeptidyl-peptidase IV activity is correlated with colorectal cancer prognosis. PLoS ONE 2015, 10, e0119436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Femia, A.P.; Raimondi, L.; Maglieri, G.; Lodovici, M.; Mannucci, E.; Caderni, G. Long-term treatment with Sitagliptin, a dipeptidyl peptidase-4 inhibitor, reduces colon carcinogenesis and reactive oxygen species in 1,2-dimethylhydrazine-induced rats. Int. J. Cancer 2013, 133, 2498–2503. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Baerts, L.; Waumans, Y.; De Meester, I.; Yamada, Y.; Limani, P.; Gil-Bazo, I.; Weder, W.; Jungraithmayr, W. Suppression of lung metastases by the CD26/DPP4 inhibitor Vildagliptin in mice. Clin. Exp. Metastasis 2015, 32, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, X.; Long, M.; Huang, Y.; Zhang, L.; Zhang, R.; Zheng, Y.; Liao, X.; Wang, Y.; Liao, Q.; et al. NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis. Sci. Transl. Med. 2016, 8, 334ra51. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.K.H.; Man, K.K.C.; Chan, E.W.Y.; Wu, T.; Tse, E.T.Y.; Wong, I.C.K.; Lam, C.L.K. DPP4i, thiazolidinediones, or insulin and risks of cancer in patients with type 2 diabetes mellitus on metformin-sulfonylurea dual therapy with inadequate control. BMJ Open Diabetes Res. Care 2020, 8. [Google Scholar] [CrossRef]
- Guo, G.; Wang, Y.; Zhou, Y.; Quan, Q.; Zhang, Y.; Wang, H.; Zhang, B.; Xia, L. Immune cell concentrations among the primary tumor microenvironment in colorectal cancer patients predicted by clinicopathologic characteristics and blood indexes. J. Immunother. Cancer 2019, 7, 179. [Google Scholar] [CrossRef]
- Dimitriou, N.; Felekouras, E.; Karavokyros, I.; Alexandrou, A.; Pikoulis, E.; Griniatsos, J. Neutrophils to lymphocytes ratio as a useful prognosticator for stage II colorectal cancer patients. BMC Cancer 2018, 18, 1202. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, Y.; Gao, P.; Chen, X.; Yu, D.; Xu, Y.; Zhao, J.; Wang, Z. The preoperative neutrophil to lymphocyte ratio is a superior indicator of prognosis compared with other inflammatory biomarkers in resectable colorectal cancer. BMC Cancer 2017, 17, 744. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Fuentes, A.; Skelton, W.I.; Wang, Y.; McGorray, S.; Shah, C.; Bishnoi, R.; Dang, L.H.; Dang, N.H. A multi-center retrospective analysis of the effect of DPP4 inhibitors on progression-free survival in advanced airway and colorectal cancers. Mol. Clin. Oncol. 2019, 10, 118–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishnoi, R.; Hong, Y.R.; Shah, C.; Ali, A.; Skelton, W.P.t.; Huo, J.; Dang, N.H.; Dang, L.H. Dipeptidyl peptidase 4 inhibitors as novel agents in improving survival in diabetic patients with colorectal cancer and lung cancer: A Surveillance Epidemiology and Endpoint Research Medicare study. Cancer Med. 2019, 8, 3918–3927. [Google Scholar] [CrossRef] [Green Version]
- Shao, S.; Xu, Q.; Yu, X.; Pan, R.; Chen, Y. Dipeptidyl peptidase 4 inhibitors and their potential immune modulatory functions. Pharmacol. Ther. 2020, 209, 107503. [Google Scholar] [CrossRef]
- Rohrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in Diabetes. Front. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef] [Green Version]
- Barreira da Silva, R.; Laird, M.E.; Yatim, N.; Fiette, L.; Ingersoll, M.A.; Albert, M.L. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat. Immunol. 2015, 16, 850–858. [Google Scholar] [CrossRef]
- Hollande, C.; Boussier, J.; Ziai, J.; Nozawa, T.; Bondet, V.; Phung, W.; Lu, B.; Duffy, D.; Paradis, V.; Mallet, V.; et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat. Immunol. 2019, 20, 257–264. [Google Scholar] [CrossRef]
- Klemann, C.; Wagner, L.; Stephan, M.; von Horsten, S. Cut to the chase: A review of CD26/dipeptidyl peptidase-4’s (DPP4) entanglement in the immune system. Clin. Exp. Immunol. 2016, 185, 1–21. [Google Scholar] [CrossRef] [Green Version]




| DPP4-Inhibitor Group | Control Group (Metformin) | |
|---|---|---|
| Gender | ||
| Male | 22 | 47 |
| Female | 4 | 45 |
| p = 0.005 | ||
| Age | ||
| Above 65 | 20 | 69 |
| Below or = 65 | 6 | 23 |
| p = 0.955 | ||
| Tumor stage * | ||
| Low | 15 | 50 |
| High | 10 | 41 |
| p = 0.823 | ||
| Single or combined DM-II treatment | ||
| Single | 11 | 92 |
| Combined (with Metformin) | 15 | |
| DPP4-inhibitor/dosage | ||
| Sitagliptin/50 mg daily | 6 | |
| Sitagliptin/100 mg daily | 7 | |
| Linagliptin/5 mg daily | 7 | |
| Vildagliptin/50 mg daily | 3 | |
| Vildagliptin/50 mg bi-daily | 2 | |
| Saxaglitptin/5 mg daily | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, L.; Foo, D.C.-C.; Wong, C.K.-H.; Man, A.T.-K.; Lo, O.S.-H.; Law, W.-L. Repurposing DPP-4 Inhibitors for Colorectal Cancer: A Retrospective and Single Center Study. Cancers 2021, 13, 3588. https://doi.org/10.3390/cancers13143588
Ng L, Foo DC-C, Wong CK-H, Man AT-K, Lo OS-H, Law W-L. Repurposing DPP-4 Inhibitors for Colorectal Cancer: A Retrospective and Single Center Study. Cancers. 2021; 13(14):3588. https://doi.org/10.3390/cancers13143588
Chicago/Turabian StyleNg, Lui, Dominic Chi-Chung Foo, Carlos King-Ho Wong, Abraham Tak-Ka Man, Oswens Siu-Hung Lo, and Wai-Lun Law. 2021. "Repurposing DPP-4 Inhibitors for Colorectal Cancer: A Retrospective and Single Center Study" Cancers 13, no. 14: 3588. https://doi.org/10.3390/cancers13143588






