Fracture Risk of Long Bone Metastases: A Review of Current and New Decision-Making Tools for Prophylactic Surgery
Abstract
:Simple Summary
Abstract
1. Introduction
2. Fracture Risk Assessment in Long Bone Metastases: Standard Radiography and Nuclear Imaging Tools
2.1. The Mirels Scoring System
2.1.1. A Clinical–Radiological Composite Prognostic Score
2.1.2. Limitations of the Mirels Scoring System
2.2. Axial Cortical Involvement (ACI) and Circumferential Cortical Involvement (CCI)
2.3. Mirels Scoring System Applied to Scintigraphy
2.3.1. 99mTc MDP SPECT-CT
2.3.2. 18F-FDG PET-CT
3. Biomechanical Models Based on Quantitative Computed Tomography
3.1. Computed Tomography–Rigidity Analysis (CT-RA)
3.1.1. Modeling Bone Rigidity
3.1.2. Assessment of Impending Fractures
3.1.3. A Step forward—Curved-Beam CT-RA
3.2. Computed Tomography–Finite Element Analysis (CT-FEA)
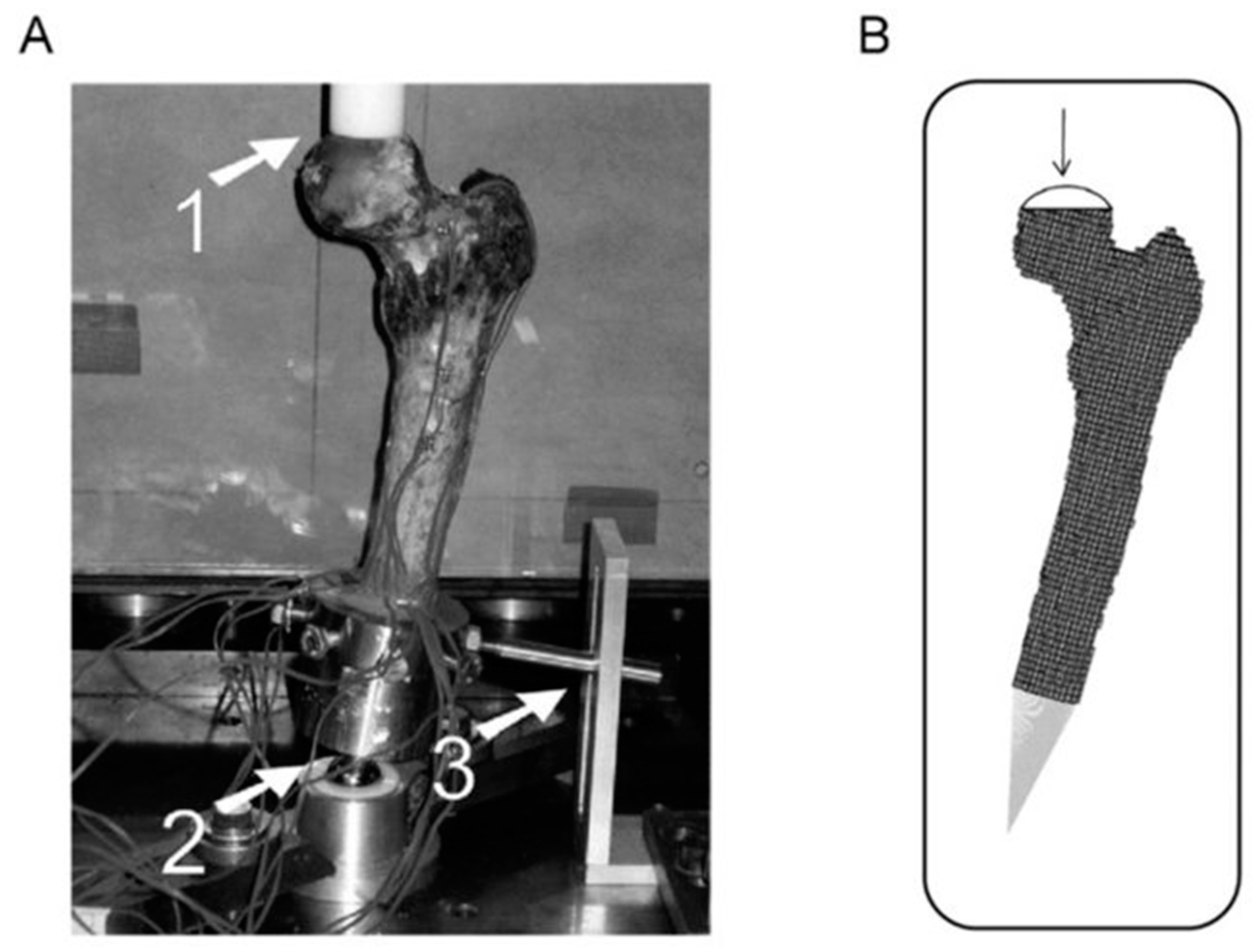
3.2.1. A Three-Dimensional Structural Modeling—Ex Vivo Studies
Femoral Load-Bearing Strength in CT-FEA
The Femoral Inner Cortex Thickness Threshold
3.2.2. Towards New Threshold Criteria: Strain Fold Ratio and Failure Load
3.2.3. FE Models: A Need for Global Standardization
Flattening the Inter-Scanner Differences in QCT Analysis
Standardized Modeling Constitutions in CT-FEA Modeling
The Particular Case of Blastic Lesions in CT-FEA Modeling
Selecting the Threshold Outcome Parameter in CT-FEA Modeling
4. What Are the Next Steps?
4.1. Net Benefit Analysis: A Help for Surgical Indications in MBD?
4.2. Machine Learning: Multimodal Data Management in a Single Decision-Making Tool
4.2.1. Real-Time FE Models Generation
4.2.2. Predicting Survival after Bone Metastases Surgery
4.2.3. Considering Both Radiological and Clinical Data at the Same Time
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roodman, G.D. Mechanisms of Bone Metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [Green Version]
- Body, J.-J.; Chevalier, P.; Gunther, O.; Hechmati, G.; Lamotte, M. The economic burden associated with skeletal-related events in patients with bone metastases secondary to solid tumors in Belgium. J. Med. Econ. 2013, 16, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Cvitkovic, F.; Mouret-Fourme, E. Épidémiologie et aspects cliniques des métastases osseuses. Bull. Cancer (Paris) 2013, 100, 1073–1081. [Google Scholar] [CrossRef]
- Carter, J.A.; Ji, X.; Botteman, M.F. Clinical, economic and humanistic burdens of skeletal-related events associated with bone metastases. Expert Rev. Pharmacoecon. Outcomes Res. 2013, 13, 483–496. [Google Scholar] [CrossRef]
- Coleman, R.E.; Rubens, R.D. The clinical course of bone metastases from breast cancer. Br. J. Cancer 1987, 55, 61–66. [Google Scholar] [CrossRef]
- Hirsh, V. Skeletal Disease Contributes Substantially to Morbidity and Mortality in Patients with Lung Cancer. Clin. Lung Cancer 2009, 10, 223–229. [Google Scholar] [CrossRef]
- Oefelein, M.G.; Ricchiuti, V.; Conrad, W.; Resnick, M.I. Skeletal Fractures Negatively Correlate With Overall Survival in Men With Prostate Cancer. J. Urol. 2002, 168, 1005–1007. [Google Scholar] [CrossRef]
- Saad, F.; Lipton, A.; Cook, R.; Chen, Y.-M.; Smith, M.; Coleman, R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 2007, 110, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.E.; De Hoedt, A.M.; Aronson, W.J.; Kane, C.J.; Amling, C.L.; Cooperberg, M.R.; Terris, M.K.; Divers, C.H.; Valderrama, A.; Freedland, S.J. Do skeletal-related events predict overall survival in men with metastatic castration-resistant prostate cancer? Prostate Cancer Prostatic Dis. 2016, 19, 380–384. [Google Scholar] [CrossRef] [Green Version]
- Saad, F.; Ivanescu, C.; Phung, D.; Loriot, Y.; Abhyankar, S.; Beer, T.M.; Tombal, B.; Holmstrom, S. Skeletal-related events significantly impact health-related quality of life in metastatic castration-resistant prostate cancer: Data from PREVAIL and AFFIRM trials. Prostate Cancer Prostatic Dis. 2017, 20, 110–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Weinfurt, K.P.; Li, Y.; Castel, L.D.; Saad, F.; Timbie, J.W.; Glendenning, G.A.; Schulman, K.A. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann. Oncol. 2005, 16, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Perisano, C.; Scaramuzzo, L.; De Santis, V.; Piccioli, A.; Ziranu, A.; Barone, C.; Maccauro, G. Quality of life following surgical treatment of lower limb metastases in long bone. J. Biol. Regul. Homeost. Agents 2015, 29, 501–507. [Google Scholar] [PubMed]
- Blank, A.T.; Lerman, D.M.; Patel, N.M.; Rapp, T.B. Is Prophylactic Intervention More Cost-effective Than the Treatment of Pathologic Fractures in Metastatic Bone Disease? Clin. Orthop. 2016, 474, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Antczak, C.; Trinh, V.Q.; Sood, A.; Ravi, P.; Roghmann, F.; Trudeau, V.; Chang, S.L.; Karakiewicz, P.I.; Kibel, A.S.; Krishna, N.; et al. The Health Care Burden of Skeletal Related Events in Patients with Renal Cell Carcinoma and Bone Metastasis. J. Urol. 2014, 191, 1678–1684. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Pala, E.; Romagnoli, C.; Romantini, M.; Calabro, T.; Ruggieri, P. Survival analysis of patients with femoral metastases. J. Surg. Oncol. 2012, 105, 135–141. [Google Scholar] [CrossRef]
- Ratasvuori, M.; Wedin, R.; Keller, J.; Nottrott, M.; Zaikova, O.; Bergh, P.; Kalen, A.; Nilsson, J.; Jonsson, H.; Laitinen, M. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg. Oncol. 2013, 22, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Errani, C.; Mavrogenis, A.F.; Cevolani, L.; Spinelli, S.; Piccioli, A.; Maccauro, G.; Baldini, N.; Donati, D. Treatment for long bone metastases based on a systematic literature review. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Piccioli, A.; Spinelli, M.S.; Maccauro, G. Impending fracture: A difficult diagnosis. Injury 2014, 45, S138–S141. [Google Scholar] [CrossRef]
- Benca, E.; Patsch, J.M.; Mayr, W.; Pahr, D.H.; Windhager, R. The insufficiencies of risk analysis of impending pathological fractures in patients with femoral metastases: A literature review. Bone Rep. 2016, 5, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Howard, E.L.; Shepherd, K.L.; Cribb, G.; Cool, P. The validity of the Mirels score for predicting impending pathological fractures of the lower limb. Bone Jt. J. 2018, 100-B, 1100–1105. [Google Scholar]
- Anract, P.; Biau, D.; Boudou-Rouquette, P. Metastatic fractures of long limb bones. Orthop. Traumatol. Surg. Res. 2017, 103, S41–S51. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.C.; Pritchard, D.J.; Sim, F.H. Surgical Treatment of Metastatic Disease of the Femur. J. Am. Acad. Orthop. Surg. 2000, 8, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.; Zeng, L.; Salvo, N.; Dennis, K.; Tsao, M.; Lutz, S. Update on the Systematic Review of Palliative Radiotherapy Trials for Bone Metastases. Clin. Oncol. 2012, 24, 112–124. [Google Scholar] [CrossRef]
- Roos, D.E. Radiotherapy for neuropathic pain due to bone metastases. Ann. Palliat. Med. 2015, 4, 5. [Google Scholar]
- Falkmer, U.; Järhult, J.; Wersäll, P.; Cavallin-ståhl, E. A Systematic Overview of Radiation Therapy Effects in Skeletal Metastases. Acta Oncol. 2003, 42, 620–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matuschek, C.; Ochtrop, T.A.; Bölke, E.; Ganswindt, U.; Fenk, R.; Gripp, S.; Kröpil, P.; Gerber, P.A.; Kammers, K.; Hamilton, J.; et al. Effects of Radiotherapy in the treatment of multiple myeloma: A retrospective analysis of a Single Institution. Radiat. Oncol. 2015, 10, 71. [Google Scholar] [CrossRef] [Green Version]
- Sze, W.M.; Shelley, M.D.; Held, I.; Wilt, T.J.; Mason, M.D. Palliation of Metastatic Bone Pain: Single Fraction versus Multifraction Radiotherapy—A Systematic Review of Randomised Trials. Clin. Oncol. 2003, 15, 345–352. [Google Scholar] [CrossRef]
- Chow, E.; Harris, K.; Fan, G.; Tsao, M.; Sze, W.M. Palliative Radiotherapy Trials for Bone Metastases: A Systematic Review. J. Clin. Oncol. 2007, 25, 1423–1436. [Google Scholar] [CrossRef]
- Rades, D.; Schild, S.E.; Abrahm, J.L. Treatment of painful bone metastases. Nat. Rev. Clin. Oncol. 2010, 7, 220–229. [Google Scholar] [CrossRef]
- Bartlow, C.M.; Mann, K.A.; Damron, T.A.; Oest, M.E. Altered mechanical behavior of demineralized bone following therapeutic radiation. J. Orthop. Res. 2021, 39, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Seinen, J.M.; Jutte, P.C.; Been, L.B.; Pras, E.; Hoekstra, H.J. Fractures after multimodality treatment of soft tissue sarcomas with isolated limb perfusion and radiation; likely to occur and hard to heal. Eur. J. Surg. Oncol. 2018, 44, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Fratto, M.E.; Vincenzi, B.; Galluzzo, S.; Tonini, G. Zoledronic acid in the management of metastatic bone disease. Expert Opin. Biol. Ther. 2006, 6, 1333–1348. [Google Scholar] [CrossRef]
- Body, J.-J.; Lipton, A.; Gralow, J.; Steger, G.G.; Gao, G.; Yeh, H.; Fizazi, K. Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. J. Bone Miner. Res. 2010, 25, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Wester, H.-J. Re-thinking the role of radiometal isotopes: Towards a future concept for theranostic radiopharmaceuticals. J. Label. Compd. Radiopharm. 2018, 61, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Barrio, M.; Fendler, W.P.; Czernin, J.; Herrmann, K. Prostate specific membrane antigen (PSMA) ligands for diagnosis and therapy of prostate cancer. Expert Rev. Mol. Diagn. 2016, 16, 1177–1188. [Google Scholar] [CrossRef]
- Pyka, T.; Okamoto, S.; Dahlbender, M.; Tauber, R.; Retz, M.; Heck, M.; Tamaki, N.; Schwaiger, M.; Maurer, T.; Eiber, M. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2114–2121. [Google Scholar] [CrossRef]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 85–90. [Google Scholar]
- Fernandez, R.; Eppard, E.; Lehnert, W.; Jimenez-Franco, L.D.; Soza-Ried, C.; Ceballos, M.; Ribbeck, J.; Kluge, A.; Roesch, F.; Meckel, M.; et al. Evaluation of safety and dosimetry of 177Lu DOTA-ZOL for therapy of bone metastases. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2021, 120, 255851. [Google Scholar]
- Mirels, H. The Classic: Metastatic Disease in Long Bones A Proposed Scoring System for Diagnosing Impending Pathologic Fractures. Clin. Orthop. 2003, 415, S4–S13. [Google Scholar] [CrossRef]
- Damron, T.A.; Morgan, H.; Prakash, D.; Grant, W.; Aronowitz, J.; Heiner, J. Critical Evaluation of Mirels’ Rating System for Impending Pathologic Fractures. Clin. Orthop. 2003, 415, S201–S207. [Google Scholar] [CrossRef]
- Evans, A.R.; Bottros, J.; Grant, W.; Chen, B.Y.; Damron, T.A. Mirels’ Rating for Humerus Lesions is Both Reproducible and Valid. Clin. Orthop. 2008, 466, 1279–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mac Niocaill, R.F.; Quinlan, J.F.; Stapleton, R.D.; Hurson, B.; Dudeney, S.; O’Toole, G.C. Inter- and intra-observer variability associated with the use of the Mirels’ scoring system for metastatic bone lesions. Int. Orthop. 2011, 35, 83–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Husseiny, M.; Coleman, N. Inter- and intra-observer variation in classification systems for impending fractures of bone metastases. Skelet. Radiol. 2010, 39, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, Y.M.; Dijkstra, P.D.S.; Kroon, H.M.; Lok, J.J.; Noordijk, E.M.; Leer, J.W.H.; Marijnen, C.A.M. Comparative analysis of risk factors for pathological fracture with femoral metastases. J. Bone Jt. Surg. Br. 2004, 86, 566–573. [Google Scholar] [CrossRef]
- Anez-Bustillos, L.; Derikx, L.C.; Verdonschot, N.; Calderon, N.; Zurakowski, D.; Snyder, B.D.; Nazarian, A.; Tanck, E. Finite element analysis and CT-based structural rigidity analysis to assess failure load in bones with simulated lytic defects. Bone 2014, 58, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Beals, R.K.; Lawton, G.D.; Snell, W.E. Prophylactic internal fixation of the femur in metastatic breast cancer. Cancer 1971, 28, 1350–1354. [Google Scholar] [CrossRef]
- Fidler, M. Incidence of Fracture Through Metastases in Long Bones. Acta Orthop. Scand. 1981, 52, 623–627. [Google Scholar] [CrossRef]
- Crenn, V.; Carlier, C.; Gouin, F.; Sailhan, F.; Bonnevialle, P. High rate of fracture in long-bone metastasis: Proposal for an improved Mirels predictive score. Orthop. Traumatol. Surg. Res. 2020, 106, 1005–1011. [Google Scholar] [CrossRef]
- Damron, T.A.; Ward, W.G. Risk of pathologic fracture: Assessment. Clin. Orthop. 2003, 415, S208–S211. [Google Scholar] [CrossRef]
- Hipp, J.A.; Springfield, D.S.; Hayes, W.C. Predicting pathologic fracture risk in the management of metastatic bone defects. Clin. Orthop. 1995, 312, 120–135. [Google Scholar]
- Lee, T. Predicting Failure Load of the Femur with Simulated Osteolytic Defects using Noninvasive Imaging Technique in a Simplified Load Case. Ann. Biomed. Eng. 2007, 35, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Keene, J.S.; Sellinger, D.S.; Mcbeath, A.A.; Engber, W.D. Metastatic Breast Cancer in the Femur A Search for the Lesion at Risk of Fracture. Clin. Orthop. 1986, 20, 282–288. [Google Scholar] [CrossRef]
- Howard, E.L.; Cool, P.; Cribb, G.L. Prediction of pathological fracture in patients with metastatic disease of the lower limb. Sci. Rep. 2019, 9, 14133. [Google Scholar] [CrossRef] [Green Version]
- Van der Wal, C.W.P.G.; Eggermont, F.; Fiocco, M.; Kroon, H.M.; Ayu, O.; Slot, A.; Snyers, A.; Rozema, T.; Verdonschot, N.J.J.; Dijkstra, P.D.S.; et al. Axial cortical involvement of metastatic lesions to identify impending femoral fractures; a clinical validation study. Radiother. Oncol. 2020, 144, 59–64. [Google Scholar] [CrossRef]
- Van der Linden, Y.M.; Kroon, H.M.; Dijkstra, S.P.D.S.; Lok, J.J.; Noordijk, E.M.; Leer, J.W.H.; Marijnen, C.A.M. Simple radiographic parameter predicts fracturing in metastatic femoral bone lesions: Results from a randomised trial. Radiother. Oncol. 2003, 69, 21–31. [Google Scholar] [CrossRef]
- Tatar, Z.; Soubrier, M.; Dillies, A.F.; Verrelle, P.; Boisgard, S.; Lapeyre, M. Assessment of the risk factors for impending fractures following radiotherapy for long bone metastases using CT scan-based virtual simulation: A retrospective study. Radiat. Oncol. 2014, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- O’rsquo, G.J.; Sullivan, F.L.C. Imaging of bone metastasis: An update. World J. Radiol. 2015, 7, 202–211. [Google Scholar]
- Ming, Y.; Wu, N.; Qian, T.; Li, X.; Wan, D.Q.; Li, C.; Li, Y.; Wu, Z.; Wang, X.; Liu, J.; et al. Progress and Future Trends in PET/CT and PET/MRI Molecular Imaging Approaches for Breast Cancer. Front. Oncol. 2020, 10, 1301. [Google Scholar] [CrossRef]
- Riaz, S.; Bashir, H.; Niazi, I.K.; Butt, S.; Qamar, F. 99mTc MDP SPECT-CT–Based Modified Mirels Classification for Evaluation of Risk of Fracture in Skeletal Metastasis: A Pilot Study. Clin. Nucl. Med. 2018, 43, e180–e183. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Zindman, A.M.; Zheng, J.; Kim, T.W.B.; Healey, J.H. FDG PET/CT Assesses the Risk of Femoral Pathological Fractures in Patients With Metastatic Breast Cancer. Clin. Nucl. Med. 2017, 42, 7. [Google Scholar] [CrossRef] [PubMed]
- Snyder, B.D.; Cordio, M.A.; Nazarian, A.; Kwak, S.D.; Chang, D.J.; Entezari, V.; Zurakowski, D.; Parker, L.M. Noninvasive Prediction of Fracture Risk in Patients with Metastatic Cancer to the Spine. Clin. Cancer Res. 2009, 15, 7676–7683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Cabe, G.D.; Tedrow, J.R.; Hipp, J.A.; Snyder, B.D. Failure of trabecular bone with simulated lytic defects can be predicted non-invasively by structural analysis. J. Orthop. Res. 2004, 22, 479–486. [Google Scholar] [CrossRef]
- Whealan, K.M.; Kwak, S.D.; Tedrow, J.R.; Inoue, K.; Snyder, B.D. Noninvasive Imaging Predicts Failure Load of the Spine with Simulated Osteolytic Defects*†. J. Bone Jt. Surg.-Am. Vol. 2000, 82, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Sas, A.; Tanck, E.; Sermon, A.; van Lenthe, G.H. Finite element models for fracture prevention in patients with metastatic bone disease. A literature review. Bone Rep. 2020, 12, 100286. [Google Scholar] [CrossRef]
- Leong, N.L.; Anderson, M.E.; Gebhardt, M.C.; Snyder, B.D. Computed Tomography-Based Structural Analysis for Predicting Fracture Risk in Children with Benign Skeletal Neoplasms: Comparison of Specificity with That of Plain Radiographs. J. Bone Jt. Surg. 2010, 92, 1827–1833. [Google Scholar] [CrossRef]
- Damron, T.A.; Nazarian, A.; Entezari, V.; Brown, C.; Grant, W.; Calderon, N.; Zurakowski, D.; Terek, R.M.; Anderson, M.E.; Cheng, E.Y.; et al. CT-based Structural Rigidity Analysis Is More Accurate Than Mirels Scoring for Fracture Prediction in Metastatic Femoral Lesions. Clin. Orthop. Relat. Res. 2016, 474, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Nazarian, A.; Entezari, V.; Zurakowski, D.; Calderon, N.; Hipp, J.A.; Villa-Camacho, J.C.; Lin, P.P.; Cheung, F.H.; Aboulafia, A.J.; Turcotte, R.; et al. Treatment Planning and Fracture Prediction in Patients with Skeletal Metastasis with CT-Based Rigidity Analysis. Clin. Cancer Res. 2015, 21, 2514–2519. [Google Scholar] [CrossRef] [Green Version]
- Oftadeh, R.; Karimi, Z.; Villa-Camacho, J.; Tanck, E.; Verdonschot, N.; Goebel, R.; Snyder, B.D.; Hashemi, H.N.; Vaziri, A.; Nazarian, A. Curved Beam Computed Tomography based Structural Rigidity Analysis of Bones with Simulated Lytic Defect: A Comparative Study with Finite Element Analysis. Sci. Rep. 2016, 6, 32397. [Google Scholar] [CrossRef] [Green Version]
- Hammer, A. The paradox of Wolff’s theories. Ir. J. Med. Sci. (1971-) 2014, 184, 13–22. [Google Scholar] [CrossRef]
- Mourtada, F.A.; Beck, T.J.; Hauser, D.L.; Ruff, C.B.; Bao, G. Curved beam model of the proximal femur for estimating stress using dual-energy x-ray absorptiometry derived structural geometry. J. Orthop. Res. 1996, 14, 483–492. [Google Scholar] [CrossRef]
- Feng, H.; Wang, J.; Xu, J.; Chen, W.; Zhang, Y. The surgical management and treatment of metastatic lesions in the proximal femur. Medicine 2016, 95, e3892. [Google Scholar] [CrossRef] [PubMed]
- Keyak, J.H.; Kaneko, T.S.; Tehranzadeh, J.; Skinner, H.B. Predicting Proximal Femoral Strength Using Structural Engineering Models . Clin. Orthop. 2005, 427, 219–228. [Google Scholar] [CrossRef]
- Tanck, E.; van Aken, J.B.; van der Linden, Y.M.; Schreuder, H.W.B.; Binkowski, M.; Huizenga, H.; Verdonschot, N. Pathological fracture prediction in patients with metastatic lesions can be improved with quantitative computed tomography based computer models. Bone 2009, 45, 777–783. [Google Scholar] [CrossRef] [PubMed]
- McBroom, R.J.; Cheal, E.J.; Hayes, W.C. Strength reductions from metastatic cortical defects in long bones. J. Orthop. Res. 1988, 6, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Cheal, E.J.; Hipp, J.A.; Hayes, W.C. Evaluation of finite element analysis for prediction of the strength reduction due to metastatic lesions in the femoral neck. J. Biomech. 1993, 26, 251–264. [Google Scholar] [CrossRef]
- Damron, T.A.; Mann, K.A. Fracture risk assessment and clinical decision making for patients with metastatic bone disease. J. Orthop. Res. 2020, 38, 1175–1190. [Google Scholar] [CrossRef] [PubMed]
- Keyak, J.H. Improved prediction of proximal femoral fracture load using nonlinear finite element models. Med. Eng. Phys. 2001, 23, 165–173. [Google Scholar] [CrossRef]
- Yosibash, Z.; Plitman Mayo, R.; Dahan, G.; Trabelsi, N.; Amir, G.; Milgrom, C. Predicting the stiffness and strength of human femurs with real metastatic tumors. Bone 2014, 69, 180–190. [Google Scholar] [CrossRef]
- Benca, E.; Synek, A.; Amini, M.; Kainberger, F.; Hirtler, L.; Windhager, R.; Mayr, W.; Pahr, D.H. QCT-based finite element prediction of pathologic fractures in proximal femora with metastatic lesions. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Derikx, L.C.; van Aken, J.B.; Janssen, D.; Snyers, A. The assessment of the risk of fracture in femora with metastatic lesions. J. Bone Jt. Surg. 2012, 94, 8. [Google Scholar] [CrossRef]
- Kawabata, Y.; Matsuo, K.; Nezu, Y.; Kamiishi, T.; Inaba, Y.; Saito, T. The risk assessment of pathological fracture in the proximal femur using a CT-based finite element method. J. Orthop. Sci. 2017, 22, 931–937. [Google Scholar] [CrossRef]
- Riglet, L.; Confavreux, C.; Chaudier, P.; Pialat, J.-B.; Bermond, F.; Gardegaront, M.; Follet, H.; Mitton, D. Ex vivo experiments on femurs to assess metastatic bone strength. Comput. Methods Biomech. Biomed. Eng. 2020, 23, S260–S261. [Google Scholar] [CrossRef]
- Sternheim, A.; Giladi, O.; Gortzak, Y.; Drexler, M.; Salai, M.; Trabelsi, N.; Milgrom, C.; Yosibash, Z. Pathological fracture risk assessment in patients with femoral metastases using CT-based finite element methods. A retrospective clinical study. Bone 2018, 110, 215–220. [Google Scholar] [CrossRef]
- Sternheim, A.; Traub, F.; Trabelsi, N.; Dadia, S.; Gortzak, Y.; Snir, N.; Gorfine, M.; Yosibash, Z. When and where do patients with bone metastases actually break their femurs? A CT-based finite element analysis. Bone Jt. J. 2020, 102-B, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, F.; van der Wal, G.; Westhoff, P.; Laar, A.; de Jong, M.; Rozema, T.; Kroon, H.M.; Ayu, O.; Derikx, L.; Dijkstra, S.; et al. Patient-specific finite element computer models improve fracture risk assessments in cancer patients with femoral bone metastases compared to clinical guidelines. Bone 2020, 130, 115101. [Google Scholar] [CrossRef] [PubMed]
- Rohlmann, A.; Mössner, U.; Bergmann, G.; Kölbel, R. Finite-Element-Analysis and experimental investigation of stresses in a femur. J. Biomed. Eng. 1982, 4, 241–247. [Google Scholar] [CrossRef]
- Polgar, K.; Gill, H.S.; Murray, D.W.; O’Connor, J.J. Strain distribution within the human femur due to physiological and simplified loading: Finite element analysis using the muscle standardized femur model. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2003, 217, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, C.; Whyne, C. Image-based finite-element modeling of the human femur. Comput. Methods Biomech. Biomed. Engin. 2020, 23, 1138–1161. [Google Scholar] [CrossRef]
- Carpenter, R.D.; Saeed, I.; Bonaretti, S.; Schreck, C.; Keyak, J.H.; Streeper, T.; Harris, T.B.; Lang, T.F. Inter-scanner differences in in vivo QCT measurements of the density and strength of the proximal femur remain after correction with anthropomorphic standardization phantoms. Med. Eng. Phys. 2014, 36, 1225–1232. [Google Scholar] [CrossRef] [Green Version]
- Paul, J.; Krauss, B.; Banckwitz, R.; Maentele, W.; Bauer, R.W.; Vogl, T.J. Relationships of clinical protocols and reconstruction kernels with image quality and radiation dose in a 128-slice CT scanner: Study with an anthropomorphic and water phantom. Eur. J. Radiol. 2012, 81, e699–e703. [Google Scholar] [CrossRef] [PubMed]
- Dragomir-Daescu, D.; Salas, C.; Uthamaraj, S.; Rossman, T. Quantitative computed tomography-based finite element analysis predictions of femoral strength and stiffness depend on computed tomography settings. J. Biomech. 2015, 48, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggermont, F.; Verdonschot, N.; van der Linden, Y.; Tanck, E. Calibration with or without phantom for fracture risk prediction in cancer patients with femoral bone metastases using CT-based finite element models. PLoS ONE 2019, 14, e0220564. [Google Scholar] [CrossRef] [Green Version]
- Helgason, B.; Gilchrist, S.; Ariza, O.; Vogt, P.; Enns-Bray, W.; Widmer, R.P.; Fitze, T.; Pálsson, H.; Pauchard, Y.; Guy, P.; et al. The influence of the modulus–density relationship and the material mapping method on the simulated mechanical response of the proximal femur in side-ways fall loading configuration. Med. Eng. Phys. 2016, 38, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Kazembakhshi, S.; Luo, Y. Constructing anisotropic finite element model of bone from computed tomography (CT). Biomed. Mater. Eng. 2014, 24, 2619–2626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panyasantisuk, J.; Dall’Ara, E.; Pretterklieber, M.; Pahr, D.H.; Zysset, P.K. Mapping anisotropy improves QCT-based finite element estimation of hip strength in pooled stance and side-fall load configurations. Med. Eng. Phys. 2018, 59, 36–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcinelli, C.; Di Martino, A.; Gizzi, A.; Vairo, G.; Denaro, V. Mechanical behavior of metastatic femurs through patient-specific computational models accounting for bone-metastasis interaction. J. Mech. Behav. Biomed. Mater. 2019, 93, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, A.; Kamm, R.D.; Moeendarbary, E. In Vitro Modeling of Mechanics in Cancer Metastasis. ACS Biomater. Sci. Eng. 2018, 4, 294–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hipp, J.A.; Rosenberg, A.E.; Hayes, W.C. Mechanical properties of trabecular bone within and adjacent to osseous metastases. J. Bone Miner. Res. 1992, 7, 1165–1171. [Google Scholar] [CrossRef]
- Kaneko, T.S.; Pejcic, M.R.; Tehranzadeh, J.; Keyak, J.H. Relationships between material properties and CT scan data of cortical bone with and without metastatic lesions. Med. Eng. 2003, 25, 445–454. [Google Scholar] [CrossRef]
- Eggermont, F.; Derikx, L.C.; Verdonschot, N.; van der Geest, I.C.M.; de Jong, M.A.A.; Snyers, A.; van der Linden, Y.M.; Tanck, E. Can patient-specific finite element models better predict fractures in metastatic bone disease than experienced clinicians? Towards computational modelling in daily clinical practice. Bone Jt. Res. 2018, 7, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Goodheart, J.R.; Cleary, R.J.; Damron, T.A.; Mann, K.A. Simulating activities of daily living with finite element analysis improves fracture prediction for patients with metastatic femoral lesions: Fracture risk of metastatic lesions. J. Orthop. Res. 2015, 33, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, G.; Deuretzbacher, G.; Heller, M.; Graichen, F.; Rohlmann, A.; Strauss, J.; Duda, G.N. Hip contact forces and gait patterns from routine activities. J. Biomech. 2001, 34, 859–871. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision Curve Analysis: A Novel Method for Evaluating Prediction Models. Med. Decis. Mak. 2006, 26, 565–575. [Google Scholar] [CrossRef] [Green Version]
- Kerr, K.F.; Brown, M.D.; Zhu, K.; Janes, H. Assessing the Clinical Impact of Risk Prediction Models With Decision Curves: Guidance for Correct Interpretation and Appropriate Use. J. Clin. Oncol. 2016, 34, 2534–2540. [Google Scholar] [CrossRef] [Green Version]
- Toci, G.R.; Bressner, J.A.; Morris, C.D.; Fayad, L.; Levin, A.S. Can a Novel Scoring System Improve on the Mirels Score in Predicting the Fracture Risk in Patients with Multiple Myeloma? Clin. Orthop. 2021, 479, 521–530. [Google Scholar] [CrossRef]
- Miotto, R.; Wang, F.; Wang, S.; Jiang, X.; Dudley, J.T. Deep learning for healthcare: Review, opportunities and challenges. Brief. Bioinform. 2018, 19, 1236–1246. [Google Scholar] [CrossRef]
- Zhou, L.; Pan, S.; Wang, J.; Vasilakos, A. Machine Learning on Big Data: Opportunities and Challenges. Neurocomputing 2017, 237, 350–361. [Google Scholar] [CrossRef] [Green Version]
- Phellan, R.; Hachem, B.; Clin, J.; Mac-Thiong, J.; Duong, L. Real-time biomechanics using the finite element method and machine learning: Review and perspective. Med. Phys. 2021, 48, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Galassi, A.; Martín-Guerrero, J.D.; Villamor, E.; Monserrat, C.; Rupérez, M.J. Risk Assessment of Hip Fracture Based on Machine Learning. Appl. Bionics Biomech. 2020, 2020, 8880786. [Google Scholar] [CrossRef] [PubMed]
- Hambli, R. Apparent damage accumulation in cancellous bone using neural networks. J. Mech. Behav. Biomed. Mater. 2011, 4, 868–878. [Google Scholar] [CrossRef]
- Forsberg, J.A.; Eberhardt, J.; Boland, P.J.; Wedin, R.; Healey, J.H. Estimating Survival in Patients with Operable Skeletal Metastases: An Application of a Bayesian Belief Network. PLoS ONE 2011, 6, e19956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meares, C.; Badran, A.; Dewar, D. Prediction of survival after surgical management of femoral metastatic bone disease—A comparison of prognostic models. J. Bone Oncol. 2019, 15, 100225. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.B.; Wedin, R.; Fabbri, N.; Boland, P.; Healey, J.; Forsberg, J.A. External Validation of PATHFx Version 3.0 in Patients Treated Surgically and Nonsurgically for Symptomatic Skeletal Metastases. Clin. Orthop. 2020, 478, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B.; Schenker, N. Multiple imputation in health-are databases: An overview and some applications. Stat. Med. 1991, 10, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Cumming, D.; Cumming, J.; Vince, A.; Benson, R. Metastatic bone disease: The requirement for improvement in a multidisciplinary approach. Int. Orthop. 2009, 33, 493–496. [Google Scholar] [CrossRef] [Green Version]
- Oh, E.; Seo, S.W.; Yoon, Y.C.; Kim, D.W.; Kwon, S.; Yoon, S. Prediction of pathologic femoral fractures in patients with lung cancer using machine learning algorithms: Comparison of computed tomography-based radiological features with clinical features versus without clinical features. J. Orthop. Surg. Hong Kong 2017, 25, 2309499017716243. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wen, X.; Lu, Y.; Yao, Y.; Zhao, H. Exploiting machine learning for predicting skeletal-related events in cancer patients with bone metastases. Oncotarget 2016, 7, 12612–12622. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Harvey, N.C.; Johansson, H.; Oden, A.; Leslie, W.D.; McCLoskey, E. FRAX Update. J. Clin. Densitom. Assess. Manag. Musculoskelet. Health 2017, 20, 360–367. [Google Scholar] [CrossRef]
- Kong, S.H.; Ahn, D.; Kim, B.; Srinivasan, K.; Ram, S.; Kim, H.; Hong, A.R.; Kim, J.H.; Cho, N.H.; Shin, C.S. A Novel Fracture Prediction Model Using Machine Learning in a Community-Based Cohort. JBMR Plus 2020, 4, e10337. [Google Scholar] [CrossRef]

| Score | Site of Lesion | Size of Lesion | Nature of Lesion | Pain |
|---|---|---|---|---|
| 1 | Upper limb | <1/3 of cortex | Blastic | Mild |
| 2 | Lower limb | 1/3–2/3 of cortex | Mixed | Moderate |
| 3 | Trochanteric region | >2/3 of cortex | Lytic | Functional |
| Fracture Risk Assessment | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Mirels score > 9 [46] | 100% | 13% | 14% | 94% |
| ACI > 30 mm [46] | 86% | 58% | 23% | 97% |
| CCI > 30% [58] | 100% | 89% | 71% | 100% |
| Predictive Tool | Population | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|
| Mirels score > 8 | |||||
| Sternheim et al., 2020 [86] | Femur palliative RT (n = 41) | 0.88 (0.47–0.99) | 0.38 (0.47–0.99) | 0.32 (0.19–0.59) | 0.90 (0.55–1.00) |
| Damron et al., 2016 [68] | Femoral MBD * (n = 78) | 0.67 (0.22–0.96) | 0.48 (0.36–0.60) | 0.10 (0.02–0.23) | 0.94 (0.81–0.99) |
| Van der Wal et al., 2020 [56] | Femur palliative RT (n = 100) | 0.77 | 0.45 | 0.17 | 0.93 |
| ACI > 30 mm | |||||
| Eggermont et al., 2020 [87] | Femur palliative RT (n = 50) | 0.86 | 0.42 | 0.19 | 0.95 |
| Van der Wal et al., 2020 [56] | Femur palliative RT (n = 100) | 0.86 | 0.50 | 0.20 | 0.96 |
| 18F-FDG PET CT 1 | |||||
| Ulaner et al., 2017 [62] | Proximal femur fracture † (n = 27) | 0.85 (0.65–0.96) | 0.80 (0.67–0.90) | 0.67 (0.48–0.82) | 0.91 (0.80–0.98) |
| CT-RA 2 | |||||
| Damron et al., 2016 [68] | Femoral MBD * (n = 78) | 0.99 (0.54–1.00) | 0.61 (0.48–0.82) | 0.18 (0.07–0.35) | 1.00 (0.92–1.00) |
| CT-FEA | |||||
| Eggermont et al., 2020 3 [87] | Femur palliative RT (n = 50) | 0.86 | 0.74 | 0.39 | 1.00 |
| Sternheim et al., 2020 4 [86] | Femur palliative RT (n = 41) | 1.00 (0.66–1.00) | 0.69 (0.45–0.84) | 0.53 (0.28–0.77) | 1.00 (0.79–1.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyễn, M.-V.; Carlier, C.; Nich, C.; Gouin, F.; Crenn, V. Fracture Risk of Long Bone Metastases: A Review of Current and New Decision-Making Tools for Prophylactic Surgery. Cancers 2021, 13, 3662. https://doi.org/10.3390/cancers13153662
Nguyễn M-V, Carlier C, Nich C, Gouin F, Crenn V. Fracture Risk of Long Bone Metastases: A Review of Current and New Decision-Making Tools for Prophylactic Surgery. Cancers. 2021; 13(15):3662. https://doi.org/10.3390/cancers13153662
Chicago/Turabian StyleNguyễn, Mỹ-Vân, Christophe Carlier, Christophe Nich, François Gouin, and Vincent Crenn. 2021. "Fracture Risk of Long Bone Metastases: A Review of Current and New Decision-Making Tools for Prophylactic Surgery" Cancers 13, no. 15: 3662. https://doi.org/10.3390/cancers13153662
APA StyleNguyễn, M.-V., Carlier, C., Nich, C., Gouin, F., & Crenn, V. (2021). Fracture Risk of Long Bone Metastases: A Review of Current and New Decision-Making Tools for Prophylactic Surgery. Cancers, 13(15), 3662. https://doi.org/10.3390/cancers13153662






