Target Heterogeneity in Oncology: The Best Predictor for Differential Response to Radioligand Therapy in Neuroendocrine Tumors and Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. General Concept of Tumor Heterogeneity
2.1. Tumor Evolution and Its Non-Invasive Assessment
2.2. Target Heterogeneity in Cancers: Inter- and Intratumor Heterogeneity
2.3. Heterogeneity and Grading of Cancers
3. Approaches to Assess Tumor Heterogeneity
3.1. In Vitro Molecular Pathology
3.2. Serum-Based Biomarkers
3.3. Molecular Imaging-Based Biomarkers
3.4. Liquid Biopsy
3.5. Pharmacogenomics-Based Markers
4. Principles of Radioligand Therapy
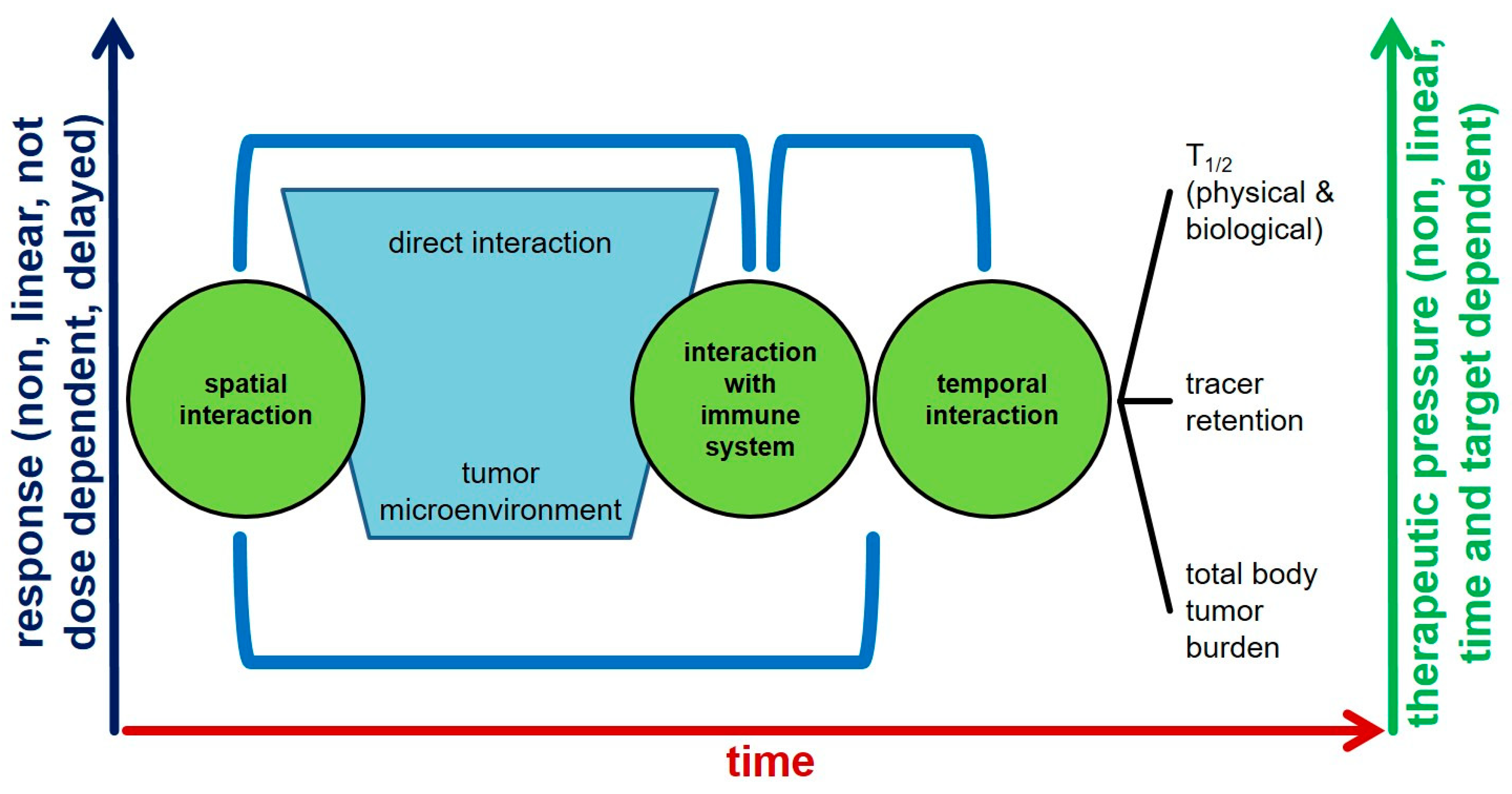
Radioligand Therapy and Tumor Heterogeneity
5. Predictors of Response to Radioligand Therapy
5.1. Imaging Features and Target Heterogeneity
5.2. Vascular Heterogeneity
5.3. Cellularity Heterogeneity
5.4. Intratumoral Heterogeneity: The Radiomics Approach
5.5. Tumor Proliferation Heterogeneity
5.6. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Hope, T.A.; Bodei, L.; Chan, J.A.; El-Haddad, G.; Fidelman, N.; Kunz, P.L.; Mailman, J.; Menda, Y.; Metz, D.C.; Mittra, E.S.; et al. NANETS/SNMMI Consensus Statement on Patient Selection and Appropriate Use of 177Lu-DOTATATE Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2020, 61, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef]
- McGranahan, N.; Swanton, C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution [published correction appears in Cancer Cell]. Cancer Cell 2015, 27, 15–26. [Google Scholar] [CrossRef]
- Von Bubnoff, N. Liquid Biopsy: Approaches to Dynamic Genotyping in Cancer. Oncol. Res. Treat. 2017, 40, 409–416. [Google Scholar] [CrossRef]
- Di Meo, A.; Bartlett, J.; Cheng, Y.; Pasic, M.D.; Yousef, G.M. Liquid biopsy: A step forward towards precision medicine in urologic malignancies. Mol. Cancer 2017, 16, 80. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Wright, N.; Rida, P.C.G.; Aneja, R. Tackling intra- and inter-tumor heterogeneity to combat triple negative breast cancer. Front. Biosci. 2017, 22, 1549–1580. [Google Scholar]
- Neuzillet, C.; Tijeras-Raballand, A.; Ragulan, C.; Cros, J.; Patil, Y.; Martinet, M.; Erkan, M.; Kleeff, J.; Wilson, J.; Apte, M.; et al. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J. Pathol. 2019, 248, 51–65. [Google Scholar] [CrossRef]
- Prasetyanti, P.R.; Medema, J.P. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol. Cancer 2017, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.; Rose, C.J.; Waterton, J.C.; Carano, R.A.; Parker, G.J.; Jackson, A. Imaging Intratumor Heterogeneity: Role in Therapy Response, Resistance, and Clinical Outcome. Clin. Cancer Res. 2015, 21, 249–257. [Google Scholar] [CrossRef]
- Walter, D.; Harter, P.N.; Battke, F.; Winkelmann, R.; Schneider, M.; Holzer, K.; Koch, C.; Bojunga, J.; Zeuzem, S.; Hansmann, M.L.; et al. Genetic heterogeneity of primary lesion and metastasis in small intestine neuroendo-crine tumors. Sci Rep. 2018, 8, 3811. [Google Scholar] [CrossRef] [PubMed]
- Tolkach, Y.; Kristiansen, G. The Heterogeneity of Prostate Cancer: A Practical Approach. Pathobiology 2018, 85, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Salazar, R.; Wiedenmann, B.; Rindi, G.; Ruszniewski, P. ENETS 2011 Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Tumors: An Update. Neuroendocrinology 2012, 95, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Kvols, L.K.; Brendtro, K.L. North American Neuroendocrine Tumor Society (NANETS). The North American Neuroendocrine Tumor Society (NANETS) guidelines: Mission, goals, and process. Pancreas 2010, 39, 705–706. [Google Scholar] [CrossRef]
- O’Toole, D.; Grossman, A.; Gross, D.; Fave, G.D.; Barkmanova, J.; Oconnor, J.M.; Pape, U.-F.; Plöckinger, U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Biochemical Markers. Neuroendocrinology 2009, 90, 194–202. [Google Scholar] [CrossRef]
- Papantoniou, D.; Grönberg, M.; Landerholm, K.; Welin, S.; Ziolkowska, B.; Nordvall, D.; Janson, E.T. Assessment of hormonal levels as prognostic markers and of their optimal cut-offs in small intestinal neuroendocrine tumours grade 2. Endocrine 2020, 72, 893–904. [Google Scholar] [CrossRef]
- Modlin, I.M.; Gustafsson, B.I.; Moss, S.F.; Pavel, M.; Tsolakis, A.V.; Kidd, M. Chromogranin A—Biological Function and Clinical Utility in Neuro Endocrine Tumor Disease. Ann. Surg. Oncol. 2010, 17, 2427–2443. [Google Scholar] [CrossRef]
- Vezzosi, D.; Walter, T.; Laplanche, A. Chromogranin A measurement in metastatic well-differentiated gastroentero-pancreatic neuroendocrine carcinoma: Screening for false positives and a prospective follow-up study. Int. J. Biol. Markers. 2011, 26, 94–101. [Google Scholar] [CrossRef]
- Sang, M.; Hulsurkar, M.; Zhang, X.; Song, H.; Zheng, D.; Zhang, Y.; Li, M.; Xu, J.; Zhang, S.; Ittmann, M.; et al. GRK3 is a direct target of CREB activation and regulates neuroendocrine differentiation of prostate cancer cells. Oncotarget 2016, 7, 45171–45185. [Google Scholar] [CrossRef]
- Li, Y.; Cozzi, P.J. Angiogenesis as a strategic target for prostate cancer therapy. Med. Res. Rev. 2009, 30, 23–66. [Google Scholar] [CrossRef]
- Desai, H.; Borges-Neto, S.; Wong, T.Z. Molecular Imaging and Therapy for Neuroendocrine Tumors. Curr. Treat. Opt. Oncol. 2019, 20, 78. [Google Scholar] [CrossRef] [PubMed]
- Hindié, E. The NETPET Score: Combining FDG and Somatostatin Receptor Imaging for Optimal Management of Patients with Metastatic Well-Differentiated Neuroendocrine Tumors. Theranostics 2017, 7, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Abdulrezzak, U.; Kurt, Y.K.; Kula, M.; Tutus, A. Combined imaging with 68Ga-DOTA-TATE and 18F-FDG PET/CT on the basis of volumetric parameters in neuroendocrine tumors. Nucl. Med. Commun. 2016, 37, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Prasad, V. “Monitoring Treatment,” in Clinical Nuclear Medicine, 4th ed.; Cook, G.J.R., Maisey, M.N., Britton, K.E., Chengazi, A.V., Eds.; Hodder Arnold: London, UK, 2006; pp. 57–78. [Google Scholar]
- Graf, J.; Pape, U.F.; Jann, H.; Denecke, T.; Arsenic, R.; Brenner, W.; Pavel, M.; Prasad, V. Prognostic Significance of Somatostatin Receptor Heterogeneity in Progressive Neuroendo-crine Tumor Treated with Lu-177 DOTATOC or Lu-177 DOTATATE. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 881–894. [Google Scholar] [CrossRef]
- Farolfi, A.; Fendler, W.; Iravani, A.; Haberkorn, U.; Hicks, R.; Herrmann, K.; Walz, J.; Fanti, S. Theranostics for Advanced Prostate Cancer: Current Indications and Future Developments. Eur. Urol. Oncol. 2019, 2, 152–162. [Google Scholar] [CrossRef]
- Ravi Kumar, A.S.; Hofman, M.S. Mechanistic Insights for Optimizing PSMA Radioligand Therapy. Clin Cancer Res. 2020, 26, 2774–2776. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Murphy, D.G.; et al. [177 Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Thang, S.P.; Violet, J.; Sandhu, S.; Iravani, A.; Akhurst, T.; Kong, G.; Hofman, M.S. Poor Outcomes for Patients with Metastatic Castration-resistant Prostate Cancer with Low Prostate-specific Membrane Antigen (PSMA) Expression Deemed Ineligible for 177Lu-labelled PSMA Radioligand Ther-apy. Eur. Urol. Oncol. 2019, 2, 670–676. [Google Scholar] [CrossRef]
- Schmidkonz, C.; Cordes, M.; Schmidt, D.; Bäuerle, T.; Goetz, T.I.; Beck, M.; Prante, O.; Cavallaro, A.; Uder, M.; Wullich, B.; et al. 68Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1862–1872. [Google Scholar] [CrossRef]
- Cheng, F.; Su, L.; Qian, C. Circulating tumor DNA: A promising biomarker in the liquid biopsy of cancer. Oncotarget 2016, 7, 48832–48841. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.G.; Gomella, L.G. Evolution of the Liquid Biopsy in Metastatic Prostate Cancer. Urology 2019, 132, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.M.; Kiezun, A.; Ramos, A.H.; Serra, S.; Pedamallu, C.S.; Qian, Z.R.; Banck, M.S.; Kanwar, R.; A Kulkarni, A.; Karpathakis, A.; et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat. Genet. 2013, 45, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Shi, C.; Edil, B.H.; De Wilde, R.F.; Klimstra, D.S.; Maitra, A.; Papadopoulos, N. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendo-crine tumors. Science 2011, 331, 1199–1203. [Google Scholar] [CrossRef]
- Oberg, K.; Modlin, I.; DeHerder, W.; Pavel, M.; Klimstra, D.; Frilling, A.; Metz, D.; Heaney, A.; Kwekkeboom, D.; Strosberg, J.; et al. Biomarkers for neuroendocrine tumor disease: A delphic consensus assessment of multianalytes, genomics, circulating cells and monoanalytes. Lancet Oncol. 2015, 16, e435–e446. [Google Scholar] [CrossRef]
- Modlin, I.M.; Kidd, M.; Malczewska, A.; Drozdov, I.; Bodei, L.; Matar, S.; Chung, K.M. The NETest: The Clinical Utility of Multigene Blood Analysis in the Diagnosis and Management of Neuroendocrine Tumors. Endocrinol. Metab. Clin. N. Am. 2018, 47, 485–504. [Google Scholar] [CrossRef]
- Pavel, M.; Jann, H.; Prasad, V.; Drozdov, I.; Modlin, I.M.; Kidd, M. NET Blood Transcript Analysis Defines the Crossing of the Clin-ical Rubicon: When Stable Disease Becomes Progressive. Neuroendocrinology 2017, 104, 170–182. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.S.; Singh, A.; Van Der Zwan, W.A.; Severi, S.; Drozdov, I.; Cwikla, J.B.; Baum, R.P.; Kwekkeboom, D.J.; Paganelli, G.; et al. PRRT genomic signature in blood for prediction of 177Lu-octreotate efficacy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1155–1169. [Google Scholar] [CrossRef]
- Kyriakopoulos, G.; Mavroeidi, V.; Chatzellis, E.; Kaltsas, G.A.; Alexandraki, K.I. Histopathological, immunohistochemical, genet-ic and molecular markers of neuroendocrine neoplasms. Ann. Transl. Med. 2018, 6, 252. [Google Scholar] [CrossRef]
- Balázs, K.; Antal, L.; Sáfrány, G.; Lumniczky, K. Blood-Derived Biomarkers of Diagnosis, Prognosis and Therapy Response in Prostate Cancer Patients. J. Pers. Med. 2021, 11, 296. [Google Scholar] [CrossRef]
- Oberg, K.; Akerström, G.; Rindi, G.; Jelic, S.; ESMO Guidelines Working Group. Neuroendocrine gastroenteropancreatic tu-mours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21, v223–v227. [Google Scholar] [CrossRef]
- Virgolini, I.; Traub, T.; Novotny, C.; Leimer, M.; Füger, B.; Li, S.; Patri, P.; Pangerl, T.; Angelberger, P.; Raderer, M.; et al. New trends in peptide receptor radioligands. Q. J. Nucl. Med. Off. Publ. Ital. Assoc. Nucl. Med. (AIMN)/Int. Assoc. Radiopharm. (IAR) 2001, 45, 153–159. [Google Scholar]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.-J. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Jalloul, M.; Azar, J.; Moubarak, M.M.; Samad, T.A.; Mukherji, D.; Al-Sayegh, M.; Abou-Kheir, W. Tumor Microenviron-ment in Prostate Cancer: Toward Identification of Novel Molecular Biomarkers for Diagnosis, Prognosis, and Therapy De-velopment. Front Genet. 2021, 12, 652747. [Google Scholar] [CrossRef]
- Lückerath, K.; Wei, L.; Fendler, W.P.; Axelsson, S.E.; Stuparu, A.D.; Slavik, R.; Mona, C.E.; Calais, J.; Rettig, M.; Reiter, R.E.; et al. Preclinical evaluation of PSMA expression in response to androgen receptor blockade for theranostics in prostate cancer. EJNMMI Res. 2018, 8, 96. [Google Scholar] [CrossRef]
- Khreish, F.; Kochems, N.; Rosar, F.; Sabet, A.; Ries, M.; Maus, S.; Ezziddin, S. Response and outcome of liver metastases in patients with metastatic castration-resistant prostate cancer (mCRPC) undergoing 177Lu-PSMA-617 radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging 2020, 48, 103–112. [Google Scholar] [CrossRef]
- Ruigrok, E.A.M.; van Weerden, W.M.; Nonnekens, J.; de Jong, M. The Future of PSMA-Targeted Radionuclide Therapy: An Over-view of Recent Preclinical Research. Pharmaceutics 2019, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Current, K.; Meyer, C.; Magyar, C.E.; Mona, C.E.; Almajano, J.; Slavik, R.; Stuparu, A.D.; Cheng, C.; Dawson, D.W.; Radu, C.G.; et al. Investigating PSMA-Targeted Radioligand Therapy Efficacy as a Function of Cellular PSMA Levels and Intratumoral PSMA Heterogeneity. Clin. Cancer Res. 2020, 26, 2946–2955. [Google Scholar] [CrossRef]
- Ronot, M.; Cuccioli, F.; Burgio, M.D.; Vullierme, M.-P.; Hentic, O.; Ruszniewski, P.; D’Assignies, G.; Vilgrain, V. Neuroendocrine liver metastases: Vascular patterns on triple-phase MDCT are indicative of primary tumour location. Eur. J. Radiol. 2017, 89, 156–162. [Google Scholar] [CrossRef]
- Dromain, C.; De Baere, T.; Lumbroso, J.; Caillet, H.; Laplanche, A.; Boige, V.; Ducreux, M.; Duvillard, P.; Elias, D.; Schlumberger, M.; et al. Detection of Liver Metastases from Endocrine Tumors: A Prospective Comparison of Somatostatin Receptor Scintigraphy, Computed Tomography, and Magnetic Resonance Imaging. J. Clin. Oncol. 2005, 23, 70–78. [Google Scholar] [CrossRef]
- Paulson, E.K.; McDermott, V.G.; Keogan, M.T.; Delong, D.M.; Frederick, M.G.; Nelson, R.C. Carcinoid metastases to the liver: Role of triple-phase helical CT. Radiology 1998, 206, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Foley, W.D.; Mallisee, T.A.; Hohenwalter, M.D.; Wilson, C.R.; Quiroz, F.A.; Taylor, A.J. Multiphase hepatic CT with a multirow detector CT scanner. AJR Am. J. Roentgenol. 2000, 175, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.H., 3rd; Baron, R.L.; Federle, M.P.; Jones, B.C.; Sheng, R. Hypervascular liver metastases: Do unenhanced and hepatic arterial phase CT images affect tumor detection? Radiology 1997, 205, 709–715. [Google Scholar] [CrossRef]
- Dromain, C.; De Baere, T.; Baudin, E.; Galline, J.; Ducreux, M.; Boige, V.; Duvillard, P.; Laplanche, A.; Caillet, H.; Lasser, P.; et al. MR Imaging of Hepatic Metastases Caused by Neuroendocrine Tumors: Comparing Four Techniques. Am. J. Roentgenol. 2003, 180, 121–128. [Google Scholar] [CrossRef]
- Elias, D.; Lefevre, J.H.; Duvillard, P.; Goéré, D.; Dromain, C.; Dumont, F.; Baudin, E. Hepatic metastases from neuroendocrine tumors with a “thin slice” pathological ex-amination: They are many more than you think. Ann. Surg. 2010, 251, 307–310. [Google Scholar] [CrossRef]
- Soyer, P.; Gueye, C.; Somveille, E.; Laissy, J.P.; Scherrer, A. MR diagnosis of hepatic metastases from neuroendocrine tumors versus hemangio-mas: Relative merits of dynamic gadolinium chelate-enhanced gradient-recalled echo and unenhanced spin-echo images. AJR Am. J. Roentgenol. 1995, 165, 1407–1413. [Google Scholar] [CrossRef]
- Marion-Audibert, A.M.; Barel, C.; Gouysse, G.; Dumortier, J.; Pilleul, F.; Pourreyron, C.; Scoazec, J.Y. Low microvessel density is an unfavorable histoprognostic factor in pan-creatic endocrine tumors. Gastroenterology 2003, 125, 1094–1104. [Google Scholar] [CrossRef]
- Takumi, K.; Fukukura, Y.; Higashi, M.; Ideue, J.; Umanodan, T.; Hakamada, H.; Kanetsuki, I.; Yoshiura, T. Pancreatic neuroendocrine tumors: Correlation between the contrast-enhanced computed tomography features and the pathological tumor grade. Eur. J. Radiol. 2015, 84, 1436–1443. [Google Scholar] [CrossRef]
- Kim, J.H.; Eun, H.W.; Kim, Y.J.; Han, J.K.; Choi, B.I. Staging accuracy of MR for pancreatic neuroendocrine tumor and imaging findings accord-ing to the tumor grade. Abdom. Imaging 2013, 38, 1106–1114. [Google Scholar] [CrossRef]
- De Robertis, R.; Cingarlini, S.; Martini, P.T.; Ortolani, S.; Butturini, G.; Landoni, L.; D’Onofrio, M. Pancreatic neuroendocrine neoplasms: Magnetic resonance imaging features according to grade and stage. World J. Gastroenterol. 2017, 23, 275–285. [Google Scholar] [CrossRef]
- Rodallec, M.; Vilgrain, V.; Couvelard, A.; Rufat, P.; O’Toole, D.; Barrau, V.; Menu, Y. Endocrine pancreatic tumours and helical CT: Contrast enhancement is correlat-ed with microvascular density, histoprognostic factors and survival. Pancreatology 2006, 6, 77–85. [Google Scholar] [CrossRef]
- d’Assignies, G.; Couvelard, A.; Bahrami, S.; Vullierme, M.P.; Hammel, P.; Hentic, O.; Vilgrain, V. Pancreatic Endocrine Tumors: Tumor Blood Flow Assessed with Perfusion CT Reflects Angiogenesis and Correlates with Prognostic Factors. Radiology 2009, 250, 407–416. [Google Scholar] [CrossRef]
- Marrache, F.; Vullierme, M.P.; Roy, C.; El Assoued, Y.; Couvelard, A.; O’Toole, D.; Mitry, E.; Hentic, O.; Hammel, P.; Lévy, P.; et al. Arterial phase enhancement and body mass index are predictors of response to chemoembolisation for liver metastases of endocrine tumours. Br. J. Cancer 2006, 96, 49–55. [Google Scholar] [CrossRef]
- Roche, A.; Girish, B.V.; De Baere, T.; Ducreux, M.; Elias, D.; Laplanche, A.; Boige, V.; Schlumberger, M.; Ruffle, P.; Baudin, E. Prognostic factors for chemoembolization in liver metastasis from endocrine tumors. Hepatogastroenterology 2004, 51, 1751–1756. [Google Scholar]
- Sahu, S.; Schernthaner, R.; Ardon, R.; Chapiro, J.; Zhao, Y.; Sohn, J.H.; Duran, R. Imaging Biomarkers of Tumor Response in Neuroendocrine Liver Metastases Treat-ed with Transarterial Chemoembolization: Can Enhancing Tumor Burden of the Whole Liver Help Predict Patient Survival? Radiology 2017, 283, 883–894. [Google Scholar] [CrossRef]
- Koh, D.-M.; Collins, D. Diffusion-Weighted MRI in the Body: Applications and Challenges in Oncology. Am. J. Roentgenol. 2007, 188, 1622–1635. [Google Scholar] [CrossRef]
- Kuroda, M.; Matsumoto, Y.; Matsuya, R.; Kato, H.; Shibuya, K.; Oita, M.; Kawabe, A.; Matsuzaki, H.; Asaumi, J.; Murakami, J.; et al. In vitro experimental study of the relationship between the apparent diffusion coefficient and changes in cellularity and cell morphology. Oncol. Rep. 2009, 22, 641–648. [Google Scholar] [CrossRef][Green Version]
- Lyng, H.; Haraldseth, O.; Rofstad, E.K. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn. Reson. Med. 2000, 43, 828–836. [Google Scholar] [CrossRef]
- Yoshikawa, M.I.; Ohsumi, S.; Sugata, S.; Kataoka, M.; Takashima, S.; Mochizuki, T.; Ikura, H.; Imai, Y. Relation between cancer cellularity and apparent diffusion coefficient values using diffusion-weighted magnetic resonance imaging in breast cancer. Radiat. Med. 2008, 26, 222–226. [Google Scholar] [CrossRef]
- Chen, L.; Liu, M.; Bao, J.; Xia, Y.; Zhang, J.; Zhang, L.; Huang, X.; Wang, J. The Correlation between Apparent Diffusion Coefficient and Tumor Cellularity in Patients: A Meta-Analysis. PLoS ONE 2013, 8, e79008. [Google Scholar] [CrossRef]
- d’Assignies, G.; Fina, P.; Bruno, O.; Vullierme, M.P.; Tubach, F.; Paradis, V.; Vilgrain, V. High sensitivity of diffusion-weighted MR imaging for the detection of liver metasta-ses from neuroendocrine tumors: Comparison with T2-weighted and dynamic gadolinium-enhanced MR imaging. Radiology 2013, 268, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Lotfalizadeh, E.; Ronot, M.; Wagner, M.; Cros, J.; Couvelard, A.; Vullierme, M.P.; Vilgrain, V. Prediction of pancreatic neuroendocrine tumour grade with MR imaging fea-tures: Added value of diffusion-weighted imaging. Eur. Radiol. 2016, 27, 1748–1759. [Google Scholar] [CrossRef]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Grenier, P.; Cabanis, E.; Laval-Jeantet, M. MR imaging of intravoxel incoherent motions: Application to diffusion and perfu-sion in neurologic disorders. Radiology 1986, 161, 401–407. [Google Scholar] [CrossRef]
- Chandarana, H.; Lee, V.S.; Hecht, E.; Taouli, B.; Sigmund, E.E. Comparison of biexponential and monoexponential model of diffusion weighted imaging in evaluation of renal lesions: Preliminary experience. Investig. Radiol. 2011, 46, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wei, M.; Han, Z.; Tang, Y.; Pan, Q.; Zhang, G.; Ren, J.; Huan, Y.; Li, N. The added value of intravoxel incoherent motion diffusion weighted imaging parameters in differentiating high-grade pancreatic neuroendocrine neoplasms from pancreatic ductal adenocarcinoma. Oncol. Lett. 2019, 18, 5448–5458. [Google Scholar] [CrossRef] [PubMed]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Limkin, E.J.; Sun, R.; Dercle, L.; Zacharaki, E.I.; Robert, C.; Reuzé, S.; Ferté, C. Promises and challenges for the implementation of computational medical imaging (radi-omics) in oncology. Ann. Oncol. 2017, 28, 1191–1206. [Google Scholar] [CrossRef]
- Guo, C.; Zhuge, X.; Wang, Z.; Wang, Q.; Sun, K.; Feng, Z.; Chen, X. Textural analysis on contrast-enhanced CT in pancreatic neuroendocrine neoplasms: Associa-tion with WHO grade. Abdom. Radiol. 2019, 44, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Hu, Y.; Ding, H.; Wei, J.; Chen, K.; Liu, H.; Zeng, M.; Tian, J. CT radiomics may predict the grade of pancreatic neuroendocrine tumors: A multicenter study. Eur. Radiol. 2019, 29, 6880–6890. [Google Scholar] [CrossRef]
- Canellas, R.; Burk, K.S.; Parakh, A.; Sahani, D.V. Prediction of Pancreatic Neuroendocrine Tumor Grade Based on CT Features and Tex-ture Analysis. AJR Am. J. Roentgenol. 2018, 210, 341–346. [Google Scholar] [CrossRef]
- Choi, T.W.; Kim, J.H.; Yu, M.H.; Park, S.J.; Han, J.K. Pancreatic neuroendocrine tumor: Prediction of the tumor grade using CT findings and computerized texture analysis. Acta Radiol. 2017, 59, 383–392. [Google Scholar] [CrossRef]
- Liang, W.; Yang, P.; Huang, R.; Xu, L.; Wang, J.; Liu, W.; Zhang, L.; Wan, D.; Huang, Q.; Lu, Y.; et al. A Combined Nomogram Model to Preoperatively Predict Histologic Grade in Pancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2019, 25, 584–594. [Google Scholar] [CrossRef]
- D’Onofrio, M.; Ciaravino, V.; Cardobi, N.; De Robertis, R.; Cingarlini, S.; Landoni, L.; Scarpa, A. CT Enhancement and 3D Texture Analysis of Pancreatic Neuroendocrine Neo-plasms. Sci. Rep. 2019, 9, 2176. [Google Scholar] [CrossRef]
- Reuzé, S.; Schernberg, A.; Orlhac, F.; Sun, R.; Chargari, C.; Dercle, L.; Deutsch, E.; Buvat, I.; Robert, C. Radiomics in Nuclear Medicine Applied to Radiation Therapy: Methods, Pitfalls, and Challenges. Int. J. Radiat. Oncol. 2018, 102, 1117–1142. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Boukheris, H.; Dromain, C.; Duvillard, P.; Leboulleux, S.; Elias, D.; Baudin, E. Prognostic factors influencing survival from metastatic (stage IV) gastroentero-pancreatic well-differentiated endocrine carcinoma. Endocr. Relat. Cancer 2009, 16, 585–597. [Google Scholar] [CrossRef]
- Palazzo, M.; Lombard-Bohas, C.; Cadiot, G.; Matysiak-Budnik, T.; Rebours, V.; Vullierme, M.-P.; Couvelard, A.; Hentic, O.; Ruszniewski, P. Ki67 proliferation index, hepatic tumor load, and pretreatment tumor growth predict the antitumoral efficacy of lanreotide in patients with malignant digestive neuroendocrine tumors. Eur. J. Gastroenterol. Hepatol. 2013, 25, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Madeira, I.; Terris, B.; Voss, M.; Denys, A.; Sauvanet, A.; Flejou, J.-F.; Vilgrain, V.; Belghiti, J.; Bernades, P.; Ruszniewski, P. Prognostic factors in patients with endocrine tumours of the duodenopancreatic area. Gut 1998, 43, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Dromain, C.; Pavel, M.E.; Ruszniewski, P.; Langley, A.; Massien, C.; Baudin, E.; Caplin, M.E. Tumor growth rate as a metric of progression, response, and prognosis in pan-creatic and intestinal neuroendocrine tumors. BMC Cancer 2019, 19, 66. [Google Scholar] [CrossRef]
- Fehr, D.; Veeraraghavan, H.; Wibmer, A.G.; Gondo, T.; Matsumoto, K.; Vargas, H.A.; Sala, E.; Hricak, H.; Deasy, J. Automatic classification of prostate cancer Gleason scores from multiparametric magnetic resonance images. Proc. Natl. Acad. Sci. USA 2015, 112, E6265–E6273. [Google Scholar] [CrossRef] [PubMed]
- Woźnicki, P.; Westhoff, N.; Huber, T.; Riffel, P.; Froelich, M.F.; Gresser, E.; Nörenberg, D. Multiparametric MRI for Prostate Cancer Characterization: Combined Use of Radi-omics Model with PI-RADS and Clinical Parameters. Cancers 2020, 12, 1767. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Zhao, W.; Wei, C.; Li, X.; Duan, S.; Ji, L.; Lu, Z.; Shen, J. Radiomics prediction model for the improved diagnosis of clinically significant prostate cancer on biparametric MRI. Quant. Imaging Med. Surg. 2020, 10, 368–379. [Google Scholar] [CrossRef]
- Xu, M.; Fang, M.; Zou, J.; Yang, S.; Yu, D.; Zhong, L.; Hu, C.; Zang, Y.; Dong, D.; Tian, J.; et al. Using biparametric MRI radiomics signature to differentiate between benign and malignant prostate lesions. Eur. J. Radiol. 2019, 114, 38–44. [Google Scholar] [CrossRef]
- Ma, S.; Xie, H.; Wang, H.; Yang, J.; Han, C.; Wang, X.; Zhang, X. Preoperative Prediction of Extracapsular Extension: Radiomics Signature Based on Magnetic Resonance Imaging to Stage Prostate Cancer. Mol. Imaging Biol. 2019, 22, 711–721. [Google Scholar] [CrossRef]
- Zhang, G.; Han, Y.; Wei, J.; Qi, Y.; Gu, D.; Lei, J.; Yan, W.; Xiao, Y.; Xue, H.; Feng, F.; et al. Radiomics Based on MRI as a Biomarker to Guide Therapy by Predicting Upgrading of Prostate Cancer from Biopsy to Radical Prostatectomy. J. Magn. Reson. Imaging 2020, 52, 1239–1248. [Google Scholar] [CrossRef]
- Gnep, K.; Fargeas, A.; Gutiérrez-Carvajal, R.E.; Commandeur, F.; Mathieu, R.; Ospina, J.D.; de Crevoisier, R. Haralick textural features on T(2)-weighted MRI are associated with bio-chemical recurrence following radiotherapy for peripheral zone prostate cancer. J. Magn. Reson. Imaging 2017, 45, 103–117. [Google Scholar] [CrossRef]
- Shiradkar, R.; Ghose, S.; Jambor, I.; Taimen, P.; Ettala, O.; Purysko, A.S.; Madabhushi, A. Radiomic features from pretreatment biparametric MRI predict prostate cancer bio-chemical recurrence: Preliminary findings. J. Magn. Reson. Imaging 2018, 48, 1626–1636. [Google Scholar] [CrossRef]
- Stoyanova, R.; Pollack, A.; Takhar, M.; Lynne, C.; Parra, N.; Lam, L.L.; Alshalalfa, M.; Buerki, C.; Castillo, R.; Jorda, M.; et al. Association of multiparametric MRI quantitative imaging features with prostate cancer gene expression in MRI-targeted prostate biopsies. Oncotarget 2016, 7, 53362–53376. [Google Scholar] [CrossRef]
- Fischer, S.; Tahoun, M.; Klaan, B.; Thierfelder, K.M.; Weber, M.-A.; Krause, B.J.; Hakenberg, O.; Fuellen, G.; Hamed, M. A Radiogenomic Approach for Decoding Molecular Mechanisms Underlying Tumor Progression in Prostate Cancer. Cancers 2019, 11, 1293. [Google Scholar] [CrossRef]
- Shi, Y.J.; Zhu, H.T.; Liu, Y.L.; Wei, Y.Y.; Qin, X.B.; Zhang, X.Y.; Sun, Y.S. Radiomics Analysis Based on Diffusion Kurtosis Imaging and T2 Weighted Imaging for Differ-entiation of Pancreatic Neuroendocrine Tumors from Solid Pseudopapillary Tumors. Front. Oncol. 2020, 10, 1624. [Google Scholar] [CrossRef]
- Bian, Y.; Li, J.; Cao, K.; Fang, X.; Jiang, H.; Ma, C.; Wang, L. Magnetic resonance imaging radiomic analysis can preoperatively predict G1 and G2/3 grades in patients with NF-pNETs. Abdom. Radiol. 2020, 46, 667–680. [Google Scholar] [CrossRef]
- Guo, C.-G.; Ren, S.; Chen, X.; Wang, Q.-D.; Xiao, W.-B.; Zhang, J.-F.; Duan, S.-F.; Wang, Z.-Q. Pancreatic neuroendocrine tumor: Prediction of the tumor grade using magnetic resonance imaging findings and texture analysis with 3-T magnetic resonance. Cancer Manag. Res. 2019, 11, 1933–1944. [Google Scholar] [CrossRef]
- Weber, M.; Kessler, L.; Schaarschmidt, B.; Fendler, W.P.; Lahner, H.; Antoch, G.; Rischpler, C. Treatment-related changes in neuroendocrine tumors as assessed by textural features derived from (68)Ga-DOTATOC PET/MRI with simultaneous acquisition of apparent diffusion coefficient. BMC Cancer 2020, 20, 326. [Google Scholar] [CrossRef]
- Paschalis, A.; Sheehan, B.; Riisnaes, R.; Rodrigues, D.N.; Gurel, B.; Bertan, C.; Ferreira, A.; Lambros, M.B.; Seed, G.; Yuan, W.; et al. Prostate-specific Membrane Antigen Heterogeneity and DNA Repair Defects in Prostate Cancer. Eur. Urol. 2019, 76, 469–478. [Google Scholar] [CrossRef]
- Busek, P.; Mateu, R.; Zubal, M.; Kotackova, L.; Sedo, A. Targeting fibroblast activation protein in cancer—Prospects and caveats. Front. Biosci. 2018, 23, 1933–1968. [Google Scholar]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Khurshid, Z.; Ahmadzadehfar, H.; Gaertner, F.C.; Papp, L.; Zsóter, N.; Essler, M.; Bundschuh, R.A. Role of textural heterogeneity parameters in patient selection for 177Lu-PSMA therapy via response prediction. Oncotarget 2018, 9, 33312–33321. [Google Scholar] [CrossRef]
- Werner, R.A.; Lapa, C.; Ilhan, H.; Higuchi, T.; Buck, A.K.; Lehner, S.; Bartenstein, P.; Bengel, F.; Schatka, I.; Muegge, D.O.; et al. Survival prediction in patients undergoing radionuclide therapy based on in-tratumoral somatostatin-receptor heterogeneity. Oncotarget 2017, 8, 7039–7049. [Google Scholar] [CrossRef]



| No of Patients | Images Phase/ROI/Sofware | Type RF | RF Correlated with G | |
|---|---|---|---|---|
| D’Onofiro et al., 2019 [84], Sci Rep | 100 | Pancreatic 2D ROI 1 slice MaZda 4.6 | 1st order | Kurtosis and entropy |
| Guo et al., 2018 [79], Abdo Imaging | 37 | Arterial 2D Five slices Matlab 2014a | 1st order | Mean grey level intensity |
| Canellas et al., 2018 [81], AJR | 101 | Portal TextRad 2D ROI | 1st order | Entropy |
| Choi et al., 2018 [82], Acta Radiol | 66 | Arterial + portal 2D ROI | 1st order | Sphericity, skewness, kurtosis |
| Gu et al., 2018 [80], Eur Radiol | 104 training 34 validation | Arterial and portal 3D ROI Pyradiomics 1.3.0 | 1st, 2nd, and 3rd order radiomics signature: 15 arterial RF + 10 portal RF from 853 RF | Radiomics signature on arterial + portal images Nomagram: Radiomics + tumor margin |
| Liang et al., 2019 [83], Clin Cancer Res | 86 training 51 validation | Arterial 3D ROI In-house software | 1st, 2nd, and 3rd order radiomics signature 8RF from 467 RF | Monogram: Radiomics signature on arterial + clinical stage |
| Author | Study Type | Application | Number of Patients | Results |
|---|---|---|---|---|
| Fehr et al., 2015 [90] | Retrospective | Cancer risk prediction | 217 | Textural features from T2WI and ADC could distinguish between different Gleason scores. Accuracy of 93% after cross-validation for discrimination of Gleason 6 (3 + 3) vs. Gleason ≥ 7, and 92% for discrimination of Gleason 3 + 4 = 7a vs. 4 + 3 = 7b. |
| Woźnicki et al., 2020 [91] | Retrospective | Cancer risk prediction | 191 | Radiomics characterizes prostatic index lesions accurate and perform comparable to radiologists for prostate cancer characterization. Prognostic machine learning models could help in detection of clinically significant prostate cancer and patient selection for MRI-guided fusion biopsy. |
| Li et al., 2020 [92] | Retrospective | Cancer risk prediction | 381 | 3 models were developed: a clinical model, a radiomics model (T2WI and ADC), and a clinical-radiomics combined model. Radiomic (AUC 0.98) and combined model (AUC 0.98) perform better in prediction of clinically significant cancer than clinical model (AUC 0.79) |
| Xu et al., 2019 [93] | Retrospective | Cancer risk prediction | 331 | 6 selected radiomics features of MRI (T2WI and ADC) performed better (AUC 0.92) than each alone (T2WI: AUC 0.81, ADC: AUC 0.89). Individual preoperative prediction model performs better when including clinical factors and radiomic features (clinical model: AUC 0.73; combined model: AUC 0.93). |
| Ma et al., 2020 [94] | Retrospective | Staging | 119 | Radiomics signature based on 17 features on T2WIs has the potential to predict preoperative risk of extracapsular extension, good performance in the validation set (AUC 0.821). |
| Zhang et al., 2020 [95] | Retrospective | Tumor grading | 166 | Radiomics model with signatures from T2WI, ADC and DCE perform better than any single sequence (AUC: radiomics model 0.87; AUC T2WI/ADC/DCE: 0.70/0.76/0.73). Combined model with radiomics signature, clinical stage, and time from biopsy to RP outperformed the clinical model and radiomics model (AUC: combined model 0.91, clinical model 0.65, radiomics model 0.87). MpMRI had the potential to predict tumor upgrade from biopsy to RP. |
| Gnep et al., 2017 [96] | Retrospective | Therapy response Biochemical recurrence | 74 | T2WI Haralick textural features appear be strongly correlate with biochemical recurrence after radiotherapy. |
| Shiradkar et al., 2018 [97] | Retrospective | Therapy response Biochemical recurrence | 120 | 10 extracted radiomic features from pretreatment T2WI and ADC are significantly correlated with BCR and could be used for BCR prediction; after radiotherapy? |
| Stoyanova et al., 2016 [98] | Retrospective | Radiogenomics | 17 | Radiomic features extracted from biopsy regions of primary tumors (?) and normal tissues correlate significant with gene signatures associated with adverse outcome. |
| Fischer et al., 2019 [99] | Retrospective | Radiogenomics | 298 | Biomarkers that play critical roles in PCa showed high correlation with aggressiveness-related imaging features extracted from mp-MRI images. The use of multi-omics data has the potential of significantly improving prediction of prostate cancer aggressiveness. |
| Author | Study Type | Application | Number of Patients | Results |
|---|---|---|---|---|
| Shi et al., 2020 [100] | Retrospective | Cancer risk prediction | 66 | Radiomics model based on diffusion kurtosis imaging (DKI) and T2 WI to discriminate pancreatic neuroendocrine tumors (PNETs) from solid pseudopapillary tumors (SPTs). 7 features of tumors were used to build radiomics model; the accuracy for diagnosis was higher than the radiologist (radiomics analysis 92.4%, radiologist 1 77.3%, radiologist 2 78.8%) and perform significantly better than of subjective diagnosis. |
| Bian et al., 2020 [101] | Retrospective | Tumor grading Primary or also mets? | 157 | 7 final radiomic features was used for rad-score calculation. Rad-score correlate significantly with NF-pNET grades. This radiomic model could help to differentiate G1 and G2/3 non-invasive. |
| Guo et al., 2019 [102] | Retrospective | Tumor grading Primary or also mets? | 77 | Preoperative T2WI and DWI was used for texture feature extraction. AUC of best predicting model on T2WI was 0.99 (Grade 1 vs. Grade 3). This radiomic model could help to predict pNETs grading. |
| Weber et al., 2020 [103] | Retrospective | Therapy response Primary or also mets? | 18 | In this small sample size, no parameter from PET or ADC predicted treatment response to PRRT on pretherapeutic 68Ga-DOTATOC-PET/MRI. Treatment responder showed a significant decrease in lesion volume on ADC maps, no other textural feature from PET or ADC was statistically significant for differentiation between responders and non-responders. |
| PET Radiopharmaceuticals | Measured Effect |
|---|---|
| F-18 fluorodeoxyglucose | Aerobic and anaerobic glycolysis, glucose consumption or metabolism |
| C-11 thymidine, F-18 fluorothymidine | DNA synthesis, tumor cell proliferation |
| C-11 methionine | Protein synthesis, tumor cell proliferation |
| C-11 choline, F-18 fluorocholine | Cell-membrane metabolism, tumor-cell proliferation |
| C-11 tyrosine, F-18 fluorotyrosine, F-18 fluoroethyltyrosine | Natural amino acid transport |
| F-18 fluorodihydroxyphenylalanine | Dopamine synthesis, natural amino acid transport |
| F-18 fluoromisonidazole | Tissue hypoxia, identification of hypoxic tumor cells |
| F-18 fluoro-17-β-estradiol | Estrogen-receptor status |
| F-18 annexin V | Apoptotic cell death |
| F-18 fluorouracil | Accumulation of 5-fluorouracil in tumor |
| C-11 acetate | Lipid synthesis |
| F-18 siTATE, Ga-68 DOTA-X, Cu-64 DOTA-X In-111-octreotide, Ga-68 somatostatin receptor antagonists | Somatostatin receptor status |
| Ga-68/In-111 herceptin affibody | HER-2 receptor status |
| Ga-68 NODAGA RGD | Tumor neoangeogenesis |
| F-18 FEBM | EGFR expression |
| Ga-68 exendin 4 | GLP 1 imaging |
| Ga-68 DOTA-mAB-F(ab’)2 cetuximab or HER3mAB105 | Receptor tyrosine kinases; resistance to PI3K and AKT inhibitors |
| F-18 PSMA, Ga-68 PSMA | Prostate-specific membrane antigen |
| Zr-89 nivolumab, F-18 BMS 986192, Cu-64 pembrolizumab, C-64 ipilimumab, etc. | PD-1, PDL-1, CTLA-4 |
| F-18, Ga-68-labeled FAPI | Tumor-associated fibroblast-activated protein |
| Ga-68 bombesin | Bombesin receptor, gastrin-releasing peptide receptors (GRPR) |
| Ga-68 pentixafor | CXCR-4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puranik, A.D.; Dromain, C.; Fleshner, N.; Sathekge, M.; Pavel, M.; Eberhardt, N.; Zengerling, F.; Marienfeld, R.; Grunert, M.; Prasad, V. Target Heterogeneity in Oncology: The Best Predictor for Differential Response to Radioligand Therapy in Neuroendocrine Tumors and Prostate Cancer. Cancers 2021, 13, 3607. https://doi.org/10.3390/cancers13143607
Puranik AD, Dromain C, Fleshner N, Sathekge M, Pavel M, Eberhardt N, Zengerling F, Marienfeld R, Grunert M, Prasad V. Target Heterogeneity in Oncology: The Best Predictor for Differential Response to Radioligand Therapy in Neuroendocrine Tumors and Prostate Cancer. Cancers. 2021; 13(14):3607. https://doi.org/10.3390/cancers13143607
Chicago/Turabian StylePuranik, Ameya D, Clarisse Dromain, Neil Fleshner, Mike Sathekge, Marianne Pavel, Nina Eberhardt, Friedemann Zengerling, Ralf Marienfeld, Michael Grunert, and Vikas Prasad. 2021. "Target Heterogeneity in Oncology: The Best Predictor for Differential Response to Radioligand Therapy in Neuroendocrine Tumors and Prostate Cancer" Cancers 13, no. 14: 3607. https://doi.org/10.3390/cancers13143607
APA StylePuranik, A. D., Dromain, C., Fleshner, N., Sathekge, M., Pavel, M., Eberhardt, N., Zengerling, F., Marienfeld, R., Grunert, M., & Prasad, V. (2021). Target Heterogeneity in Oncology: The Best Predictor for Differential Response to Radioligand Therapy in Neuroendocrine Tumors and Prostate Cancer. Cancers, 13(14), 3607. https://doi.org/10.3390/cancers13143607







