Lobular Breast Cancer: Histomorphology and Different Concepts of a Special Spectrum of Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Concepts and Perceptions of ILC and Their Influence on BC Diagnosis
2.1. ILC as Carcinoma Arising in Lobules
2.2. ILC as Carcinoma Growing in Single Files
2.3. ILC as a Morpho-Molecular Entity
3. Histomorphology
3.1. Classic ILC
3.2. Related In Situ Lesions
3.3. Immunohistochemical Features
4. Histologic ILC Variants
4.1. Relevance of ILC Variants
4.2. Histiocytoid ILC
4.3. Solid ILC and Solid-Papillary ILC
4.4. Signet Ring Cell-Rich ILC
4.5. Tubulolobular BC—Possibly Not an ILC Variant
4.6. ILC with Tubular Elements
4.7. Alveolar ILC
4.8. Trabecular and Plexiform ILC
4.9. Mixed Non-Classical ILC
4.10. Pleomorphic ILC
4.11. ILC with Extracellular Mucin
4.12. ILC with Neuroendocrine Features
4.13. ILC of the Diffuse Type
5. Advances
5.1. Laminin and Pagetoid Extension of LCIS
5.2. E-cadherin to P-cadherin Switching and Tubular Elements
5.3. ILC and the Microenvironment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| BC | |||||
|---|---|---|---|---|---|
| Cases | NST | Mixed (NST/ILC) | ILC | Ref. | |
| Christgen et al. (2020) a | 2515 | 86% | <1% | 14% | [61] |
| Flores-Diaz et al. (2019) b | 4733 | 86% | 4% | 10% | [70] |
| Ciriello et al. (2015) c | 705 | 70% | 12% | 18% | [42] |
| Braunstein et al. (2015) d | 998 | 74% | 18% | 8% | [71] |
| Bharat et al. (2009) e | 4336 | 83% | 6% | 11% | [69] |
| Louwman et al. (2007) f | 145,180 | 84% | 4% | 12% | [68] |
| Sastre-Garau et al. (1996) g | 11,036 | 91% | 2% | 7% | [27] |
| Martinez and Azzopardi (1979) h | 203 | 80% | 5% | 15% | [67] |
| Study | Cases | Loss of E-Cadherin | CDH1 Mutation | Ref. |
|---|---|---|---|---|
| Rinaldi et al. (2020) | 611 | 79% | [119] | |
| Christgen et al. (2020) | 353 | 96% | [61] | |
| Christgen et al. (2020) | 13 | 100% | 85% | [59] |
| Sokol et al. (2019) | 180 | 77% | [118] | |
| Christgen et al. (2019) | 106 | 99% | 41% | [117] |
| Zhu et al. (2018) | 17 | 59% | [116] | |
| Desmedt et al. (2016) | 413 | 85% | 65% | [43] |
| Sakr et al. (2016) | 21 | 100% | 66% | [82] |
| McCart-Reed et al. (2015) | 148 | 76% | [115] | |
| Ciriello et al. (2015) | 127 | 63% | [42] | |
| Christgen et al. (2013) | 43 | 98% | [114] | |
| Boyault et al. (2012) | 12 | 58% | [113] | |
| Koboldt et al. (2012) | 36 | 83% | [112] | |
| Lips et al. (2012) | 75 | 85% | [111] | |
| Ellis et al. (2012) | 40 | 50% | [110] | |
| Rakha et al. (2010) | 239 | 84% | [109] | |
| Zou et al. (2009) | 20 | 100% | [108] | |
| Bertucci et al. (2008) | 21 | 86% | 62% | [41] |
| Da Silva et al. (2008) | 25 | 85% | [90] | |
| Turashvili et al. (2007) | 29 | 93% | [107] | |
| Reis-Filho et al. (2006) | 13 | 92% | 15% | [106] |
| Caldeira et al. (2006) | 5 | 100% | [105] | |
| Qureshi et al. (2006) | 49 | 90% | [104] | |
| Sarrio et al. (2004) | 69 | 78% | [103] | |
| Sarrio et al. (2003) | 51 | 76% | 22% | [99] |
| Lei et al. (2002) | 25 | 12% | [102] | |
| Droufakou et al. (2001) | 22 | 55% | 27% | [88] |
| Huiping et al. (1999) | 40 | 94% | 15% | [101] |
| Leeuw et al. (1997) | 38 | 84% | 55% | [100] |
| Berx et al. (1996) | 41 | 84% | 56% | [12] |
| Berx et al. (1995) | 7 | 86% | 57% | [11] |
| Moll et al. (1993) | 22 | 86% | [9] | |
| Gamallo et al. (1993) | 7 | 100% | [10] | |
| median 2011–2020 | 97% | 65% | ||
| median 1993–2010 | 86% | 27% |
| Study | Cases | Histology | Surgery | Follow-Up | Metachronous Invasive Cancer | Metachronous Ipsilateral ILC | Ref. |
|---|---|---|---|---|---|---|---|
| Lo et al. (2018) | 732 | LCIS | n.a. | >9 yrs | 10% | n.a. | [150] |
| DeBrot et al. (2017) | 7 | pleo. LCIS | loc. ex. | >5 yrs | n.a. | 29% | [149] |
| King et al. (2015) | 1004 | LCIS | loc. ex. | >6 yrs | 11% | 3% | [137] |
| Cutui et al. (2015) | 159 | LCIS | loc. ex. | 6 yrs | 10% | 2% | [148] |
| Hartmann et al. (2014) | 327 | ALH | var. | >12 yrs | 18% | 2% | [147] |
| To et al. (2014) | 35 | LCIS | var. | 20 yrs | 21% | n.a. | [146] |
| Provencher et al. (2012) | 275 | LN | CNB | 5 yrs | n.a. | 1% * | [145] |
| Aulmann et al. (2008) | 88 | LCIS | var. | >10 yrs | 10% | 6% | [144] |
| Chuba et al. (2005) | 4853 | LCIS | n.a. | n.a. | 7% | <1% | [143] |
| Fisher et al. (2004) | 180 | LCIS | loc. ex. | 12 yrs | 10% | 4% | [142] |
| Fisher et al. (1996) | 182 | LCIS | loc. ex. | 5 yrs | 3% | 2% | [141] |
| Haagensen et al. (1978) | 209 | LCIS | var. | 11–42 yrs | 17% | 6% | [140] |
| Rosen et al. (1978) | 99 | LCIS | loc. ex. | 24 yrs | 32% | 8% | [6] |
| Wheeler et al. (1974) | 25 | LCIS | var. | 7–25 yrs | 4% | 4% | [139] |
| McDivitt and Stewart (1967) | 40 | LCIS | loc. ex. | 10 yrs | 15% | n.a. | [152] |
| Godwin et al. (1952) | 1 | LCIS | loc. ex. | 15 yrs | 100% | 100% | [138] |
| ILC | |||||
|---|---|---|---|---|---|
| Cases | Classic | Variants | Reported Histologies * | Ref. | |
| Tille et al. (2020) | 459 | 68% | 32% | s, a, m | [46] |
| Christgen et al. (2020) | 353 | 90% | 10% | s, a, p, o | [61] |
| Desmedt et al. (2016) | 413 | 48% | 52% | s, t, a, m | [43] |
| Iorfida et al. (2012) | 981 | 56% | 44% | s, t, m | [62] |
| Orvieto et al. (2008) | 530 | 57% | 43% | s, a, p, tl, o | [63] |
| Rakha et al. (2008) | 517 | 55% | 45% | s, a, m, tl, o | [64] |
| Du Toit et al. (1989) | 171 | 30% | 70% | s, a, m, tl, o | [65] |
| Fechner et al. (1975) | 26 | 77% | 23% | s | [66] |
| Diagnosis | Collision BC (NST + ILC) | Tubulo- Lobular BC | ILC with Tubular Elements | |||
|---|---|---|---|---|---|---|
| Component | Dissociated | Tubules | Dissociated | Tubules | Dissociated | Tubules |
| E-cadherin | neg. | pos. | pos. | pos. | neg. | neg. |
| β-catenin | neg. | pos. | pos. | pos. | neg. | pos. |
| P-cadherin | neg. | pos. | ||||
| IHC Markers | Mutation | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | Cases | ER pos. | HER2 pos. | p53 pos. | E-cad. neg. | TP53 | ERBB2 | Ref. |
| Riedlinger et al. (2021) | 16 | 81% | 25% | 25% | 19% | [256] | ||
| Rosa-Rosa et al. (2019) | 27 | 74% | 3% | 85% | 26% | [255] | ||
| Christgen et al. (2019) | 27 | 88% | 0% | 4% | 100% | 11% | 7% | [117] |
| Zhu et al. (2018) | 17 | 81% | 25% | 12% | 17% | [116] | ||
| Liu et al. (2018) | 46 | 85% | 9% | [254] | ||||
| Ilic et al. (2016) | 53 | 64% | 18% | 42% | 61% | [253] | ||
| Lien et al. (2015) | 21 | 46% | 33% | 100% | 14% | [252] | ||
| Rakha et al. (2013) | 16 | 86% | 0% | 82% | [245] | |||
| Monhollen et al. (2012) | 26 | 82% | 35% | 42% | 100% | [177] | ||
| Ercan et al. (2012) | 19 | 42% | [257] | |||||
| Simpson et al. (2008) | 26 | 76% | 14% | 13% | 100% | [176] | ||
| Palacios et al. (2003) | 29 | 100% | [251] | |||||
| Sneige et al. (2002) | 14 | 100% | 8% | 29% | 100% | [250] | ||
| Frolik et al. (2001) | 30 | 93% | 13% | 3% | [249] | |||
| Radhi et al. (2000) | 10 | 20% | 80% | [248] | ||||
| Middleton et al. (2000) | 38 | 81% | 81% | 48% | [247] | |||
References
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304.e296. [Google Scholar] [CrossRef] [Green Version]
- Hoberger, M.; von Laffert, M.; Heim, D.; Klauschen, F. Histomorphological and molecular profiling: Friends not foes! Morpho-molecular analysis reveals agreement between histological and molecular profiling. Histopathology 2019, 75, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Hyman, D.M.; Piha-Paul, S.A.; Won, H.; Rodon, J.; Saura, C.; Shapiro, G.I.; Juric, D.; Quinn, D.I.; Moreno, V.; Doger, B.; et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 2018, 554, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Cheatle, G.L.; Cutler, M. Tumors of the Breast. Their Pathology, Symptoms, Diagnosis and Treatment; Lippincott Company: Philadelphia, PA, USA; Montreal, QC, Canada, 1930. [Google Scholar]
- Cornil, V. Les Tumeurs du Sein; Librairie Germer Bailliere and Co.: Paris, France, 1908. [Google Scholar]
- Rosen, P.P.; Kosloff, C.; Lieberman, P.H.; Adair, F.; Braun, D.W., Jr. Lobular carcinoma in situ of the breast. Detailed analysis of 99 patients with average follow-up of 24 years. Am. J. Surg. Pathol. 1978, 2, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Foote, F.W.; Stewart, F.W. Lobular carcinoma in situ: A rare form of mammary cancer. Am. J. Pathol. 1941, 17, 491–496. [Google Scholar] [CrossRef]
- Rosen, P.P.; Menendez-Botet, C.J.; Nisselbaum, J.S.; Urban, J.A.; Mike, V.; Fracchia, A.; Schwartz, M.K. Pathological review of breast lesions analyzed for estrogen receptor protein. Cancer Res. 1975, 35, 3187–3194. [Google Scholar] [PubMed]
- Moll, R.; Mitze, M.; Frixen, U.H.; Birchmeier, W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am. J. Pathol. 1993, 143, 1731–1742. [Google Scholar] [PubMed]
- Gamallo, C.; Palacios, J.; Suarez, A.; Pizarro, A.; Navarro, P.; Quintanilla, M.; Cano, A. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am. J. Pathol. 1993, 142, 987–993. [Google Scholar]
- Berx, G.; Cleton-Jansen, A.M.; Nollet, F.; de Leeuw, W.J.; van de Vijver, M.; Cornelisse, C.; van Roy, F. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J. 1995, 14, 6107–6115. [Google Scholar] [CrossRef]
- Berx, G.; Cleton-Jansen, A.M.; Strumane, K.; de Leeuw, W.J.; Nollet, F.; van Roy, F.; Cornelisse, C. E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene 1996, 13, 1919–1925. [Google Scholar] [PubMed]
- Derksen, P.W.; Liu, X.; Saridin, F.; van der Gulden, H.; Zevenhoven, J.; Evers, B.; van Beijnum, J.R.; Griffioen, A.W.; Vink, J.; Krimpenfort, P.; et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell 2006, 10, 437–449. [Google Scholar] [CrossRef] [Green Version]
- Tasdemir, N.; Ding, K.; Savariau, L.; Levine, K.M.; Du, T.; Elangovan, A.; Bossart, E.A.; Lee, A.V.; Davidson, N.E.; Oesterreich, S. Proteomic and transcriptomic profiling identifies mediators of anchorage-independent growth and roles of inhibitor of differentiation proteins in invasive lobular carcinoma. Sci. Rep. 2020, 10, 11487. [Google Scholar] [CrossRef]
- Schackmann, R.C.; van Amersfoort, M.; Haarhuis, J.H.; Vlug, E.J.; Halim, V.A.; Roodhart, J.M.; Vermaat, J.S.; Voest, E.E.; van der Groep, P.; van Diest, P.J.; et al. Cytosolic p120-catenin regulates growth of metastatic lobular carcinoma through Rock1-mediated anoikis resistance. J. Clin. Investig. 2011, 121, 3176–3188. [Google Scholar] [CrossRef]
- Hornsveld, M.; Tenhagen, M.; van de Ven, R.A.; Smits, A.M.; van Triest, M.H.; van Amersfoort, M.; Kloet, D.E.; Dansen, T.B.; Burgering, B.M.; Derksen, P.W. Restraining FOXO3-dependent transcriptional BMF activation underpins tumour growth and metastasis of E-cadherin-negative breast cancer. Cell Death Differ. 2016, 23, 1483–1492. [Google Scholar] [CrossRef] [Green Version]
- Nagle, A.M.; Levine, K.M.; Tasdemir, N.; Scott, J.A.; Burlbaugh, K.; Kehm, J.; Katz, T.A.; Boone, D.N.; Jacobsen, B.M.; Atkinson, J.M.; et al. Loss of E-cadherin Enhances IGF1-IGF1R Pathway Activation and Sensitizes Breast Cancers to Anti-IGF1R/InsR Inhibitors. Clin. Cancer Res. 2018, 24, 5165–5177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, K.; Gomez-Cuadrado, L.; Tenhagen, M.; Byron, A.; Ratze, M.; van Amersfoort, M.; Renes, J.; Strengman, E.; Mandoli, A.; Singh, A.A.; et al. E-cadherin loss induces targetable autocrine activation of growth factor signalling in lobular breast cancer. Sci. Rep. 2018, 8, 15454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, K.H.; Brogi, E.; Ellis, I.O.; Fox, S.B.; Morris, E.A.; Sahin, A.; Salgado, R.; Sapino, A.; Sasano, H.; Schnitt, S.; et al. WHO Classification of Tumours Editorial Board. Breast Tumours; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Christgen, M.; Steinemann, D.; Kuhnle, E.; Langer, F.; Gluz, O.; Harbeck, N.; Kreipe, H. Lobular breast cancer: Clinical, molecular and morphological characteristics. Pathol. Res. Pract. 2016, 212, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Tsuchiya, S.; Matsuyama, I.; Ishii, K. Clinicopathologic features and incidence of invasive lobular carcinoma in Japanese women. Pathol. Int. 1998, 48, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, K.D.; Fan, L.; Hou, Y.F.; Shao, Z.M. Predicting breast cancer recurrence following breast-conserving therapy: A single-institution analysis consisting of 764 Chinese breast cancer cases. Ann. Surg. Oncol. 2011, 18, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.Y.; Lee, H.B.; Lee, Y.J.; Seong, M.K.; Paik, N.; Park, W.C.; Park, S.; Jung, S.P.; Bae, S.Y. Characteristics and prognosis of 17 special histologic subtypes of invasive breast cancers according to World Health Organization classification: Comparative analysis to invasive carcinoma of no special type. Breast Cancer Res. Treat. 2020, 184, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, J.; Li, S.; Lv, M.; Shen, Y.; Wang, B.; Li, P.; Yi, M.; Zhao, X.; Zhang, L.; et al. Invasive lobular carcinoma of the breast: A special histological type compared with invasive ductal carcinoma. PLoS ONE 2017, 12, e0182397. [Google Scholar] [CrossRef]
- Barinoff, J.; Hils, R.; Bender, A.; Gross, J.; Kurz, C.; Tauchert, S.; Mann, E.; Schwidde, I.; Ipsen, B.; Sawitzki, K.; et al. Clinicopathological differences between breast cancer in patients with primary metastatic disease and those without: A multicentre study. Eur. J. Cancer 2013, 49, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Howell, A.; Chrissohou, M.; Swindell, R.I.; Hudson, M.; Sellwood, R.A. A comparison of the metastatic pattern of infiltrating lobular carcinoma and infiltrating duct carcinoma of the breast. Br. J. Cancer 1984, 50, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sastre-Garau, X.; Jouve, M.; Asselain, B.; Vincent-Salomon, A.; Beuzeboc, P.; Dorval, T.; Durand, J.C.; Fourquet, A.; Pouillart, P. Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer 1996, 77, 113–120. [Google Scholar] [CrossRef]
- Arpino, G.; Bardou, V.J.; Clark, G.M.; Elledge, R.M. Infiltrating lobular carcinoma of the breast: Tumor characteristics and clinical outcome. Breast Cancer Res. 2004, 6, R149–R156. [Google Scholar] [CrossRef] [Green Version]
- Kwast, A.B.; Groothuis-Oudshoorn, K.C.; Grandjean, I.; Ho, V.K.; Voogd, A.C.; Menke-Pluymers, M.B.; van der Sangen, M.J.; Tjan-Heijnen, V.C.; Kiemeney, L.A.; Siesling, S. Histological type is not an independent prognostic factor for the risk pattern of breast cancer recurrences. Breast Cancer Res. Treat. 2012, 135, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, T.; Kuukasjarvi, T.; Huhtala, H.; Alarmo, E.L.; Holli, K.; Kallioniemi, A.; Pylkkanen, L. The impact of lobular and ductal breast cancer histology on the metastatic behavior and long term survival of breast cancer patients. Breast 2013, 22, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Raap, M.; Antonopoulos, W.; Dammrich, M.; Christgen, H.; Steinmann, D.; Langer, F.; Lehmann, U.; Kreipe, H.; Christgen, M. High frequency of lobular breast cancer in distant metastases to the orbit. Cancer Med. 2015, 4, 104–111. [Google Scholar] [CrossRef]
- El-Hage, A.; Ruel, C.; Afif, W.; Wissanji, H.; Hogue, J.C.; Desbiens, C.; Leblanc, G.; Poirier, E. Metastatic pattern of invasive lobular carcinoma of the breast-Emphasis on gastric metastases. J. Surg. Oncol. 2016, 114, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Montagna, E.; Pirola, S.; Maisonneuve, P.; De Roberto, G.; Cancello, G.; Palazzo, A.; Viale, G.; Colleoni, M. Lobular Metastatic Breast Cancer Patients With Gastrointestinal Involvement: Features and Outcomes. Clin. Breast Cancer 2018, 18, 401–405. [Google Scholar] [CrossRef] [PubMed]
- DiPiro, P.J.; Tirumani, S.H.; Cruz, G.P.; Ramaiya, N.H.; Lester, S.C.; Shinagare, A.B. Lobular breast cancer: Patterns of intraabdominal metastatic spread on imaging and prognostic significance. Abdom. Radiol. 2019, 44, 362–369. [Google Scholar] [CrossRef]
- Ferlicot, S.; Vincent-Salomon, A.; Medioni, J.; Genin, P.; Rosty, C.; Sigal-Zafrani, B.; Freneaux, P.; Jouve, M.; Thiery, J.P.; Sastre-Garau, X. Wide metastatic spreading in infiltrating lobular carcinoma of the breast. Eur. J. Cancer 2004, 40, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Delpech, Y.; Coutant, C.; Hsu, L.; Barranger, E.; Iwamoto, T.; Barcenas, C.H.; Hortobagyi, G.N.; Rouzier, R.; Esteva, F.J.; Pusztai, L. Clinical benefit from neoadjuvant chemotherapy in oestrogen receptor-positive invasive ductal and lobular carcinomas. Br. J. Cancer 2013, 108, 285–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiche, M.; Scabia, V.; Aouad, P.; Battista, L.; Treboux, A.; Stravodimou, A.; Zaman, K.; Dormoy, V.; Ayyanan, A.; Sflomos, G.; et al. Intraductal patient derived xenografts of estrogen receptor alpha positive (ER+) breast cancer recapitulate the histopathological spectrum and metastatic potential of human lesions. J. Pathol. 2018, 247, 287–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loibl, S.; Volz, C.; Mau, C.; Blohmer, J.U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Hanusch, C.; Jackisch, C.; et al. Response and prognosis after neoadjuvant chemotherapy in 1,051 patients with infiltrating lobular breast carcinoma. Breast Cancer Res. Treat. 2014, 144, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Korkola, J.E.; DeVries, S.; Fridlyand, J.; Hwang, E.S.; Estep, A.L.; Chen, Y.Y.; Chew, K.L.; Dairkee, S.H.; Jensen, R.M.; Waldman, F.M. Differentiation of lobular versus ductal breast carcinomas by expression microarray analysis. Cancer Res. 2003, 63, 7167–7175. [Google Scholar] [PubMed]
- McCart Reed, A.E.; Kalinowski, L.; Simpson, P.T.; Lakhani, S.R. Invasive lobular carcinoma of the breast: The increasing importance of this special subtype. Breast Cancer Res. 2021, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, F.; Orsetti, B.; Negre, V.; Finetti, P.; Rouge, C.; Ahomadegbe, J.C.; Bibeau, F.; Mathieu, M.C.; Treilleux, I.; Jacquemier, J.; et al. Lobular and ductal carcinomas of the breast have distinct genomic and expression profiles. Oncogene 2008, 27, 5359–5372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciriello, G.; Gatza, M.L.; Beck, A.H.; Wilkerson, M.D.; Rhie, S.K.; Pastore, A.; Zhang, H.; McLellan, M.; Yau, C.; Kandoth, C.; et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell 2015, 163, 506–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmedt, C.; Zoppoli, G.; Gundem, G.; Pruneri, G.; Larsimont, D.; Fornili, M.; Fumagalli, D.; Brown, D.; Rothe, F.; Vincent, D.; et al. Genomic Characterization of Primary Invasive Lobular Breast Cancer. J. Clin. Oncol. 2016, 34, 1872–1881. [Google Scholar] [CrossRef] [Green Version]
- Desmedt, C.; Salgado, R.; Fornili, M.; Pruneri, G.; Van den Eynden, G.; Zoppoli, G.; Rothe, F.; Buisseret, L.; Garaud, S.; Willard-Gallo, K.; et al. Immune Infiltration in Invasive Lobular Breast Cancer. J. Natl. Cancer Inst. 2018, 110, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Zhu, L.; Levine, K.M.; Tasdemir, N.; Lee, A.V.; Vignali, D.A.A.; Houten, B.V.; Tseng, G.C.; Oesterreich, S. Invasive lobular and ductal breast carcinoma differ in immune response, protein translation efficiency and metabolism. Sci. Rep. 2018, 8, 7205. [Google Scholar] [CrossRef] [PubMed]
- Tille, J.C.; Vieira, A.F.; Saint-Martin, C.; Djerroudi, L.; Furhmann, L.; Bidard, F.C.; Kirova, Y.; Tardivon, A.; Reyal, F.; Carton, M.; et al. Tumor-infiltrating lymphocytes are associated with poor prognosis in invasive lobular breast carcinoma. Mod. Pathol. 2020, 33, 2198–2207. [Google Scholar] [CrossRef]
- Warner, N.E. Lobular carcinoma of the breast. Cancer 1969, 23, 840–846. [Google Scholar] [CrossRef]
- Newman, W. Lobular carcinoma of the female breast. Report of 73 cases. Ann. Surg. 1966, 164, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, R.M.; Li, T.; Freedman, G.M.; Morrow, M. Presence of lobular carcinoma in situ does not increase local recurrence in patients treated with breast-conserving therapy. Ann. Surg. Oncol. 2008, 15, 2263–2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, A.S.; Xiang, D.; Hockman, L.; Arya, M.; Jeffress, J.; Wang, Z.; Dale, P.S. Synchronous lobular carcinoma in situ and invasive lobular cancer: Marker or precursor for invasive lobular carcinoma. Eur. J. Surg. Oncol. 2014, 40, 1245–1249. [Google Scholar] [CrossRef]
- Harbhajanka, A.; Lamzabi, I.; Syed, S.; Jain, R.; Ghai, R.; Reddy, V.B.; Bitterman, P.; Gattuso, P. Prognostic Value of Coexisting Lobular Carcinoma In Situ With Invasive Lobular Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Moten, A.; Obirieze, A.; Wilson, L.L. Characterizing lobular carcinoma of the male breast using the SEER database. J. Surg. Res. 2013, 185, e71–e76. [Google Scholar] [CrossRef]
- Senger, J.L.; Adams, S.J.; Kanthan, R. Invasive lobular carcinoma of the male breast—A systematic review with an illustrative case study. Breast Cancer 2017, 9, 337–345. [Google Scholar] [CrossRef] [Green Version]
- McBryan, J.; Howlin, J. Pubertal Mammary Gland Development: Elucidation of In Vivo Morphogenesis Using Murine Models. Methods Mol. Biol. 2017, 1501, 77–114. [Google Scholar]
- Ninkovic, S.; Azanjac, G.; Knezevic, M.; Radovanovic, D.; Canovic, D.; Nedovic, J.; Mitrovic, S. Lobular Breast Cancer in a Male Patient with a Previous History of Irradiation Due to Hodgkin’s Disease. Breast Care 2012, 7, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Fechner, R.E. Infiltrating lobular carcinoma without lobular carcinoma in situ. Cancer 1972, 29, 1539–1545. [Google Scholar] [CrossRef]
- Fitzgerald, P.J. In memoriam: Frank W. Foote, Jr, MD (1911–1989). Am. J. Clin. Pathol. 1990, 94, 238–239. [Google Scholar] [CrossRef]
- McCart Reed, A.E.; Kutasovic, J.R.; Nones, K.; Saunus, J.M.; Da Silva, L.; Newell, F.; Kazakoff, S.; Melville, L.; Jayanthan, J.; Vargas, A.C.; et al. Mixed ductal-lobular carcinomas: Evidence for progression from ductal to lobular morphology. J. Pathol. 2018, 244, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Christgen, M.; Bartels, S.; van Luttikhuizen, J.L.; Bublitz, J.; Rieger, L.U.; Christgen, H.; Stark, H.; Sander, B.; Lehmann, U.; Steinemann, D.; et al. E-cadherin to P-cadherin switching in lobular breast cancer with tubular elements. Mod. Pathol. 2020, 33, 2483–2498. [Google Scholar] [CrossRef]
- Azzopardi, J.G. Problems in Breast Pathology; WB Saunders Company LTD: Eastbourne, UK, 1979. [Google Scholar]
- Christgen, M.; Gluz, O.; Harbeck, N.; Kates, R.E.; Raap, M.; Christgen, H.; Clemens, M.; Malter, W.; Nuding, B.; Aktas, B.; et al. Differential impact of prognostic parameters in hormone receptor-positive lobular breast cancer. Cancer 2020, 126, 4847–4858. [Google Scholar] [CrossRef] [PubMed]
- Iorfida, M.; Maiorano, E.; Orvieto, E.; Maisonneuve, P.; Bottiglieri, L.; Rotmensz, N.; Montagna, E.; Dellapasqua, S.; Veronesi, P.; Galimberti, V.; et al. Invasive lobular breast cancer: Subtypes and outcome. Breast Cancer Res. Treat. 2012, 133, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Orvieto, E.; Maiorano, E.; Bottiglieri, L.; Maisonneuve, P.; Rotmensz, N.; Galimberti, V.; Luini, A.; Brenelli, F.; Gatti, G.; Viale, G. Clinicopathologic characteristics of invasive lobular carcinoma of the breast: Results of an analysis of 530 cases from a single institution. Cancer 2008, 113, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; El-Sayed, M.E.; Menon, S.; Green, A.R.; Lee, A.H.; Ellis, I.O. Histologic grading is an independent prognostic factor in invasive lobular carcinoma of the breast. Breast Cancer Res. Treat. 2008, 111, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, R.S.; Locker, A.P.; Ellis, I.O.; Elston, C.W.; Nicholson, R.I.; Blamey, R.W. Invasive lobular carcinomas of the breast—The prognosis of histopathological subtypes. Br. J. Cancer 1989, 60, 605–609. [Google Scholar] [CrossRef] [Green Version]
- Fechner, R.E. Histologic variants of infiltrating lobular carcinoma of the breast. Hum. Pathol. 1975, 6, 373–378. [Google Scholar] [CrossRef]
- Martinez, V.; Azzopardi, J.G. Invasive lobular carcinoma of the breast: Incidence and variants. Histopathology 1979, 3, 467–488. [Google Scholar] [CrossRef] [PubMed]
- Louwman, M.W.; Vriezen, M.; van Beek, M.W.; Nolthenius-Puylaert, M.C.; van der Sangen, M.J.; Roumen, R.M.; Kiemeney, L.A.; Coebergh, J.W. Uncommon breast tumors in perspective: Incidence, treatment and survival in The Netherlands. Int. J. Cancer 2007, 121, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Bharat, A.; Gao, F.; Margenthaler, J.A. Tumor characteristics and patient outcomes are similar between invasive lobular and mixed invasive ductal/lobular breast cancers but differ from pure invasive ductal breast cancers. Am. J. Surg. 2009, 198, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Flores-Diaz, D.; Arce, C.; Flores-Luna, L.; Reynoso-Noveron, N.; Lara-Medina, F.; Matus, J.A.; Bargallo-Rocha, E.; Perez, V.; Villarreal-Garza, C.; Cabrera-Galeana, P.; et al. Impact of invasive lobular carcinoma on long-term outcomes in Mexican breast cancer patients. Breast Cancer Res. Treat. 2019, 176, 243–249. [Google Scholar] [CrossRef]
- Braunstein, L.Z.; Brock, J.E.; Chen, Y.H.; Truong, L.; Russo, A.L.; Arvold, N.D.; Harris, J.R. Invasive lobular carcinoma of the breast: Local recurrence after breast-conserving therapy by subtype approximation and surgical margin. Breast Cancer Res. Treat. 2015, 149, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.R.; Ellis, I.; Schnitt, S.; Tan, P.H.; van de Vijver, M. WHO Classification of Tumours of the Breast; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- Pestalozzi, B.C.; Zahrieh, D.; Mallon, E.; Gusterson, B.A.; Price, K.N.; Gelber, R.D.; Holmberg, S.B.; Lindtner, J.; Snyder, R.; Thurlimann, B.; et al. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: Combined results of 15 International Breast Cancer Study Group clinical trials. J. Clin. Oncol. 2008, 26, 3006–3014. [Google Scholar] [CrossRef] [PubMed]
- Canas-Marques, R.; Schnitt, S.J. E-cadherin immunohistochemistry in breast pathology: Uses and pitfalls. Histopathology 2016, 68, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Cserni, G. Reproducibility of a diagnosis of invasive lobular carcinoma. J. Surg. Oncol. 1999, 70, 217–221. [Google Scholar] [CrossRef]
- Longacre, T.A.; Ennis, M.; Quenneville, L.A.; Bane, A.L.; Bleiweiss, I.J.; Carter, B.A.; Catelano, E.; Hendrickson, M.R.; Hibshoosh, H.; Layfield, L.J.; et al. Interobserver agreement and reproducibility in classification of invasive breast carcinoma: An NCI breast cancer family registry study. Mod. Pathol. 2006, 19, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Kiaer, H.; Andersen, J.A.; Rank, F.; Pedersen, B.V. Quality control of patho-anatomical diagnosis of carcinoma of the breast. Acta Oncol. 1988, 27, 745–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzger, O.; Cardoso, F.; Poncet, C.; Desmedt, C.; Linn, S.; Wesseling, J.; Hilbers, F.; Aalders, K.; Delorenzi, M.; Delaloge, S.; et al. Clinical Utility of MammaPrint testing in invasive lobular carcinoma: Results from the MINDACT phase III trial. In Proceedings of the San Antonio Breast Cancer Conference, Online, 10 December 2019. [Google Scholar]
- Vos, C.B.; Cleton-Jansen, A.M.; Berx, G.; de Leeuw, W.J.; ter Haar, N.T.; van Roy, F.; Cornelisse, C.J.; Peterse, J.L.; van de Vijver, M.J. E-cadherin inactivation in lobular carcinoma in situ of the breast: An early event in tumorigenesis. Br. J. Cancer 1997, 76, 1131–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastracci, T.L.; Tjan, S.; Bane, A.L.; O’Malley, F.P.; Andrulis, I.L. E-cadherin alterations in atypical lobular hyperplasia and lobular carcinoma in situ of the breast. Mod. Pathol. 2005, 18, 741–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastracci, T.L.; Shadeo, A.; Colby, S.M.; Tuck, A.B.; O’Malley, F.P.; Bull, S.B.; Lam, W.L.; Andrulis, I.L. Genomic alterations in lobular neoplasia: A microarray comparative genomic hybridization signature for early neoplastic proliferationin the breast. Genes Chromosomes Cancer 2006, 45, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Sakr, R.A.; Schizas, M.; Carniello, J.V.; Ng, C.K.; Piscuoglio, S.; Giri, D.; Andrade, V.P.; De Brot, M.; Lim, R.S.; Towers, R.; et al. Targeted capture massively parallel sequencing analysis of LCIS and invasive lobular cancer: Repertoire of somatic genetic alterations and clonal relationships. Mol. Oncol. 2016, 10, 360–370. [Google Scholar] [CrossRef] [Green Version]
- Simpson, P.T.; Gale, T.; Fulford, L.G.; Reis-Filho, J.S.; Lakhani, S.R. The diagnosis and management of pre-invasive breast disease: Pathology of atypical lobular hyperplasie and lobular carcinoma in situ. Breast Cancer Res. 2003, 5, 258–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morandi, L.; Marucci, G.; Foschini, M.P.; Cattani, M.G.; Pession, A.; Riva, C.; Eusebi, V. Genetic similarities and differences between lobular in situ neoplasia (LN) and invasive lobular carcinoma of the breast. Virchows Arch. 2006, 449, 14–23. [Google Scholar] [CrossRef]
- Lee, J.Y.; Schizas, M.; Geyer, F.C.; Selenica, P.; Piscuoglio, S.; Sakr, R.A.; Ng, C.K.Y.; Carniello, J.V.S.; Towers, R.; Giri, D.D.; et al. Lobular Carcinomas In Situ Display Intralesion Genetic Heterogeneity and Clonal Evolution in the Progression to Invasive Lobular Carcinoma. Clin. Cancer Res. 2019, 25, 674–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karam, R.; Carvalho, J.; Bruno, I.; Graziadio, C.; Senz, J.; Huntsman, D.; Carneiro, F.; Seruca, R.; Wilkinson, M.F.; Oliveira, C. The NMD mRNA surveillance pathway downregulates aberrant E-cadherin transcripts in gastric cancer cells and in CDH1 mutation carriers. Oncogene 2008, 27, 4255–4260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004, 117, 927–939. [Google Scholar] [CrossRef] [Green Version]
- Droufakou, S.; Deshmane, V.; Roylance, R.; Hanby, A.; Tomlinson, I.; Hart, I.R. Multiple ways of silencing E-cadherin gene expression in lobular carcinoma of the breast. Int. J. Cancer 2001, 92, 404–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCart Reed, A.E.; Foong, S.; Kutasovic, J.R.; Nones, K.; Waddell, N.; Lakhani, S.R.; Simpson, P.T. The genomic landscape of lobular breast cancer. Cancers 2021, 13, 1950. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.; Parry, S.; Reid, L.; Keith, P.; Waddell, N.; Kossai, M.; Clarke, C.; Lakhani, S.R.; Simpson, P.T. Aberrant expression of E-cadherin in lobular carcinomas of the breast. Am. J. Surg. Pathol. 2008, 32, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Grabenstetter, A.; Mohanty, A.S.; Rana, S.; Zehir, A.; Brannon, A.R.; D’Alfonso, T.M.; DeLair, D.F.; Tan, L.K.; Ross, D.S. E-cadherin immunohistochemical expression in invasive lobular carcinoma of the breast: Correlation with morphology and CDH1 somatic alterations. Hum. Pathol. 2020, 102, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Takeichi, M. Adherens junction: Molecular architecture and regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a002899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollestelle, A.; Elstrodt, F.; Timmermans, M.; Sieuwerts, A.M.; Klijn, J.G.; Foekens, J.A.; den Bakker, M.A.; Schutte, M. Four human breast cancer cell lines with biallelic inactivating alpha-catenin gene mutations. Breast Cancer Res. Treat. 2010, 122, 125–133. [Google Scholar] [CrossRef]
- Majewski, I.J.; Kluijt, I.; Cats, A.; Scerri, T.S.; de Jong, D.; Kluin, R.J.; Hansford, S.; Hogervorst, F.B.; Bosma, A.J.; Hofland, I.; et al. An alpha-E-catenin (CTNNA1) mutation in hereditary diffuse gastric cancer. J. Pathol. 2013, 229, 621–629. [Google Scholar] [CrossRef]
- De Groot, J.S.; Ratze, M.A.; van Amersfoort, M.; Eisemann, T.; Vlug, E.J.; Niklaas, M.T.; Chin, S.F.; Caldas, C.; van Diest, P.J.; Jonkers, J.; et al. alphaE-catenin is a candidate tumor suppressor for the development of E-cadherin-expressing lobular-type breast cancer. J. Pathol. 2018, 245, 456–467. [Google Scholar] [CrossRef] [PubMed]
- DeLair, D.F.; Burke, K.A.; Selenica, P.; Lim, R.S.; Scott, S.N.; Middha, S.; Mohanty, A.S.; Cheng, D.T.; Berger, M.F.; Soslow, R.A.; et al. The genetic landscape of endometrial clear cell carcinomas. J. Pathol. 2017, 243, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jeong, S. Mutation hotspots in the beta-catenin gene: Lessons from the human cancer genome database. Mol. Cells 2019, 42, 8–16. [Google Scholar] [PubMed]
- Abraham, S.C.; Reynolds, C.; Lee, J.H.; Montgomery, E.A.; Baisden, B.L.; Krasinskas, A.; Wu, T.T. Fibromatosis of the breast and mutations involving the APC/beta-catenin pathway. Hum. Pathol. 2002, 33, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Sarrio, D.; Moreno-Bueno, G.; Hardisson, D.; Sanchez-Estevez, C.; Guo, M.; Herman, J.G.; Gamallo, C.; Esteller, M.; Palacios, J. Epigenetic and genetic alterations of APC and CDH1 genes in lobular breast cancer: Relationships with abnormal E-cadherin and catenin expression and microsatellite instability. Int. J. Cancer 2003, 106, 208–215. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, W.J.; Berx, G.; Vos, C.B.; Peterse, J.L.; Van de Vijver, M.J.; Litvinov, S.; Van Roy, F.; Cornelisse, C.J.; Cleton-Jansen, A.M. Simultaneous loss of E-cadherin and catenins in invasive lobular breast cancer and lobular carcinoma in situ. J. Pathol. 1997, 183, 404–411. [Google Scholar] [CrossRef]
- Huiping, C.; Sigurgeirsdottir, J.R.; Jonasson, J.G.; Eiriksdottir, G.; Johannsdottir, J.T.; Egilsson, V.; Ingvarsson, S. Chromosome alterations and E-cadherin gene mutations in human lobular breast cancer. Br. J. Cancer 1999, 81, 1103–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, H.; Sjoberg-Margolin, S.; Salahshor, S.; Werelius, B.; Jandakova, E.; Hemminki, K.; Lindblom, A.; Vorechovsky, I. CDH1 mutations are present in both ductal and lobular breast cancer, but promoter allelic variants show no detectable breast cancer risk. Int. J. Cancer 2002, 98, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Sarrio, D.; Perez-Mies, B.; Hardisson, D.; Moreno-Bueno, G.; Suarez, A.; Cano, A.; Martin-Perez, J.; Gamallo, C.; Palacios, J. Cytoplasmic localization of p120ctn and E-cadherin loss characterize lobular breast carcinoma from preinvasive to metastatic lesions. Oncogene 2004, 23, 3272–3283. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, H.S.; Linden, M.D.; Divine, G.; Raju, U.B. E-cadherin status in breast cancer correlates with histologic type but does not correlate with established prognostic parameters. Am. J. Clin. Pathol. 2006, 125, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, J.R.; Prando, E.C.; Quevedo, F.C.; Neto, F.A.; Rainho, C.A.; Rogatto, S.R. CDH1 promoter hypermethylation and E-cadherin protein expression in infiltrating breast cancer. BMC Cancer 2006, 6, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis-Filho, J.S.; Simpson, P.T.; Turner, N.C.; Lambros, M.B.; Jones, C.; Mackay, A.; Grigoriadis, A.; Sarrio, D.; Savage, K.; Dexter, T.; et al. FGFR1 emerges as a potential therapeutic target for lobular breast carcinomas. Clin. Cancer Res. 2006, 12, 6652–6662. [Google Scholar] [CrossRef] [Green Version]
- Turashvili, G.; Bouchal, J.; Ehrmann, J.; Fridman, E.; Skarda, J.; Kolar, Z. Novel immunohistochemical markers for the differentiation of lobular and ductal invasive breast carcinomas. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc 2007, 151, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, D.; Yoon, H.S.; Perez, D.; Weeks, R.J.; Guilford, P.; Humar, B. Epigenetic silencing in non-neoplastic epithelia identifies E-cadherin (CDH1) as a target for chemoprevention of lobular neoplasia. J. Pathol. 2009, 218, 265–272. [Google Scholar] [CrossRef]
- Rakha, E.A.; Patel, A.; Powe, D.G.; Benhasouna, A.; Green, A.R.; Lambros, M.B.; Reis-Filho, J.S.; Ellis, I.O. Clinical and biological significance of E-cadherin protein expression in invasive lobular carcinoma of the breast. Am. J. Surg. Pathol. 2010, 34, 1472–1479. [Google Scholar] [CrossRef]
- Ellis, M.J.; Ding, L.; Shen, D.; Luo, J.; Suman, V.J.; Wallis, J.W.; Van Tine, B.A.; Hoog, J.; Goiffon, R.J.; Goldstein, T.C.; et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 2012, 486, 353–360. [Google Scholar] [CrossRef]
- Lips, E.H.; Mukhtar, R.A.; Yau, C.; de Ronde, J.J.; Livasy, C.; Carey, L.A.; Loo, C.E.; Vrancken-Peeters, M.J.; Sonke, G.S.; Berry, D.A.; et al. Lobular histology and response to neoadjuvant chemotherapy in invasive breast cancer. Breast Cancer Res. Treat. 2012, 136, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koboldt, D.C. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar]
- Boyault, S.; Drouet, Y.; Navarro, C.; Bachelot, T.; Lasset, C.; Treilleux, I.; Tabone, E.; Puisieux, A.; Wang, Q. Mutational characterization of individual breast tumors: TP53 and PI3K pathway genes are frequently and distinctively mutated in different subtypes. Breast Cancer Res. Treat. 2012, 132, 29–39. [Google Scholar] [CrossRef]
- Christgen, M.; Noskowicz, M.; Schipper, E.; Christgen, H.; Heil, C.; Krech, T.; Langer, F.; Kreipe, H.; Lehmann, U. Oncogenic PIK3CA mutations in lobular breast cancer progression. Genes Chromosomes Cancer 2013, 52, 69–80. [Google Scholar] [CrossRef] [PubMed]
- McCart Reed, A.E.; Kutasovic, J.R.; Vargas, A.C.; Jayanthan, J.; Al-Murrani, A.; Reid, L.E.; Chambers, R.; Silva, L.D.; Melville, L.; Evans, E.; et al. An Epithelial to Mesenchymal Transition programme does not usually drive the phenotype of Invasive Lobular Carcinomas. J. Pathol. 2015, in press. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Ward, B.M.; Yu, J.; Matthew-Onabanjo, A.N.; Janusis, J.; Hsieh, C.C.; Tomaszewicz, K.; Hutchinson, L.; Zhu, L.J.; Kandil, D.; et al. IRS2 mutations linked to invasion in pleomorphic invasive lobular carcinoma. JCI Insight 2018, 3, e97398. [Google Scholar] [CrossRef] [Green Version]
- Christgen, M.; Bartels, S.; Radner, M.; Raap, M.; Rieger, L.; Christgen, H.; Gluz, O.; Nitz, U.; Harbeck, N.; Lehmann, U.; et al. ERBB2 mutation frequency in lobular breast cancer with pleomorphic histology or high-risk characteristics by molecular expression profiling. Genes Chromosomes Cancer 2019, I58, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Sokol, E.S.; Feng, Y.X.; Jin, D.X.; Basudan, A.; Lee, A.V.; Atkinson, J.M.; Chen, J.; Stephens, P.J.; Frampton, G.M.; Gupta, P.B.; et al. Loss of function of NF1 is a mechanism of acquired resistance to endocrine therapy in lobular breast cancer. Ann. Oncol. 2019, 30, 115–123. [Google Scholar] [CrossRef]
- Rinaldi, J.; Sokol, E.S.; Hartmaier, R.J.; Trabucco, S.E.; Frampton, G.M.; Goldberg, M.E.; Albacker, L.A.; Daemen, A.; Manning, G. The genomic landscape of metastatic breast cancer: Insights from 11,000 tumors. PLoS ONE 2020, 15, e0231999. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, A.; Desmoulins, I.; Beltjens, F.; Causeret, S.; Charon-Barra, C.; Martin, E.; Richard, C.; Boidot, R.; Arnould, L. An exceptional metaplastic lobular breast carcinoma diagnosed through exome sequencing. J. Clin. Pathol. 2020, 73, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Mateus, A.R.; Simoes-Correia, J.; Figueiredo, J.; Heindl, S.; Alves, C.C.; Suriano, G.; Luber, B.; Seruca, R. E-cadherin mutations and cell motility: A genotype-phenotype correlation. Exp. Cell Res. 2009, 315, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Dabbs, D.J.; Schnitt, S.J.; Geyer, F.C.; Weigelt, B.; Baehner, F.L.; Decker, T.; Eusebi, V.; Fox, S.B.; Ichihara, S.; Lakhani, S.R.; et al. Lobular neoplasia of the breast revisited with emphasis on the role of E-cadherin immunohistochemistry. Am. J. Surg. Pathol. 2013, 37, e1–e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harigopal, M.; Shin, S.J.; Murray, M.P.; Tickoo, S.K.; Brogi, E.; Rosen, P.P. Aberrant E-cadherin staining patterns in invasive mammary carcinoma. World J. Surg. Oncol. 2005, 3, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.J.; Pinto, M.M.; Hao, L.; Riba, A.K. Interobserver variability and aberrant E-cadherin immunostaining of lobular neoplasia and infiltrating lobular carcinoma. Mod. Pathol. 2008, 21, 1224–1237. [Google Scholar] [CrossRef]
- Mahler-Araujo, B.; Savage, K.; Parry, S.; Reis-Filho, J.S. Reduction of E-cadherin expression is associated with non-lobular breast carcinomas of basal-like and triple negative phenotype. J. Clin. Pathol. 2008, 61, 615–620. [Google Scholar] [CrossRef]
- Alsaleem, M.; Toss, M.S.; Joseph, C.; Aleskandarany, M.; Kurozumi, S.; Alshankyty, I.; Ogden, A.; Rida, P.C.G.; Ellis, I.O.; Aneja, R.; et al. The molecular mechanisms underlying reduced E-cadherin expression in invasive ductal carcinoma of the breast: High throughput analysis of large cohorts. Mod. Pathol. 2019, 32, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Couture, H.D.; Williams, L.A.; Geradts, J.; Nyante, S.J.; Butler, E.N.; Marron, J.S.; Perou, C.M.; Troester, M.A.; Niethammer, M. Image analysis with deep learning to predict breast cancer grade, ER status, histologic subtype, and intrinsic subtype. NPJ Breast Cancer 2018, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Dabbs, D.J.; Bhargava, R.; Chivukula, M. Lobular versus ductal breast neoplasms: The diagnostic utility of p120 catenin. Am. J. Surg. Pathol. 2007, 31, 427–437. [Google Scholar] [CrossRef]
- Metzger-Filho, O.; Ferreira, A.R.; Jeselsohn, R.; Barry, W.T.; Dillon, D.A.; Brock, J.E.; Vaz-Luis, I.; Hughes, M.E.; Winer, E.P.; Lin, N.U. Mixed Invasive Ductal and Lobular Carcinoma of the Breast: Prognosis and the Importance of Histologic Grade. Oncologist 2019, 24, e441. [Google Scholar] [CrossRef] [Green Version]
- Eusebi, V.; Magalhaes, F.; Azzopardi, J.G. Pleomorphic lobular carcinoma of the breast: An aggressive tumor showing apocrine differentiation. Hum. Pathol. 1992, 23, 655–662. [Google Scholar] [CrossRef]
- Bianchi, S.; Vezzosi, V. Microinvasive carcinoma of the breast. Pathol. Oncol. Res. 2008, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.M.; Young, G.Q.; Hoda, S.A. The “Rosen Triad”: Tubular carcinoma, lobular carcinoma in situ, and columnar cell lesions. Adv. Anat. Pathol. 2008, 15, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Richter-Ehrenstein, C.; Maak, K.; Roger, S.; Ehrenstein, T. Lesions of “uncertain malignant potential” in the breast (B3) identified with mammography screening. BMC Cancer 2018, 18, 829. [Google Scholar] [CrossRef]
- Rageth, C.J.; O’Flynn, E.A.M.; Pinker, K.; Kubik-Huch, R.A.; Mundinger, A.; Decker, T.; Tausch, C.; Dammann, F.; Baltzer, P.A.; Fallenberg, E.M.; et al. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res. Treat. 2019, 174, 279–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merkkola-von Schantz, P.A.; Jahkola, T.A.; Krogerus, L.A.; Hukkinen, K.S.; Kauhanen, S.M. Should we routinely analyze reduction mammaplasty specimens? J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, B.; Xu, J.Y.; Jiang, L.; Wang, Z. Fibroadenoma with an unexpected lobular carcinoma: A case report and review of the literature. Oncol. Lett. 2015, 10, 1397–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, T.A.; Pilewskie, M.; Muhsen, S.; Patil, S.; Mautner, S.K.; Park, A.; Oskar, S.; Guerini-Rocco, E.; Boafo, C.; Gooch, J.C.; et al. Lobular Carcinoma in Situ: A 29-Year Longitudinal Experience Evaluating Clinicopathologic Features and Breast Cancer Risk. J. Clin. Oncol. 2015, 33, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.T. Chronology of lobular carcinoma of the breast; report of a case. Cancer 1952, 5, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, J.E.; Enterline, H.T.; Roseman, J.M.; Tomasulo, J.P.; McIlvaine, C.H.; Fitts, W.T., Jr.; Kirshenbaum, J. Lobular carcinoma in situ of the breast. Long-term followup. Cancer 1974, 34, 554–563. [Google Scholar] [CrossRef]
- Haagensen, C.D.; Lane, N.; Lattes, R.; Bodian, C. Lobular neoplasia (so-called lobular carcinoma in situ) of the breast. Cancer 1978, 42, 737–769. [Google Scholar] [CrossRef]
- Fisher, E.R.; Costantino, J.; Fisher, B.; Palekar, A.S.; Paik, S.M.; Suarez, C.M.; Wolmark, N. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) Protocol B-17. Five-year observations concerning lobular carcinoma in situ. Cancer 1996, 78, 1403–1416. [Google Scholar] [CrossRef]
- Fisher, E.R.; Land, S.R.; Fisher, B.; Mamounas, E.; Gilarski, L.; Wolmark, N. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: Twelve-year observations concerning lobular carcinoma in situ. Cancer 2004, 100, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Chuba, P.J.; Hamre, M.R.; Yap, J.; Severson, R.K.; Lucas, D.; Shamsa, F.; Aref, A. Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: Analysis of surveillance, epidemiology, and end results data. J. Clin. Oncol. 2005, 23, 5534–5541. [Google Scholar] [CrossRef]
- Aulmann, S.; Penzel, R.; Longerich, T.; Funke, B.; Schirmacher, P.; Sinn, H.P. Clonality of lobular carcinoma in situ (LCIS) and metachronous invasive breast cancer. Breast Cancer Res. Treat. 2008, 107, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Provencher, L.; Jacob, S.; Cote, G.; Hogue, J.C.; Desbiens, C.; Poirier, B.; Raiche, I.; Le Regent, L.; Diorio, C. Low frequency of cancer occurrence in same breast quadrant diagnosed with lobular neoplasia at percutaneous needle biopsy. Radiology 2012, 263, 43–52. [Google Scholar] [CrossRef] [Green Version]
- To, T.; Wall, C.; Baines, C.J.; Miller, A.B. Is carcinoma in situ a precursor lesion of invasive breast cancer? Int. J. Cancer 2014, 135, 1646–1652. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, L.C.; Radisky, D.C.; Frost, M.H.; Santen, R.J.; Vierkant, R.A.; Benetti, L.L.; Tarabishy, Y.; Ghosh, K.; Visscher, D.W.; Degnim, A.C. Understanding the premalignant potential of atypical hyperplasia through its natural history: A longitudinal cohort study. Cancer Prev. Res. 2014, 7, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutuli, B.; De Lafontan, B.; Kirova, Y.; Auvray, H.; Tallet, A.; Avigdor, S.; Brunaud, C.; Delva, C. Lobular carcinoma in situ (LCIS) of the breast: Is long-term outcome similar to ductal carcinoma in situ (DCIS)? Analysis of 200 cases. Radiat. Oncol. 2015, 10, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Brot, M.; Koslow Mautner, S.; Muhsen, S.; Andrade, V.P.; Mamtani, A.; Murray, M.; Giri, D.; Sakr, R.A.; Brogi, E.; King, T.A. Pleomorphic lobular carcinoma in situ of the breast: A single institution experience with clinical follow-up and centralized pathology review. Breast Cancer Res. Treat. 2017, 165, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Lo, L.L.; Milne, R.L.; Liao, Y.; Cuzick, J.; Terry, M.B.; Phillips, K.A. Validation of the IBIS breast cancer risk evaluator for women with lobular carcinoma in-situ. Br. J. Cancer 2018, 119, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.; Lakhani, S.R. Lobular carcinoma in situ: Diagnostic criteria and molecular correlates. Mod. Pathol. 2021, 34, 8–14. [Google Scholar] [CrossRef] [PubMed]
- McDivitt, R.W.; Hutter, R.V.; Foote, F.W., Jr.; Stewart, F.W. In situ lobular carcinoma. A prospective follow-up study indicating cumulative patient risks. JAMA 1967, 201, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Schaumann, N.; Raap, M.; Hinze, L.; Rieger, L.; Schurch, C.M.; Antonopoulos, W.; Avril, S.; Krech, T.; Dammrich, M.; Kayser, G.; et al. Lobular neoplasia and invasive lobular breast cancer: Inter-observer agreement for histological grading and subclassification. Pathol. Res. Pract. 2019, 215, 152611. [Google Scholar] [CrossRef]
- Bratthauer, G.L.; Tavassoli, F.A. Lobular intraepithelial neoplasia: Previously unexplored aspects assessed in 775 cases and their clinical implications. Virchows Arch. 2002, 440, 134–138. [Google Scholar] [CrossRef]
- Kreipe, H.; Länger, F.; Barth, P. Stanzbioptische Mammapathologie im Mammographie-Screening; International Academy of Pathology, German Division: Bonn, Germany, 2015. [Google Scholar]
- Alvarado-Cabrero, I.; Picon Coronel, G.; Valencia Cedillo, R.; Canedo, N.; Tavassoli, F.A. Florid lobular intraepithelial neoplasia with signet ring cells, central necrosis and calcifications: A clinicopathological and immunohistochemical analysis of ten cases associated with invasive lobular carcinoma. Arch. Med. Res. 2010, 41, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Fadare, O.; Dadmanesh, F.; Alvarado-Cabrero, I.; Snyder, R.; Stephen Mitchell, J.; Tot, T.; Wang, S.A.; Ghofrani, M.; Eusebi, V.; Martel, M.; et al. Lobular intraepithelial neoplasia [lobular carcinoma in situ] with comedo-type necrosis: A clinicopathologic study of 18 cases. Am. J. Surg. Pathol. 2006, 30, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A. Why is LCIS important—Pathological review. Curr. Breast Cancer Rep. 2021, in press. [Google Scholar] [CrossRef]
- Shin, S.J.; Lal, A.; De Vries, S.; Suzuki, J.; Roy, R.; Hwang, E.S.; Schnitt, S.J.; Waldman, F.M.; Chen, Y.Y. Florid lobular carcinoma in situ: Molecular profiling and comparison to classic lobular carcinoma in situ and pleomorphic lobular carcinoma in situ. Hum. Pathol. 2013, 44, 1998–2009. [Google Scholar] [CrossRef]
- Chaudhary, S.; Lawrence, L.; McGinty, G.; Kostroff, K.; Bhuiya, T. Classic lobular neoplasia on core biopsy: A clinical and radio-pathologic correlation study with follow-up excision biopsy. Mod. Pathol. 2013, 26, 762–771. [Google Scholar] [CrossRef] [Green Version]
- Harrison, B.T.; Nakhlis, F.; Dillon, D.A.; Soong, T.R.; Garcia, E.P.; Schnitt, S.J.; King, T.A. Genomic profiling of pleomorphic and florid lobular carcinoma in situ reveals highly recurrent ERBB2 and ERRB3 alterations. Mod. Pathol. 2020, 33, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, T.M.; Silverman, J.F. Follow-up surgical excision is indicated when breast core needle biopsies show atypical lobular hyperplasia or lobular carcinoma in situ: A correlative study of 33 patients with review of the literature. Am. J. Surg. Pathol. 2005, 29, 534–543. [Google Scholar] [CrossRef]
- Chivukula, M.; Haynik, D.M.; Brufsky, A.; Carter, G.; Dabbs, D.J. Pleomorphic lobular carcinoma in situ (PLCIS) on breast core needle biopsies: Clinical significance and immunoprofile. Am. J. Surg. Pathol. 2008, 32, 1721–1726. [Google Scholar] [CrossRef]
- El-Sayed, M.E.; Rakha, E.A.; Reed, J.; Lee, A.H.; Evans, A.J.; Ellis, I.O. Predictive value of needle core biopsy diagnoses of lesions of uncertain malignant potential (B3) in abnormalities detected by mammographic screening. Histopathology 2008, 53, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.L.; Lee, D.Y.; Tartter, P.I. The significance of lobular carcinoma in situ and atypical lobular hyperplasia of the breast. Ann. Surg. Oncol. 2012, 19, 4124–4128. [Google Scholar] [CrossRef]
- Masannat, Y.A.; Husain, E.; Roylance, R.; Heys, S.D.; Carder, P.J.; Ali, H.; Maurice, Y.; Pinder, S.E.; Sawyer, E.; Shaaban, A.M. Pleomorphic LCIS what do we know? A UK multicenter audit of pleomorphic lobular carcinoma in situ. Breast 2018, 38, 120–124. [Google Scholar] [CrossRef]
- Foschini, M.P.; Miglio, R.; Fiore, R.; Baldovini, C.; Castellano, I.; Callagy, G.; Bianchi, S.; Kaya, H.; Amendoeira, I.; Querzoli, P.; et al. Pre-operative management of Pleomorphic and florid lobular carcinoma in situ of the breast: Report of a large multi-institutional series and review of the literature. Eur. J. Surg. Oncol. 2019, 45, 2279–2286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, L.Z.; Thurow, T.A.; Mahajan, A.; Susnik, B.; Helenowski, I.; Chmiel, J.S.; Sullivan, M.E. Multinucleation is an objective feature useful in the diagnosis of pleomorphic lobular carcinoma in situ. Am. J. Clin. Pathol. 2015, 144, 722–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent-Salomon, A.; Hajage, D.; Rouquette, A.; Cedenot, A.; Gruel, N.; Alran, S.; Sastre-Garau, X.; Sigal-Zafrani, B.; Fourquet, A.; Kirova, Y. High Ki67 expression is a risk marker of invasive relapse for classical lobular carcinoma in situ patients. Breast 2012, 21, 380–383. [Google Scholar] [CrossRef]
- Andrade, V.P.; Morrogh, M.; Qin, L.X.; Olvera, N.; Giri, D.; Muhsen, S.; Sakr, R.A.; Schizas, M.; Ng, C.K.; Arroyo, C.D.; et al. Gene expression profiling of lobular carcinoma in situ reveals candidate precursor genes for invasion. Mol. Oncol. 2015, 9, 772–782. [Google Scholar] [CrossRef]
- Ibrahim, A.E.; Bateman, A.C.; Theaker, J.M.; Low, J.L.; Addis, B.; Tidbury, P.; Rubin, C.; Briley, M.; Royle, G.T. The role and histological classification of needle core biopsy in comparison with fine needle aspiration cytology in the preoperative assessment of impalpable breast lesions. J. Clin. Pathol. 2001, 54, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Pinder, S.E.; Shaaban, A.; Deb, R.; Desai, A.; Gandhi, A.; Lee, A.H.S.; Pain, S.; Wilkinson, L.; Sharma, N. NHS Breast Screening multidisciplinary working group guidelines for the diagnosis and management of breast lesions of uncertain malignant potential on core biopsy (B3 lesions). Clin. Radiol. 2018, 73, 682–692. [Google Scholar] [CrossRef] [Green Version]
- Chester, R.; Bokinni, O.; Ahmed, I.; Kasem, A. UK national survey of management of breast lobular carcinoma in situ. Ann. R. Coll. Surg. Engl. 2015, 97, 574–577. [Google Scholar] [CrossRef] [Green Version]
- Colleoni, M.; Rotmensz, N.; Maisonneuve, P.; Mastropasqua, M.G.; Luini, A.; Veronesi, P.; Intra, M.; Montagna, E.; Cancello, G.; Cardillo, A.; et al. Outcome of special types of luminal breast cancer. Ann. Oncol. 2012, 23, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; El-Sayed, M.E.; Powe, D.G.; Green, A.R.; Habashy, H.; Grainge, M.J.; Robertson, J.F.; Blamey, R.; Gee, J.; Nicholson, R.I.; et al. Invasive lobular carcinoma of the breast: Response to hormonal therapy and outcomes. Eur. J. Cancer 2008, 44, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Simpson, P.T.; Reis-Filho, J.S.; Lambros, M.B.; Jones, C.; Steele, D.; Mackay, A.; Iravani, M.; Fenwick, K.; Dexter, T.; Jones, A.; et al. Molecular profiling pleomorphic lobular carcinomas of the breast: Evidence for a common molecular genetic pathway with classic lobular carcinomas. J. Pathol. 2008, 215, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Monhollen, L.; Morrison, C.; Ademuyiwa, F.O.; Chandrasekhar, R.; Khoury, T. Pleomorphic lobular carcinoma: A distinctive clinical and molecular breast cancer type. Histopathology 2012, 61, 365–377. [Google Scholar] [CrossRef]
- Deniziaut, G.; Tille, J.C.; Bidard, F.C.; Vacher, S.; Schnitzler, A.; Chemlali, W.; Tremoulet, L.; Fuhrmann, L.; Cottu, P.; Rouzier, R.; et al. ERBB2 mutations associated with solid variant of high-grade invasive lobular breast carcinomas. Oncotarget 2016, 7, 73337–73346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christgen, M.; Bartels, S.; Luft, A.; Persing, S.; Henkel, D.; Lehmann, U.; Kreipe, H. Activating human epidermal growth factor receptor 2 (HER2) gene mutation in bone metastases from breast cancer. Virchows Arch. 2018, 473, 577–582. [Google Scholar] [CrossRef]
- Jongen, L.; Floris, G.; Boeckx, B.; Smeets, D.; Lambrechts, D.; Vander Borght, S.; Laenen, A.; Mann, G.; Cutler, R.E., Jr.; Lalani, A.S.; et al. Identification, clinical-pathological characteristics and treatment outcomes of patients with metastatic breast cancer and somatic human epidermal growth factor receptor 2 (ERBB2) mutations. Breast Cancer Res. Treat. 2019, 174, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kurozumi, S.; Alsaleem, M.; Monteiro, C.J.; Bhardwaj, K.; Joosten, S.E.P.; Fujii, T.; Shirabe, K.; Green, A.R.; Ellis, I.O.; Rakha, E.A.; et al. Targetable ERBB2 mutation status is an independent marker of adverse prognosis in estrogen receptor positive, ERBB2 non-amplified primary lobular breast carcinoma: A retrospective in silico analysis of public datasets. Breast Cancer Res. 2020, 22, 85. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Wang, K.; Sheehan, C.E.; Boguniewicz, A.B.; Otto, G.; Downing, S.R.; Sun, J.; He, J.; Curran, J.A.; Ali, S.; et al. Relapsed classic E-cadherin (CDH1)-mutated invasive lobular breast cancer shows a high frequency of HER2 (ERBB2) gene mutations. Clin. Cancer Res. 2013, 19, 2668–2676. [Google Scholar] [CrossRef] [Green Version]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, R.; Kavuri, S.M.; Searleman, A.C.; Shen, W.; Shen, D.; Koboldt, D.C.; Monsey, J.; Goel, N.; Aronson, A.B.; Li, S.; et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov 2013, 3, 224–237. [Google Scholar] [CrossRef] [Green Version]
- Van Maaren, M.C.; de Munck, L.; Strobbe, L.J.A.; Sonke, G.S.; Westenend, P.J.; Smidt, M.L.; Poortmans, P.M.P.; Siesling, S. Ten-year recurrence rates for breast cancer subtypes in The Netherlands: A large population-based study. Int. J. Cancer 2019, 144, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Christgen, M.; Geffers, R.; Kreipe, H.; Lehmann, U. IPH-926 lobular breast cancer cells are triple-negative but their microarray profile uncovers a luminal subtype. Cancer Sci. 2013, 104, 1726–1730. [Google Scholar] [CrossRef] [Green Version]
- Bergeron, A.; MacGrogan, G.; Bertaut, A.; Ladoire, S.; Arveux, P.; Desmoulins, I.; Bonnefoi, H.; Loustalot, C.; Auriol, S.; Beltjens, F.; et al. Triple-negative breast lobular carcinoma: A luminal androgen receptor carcinoma with specific ESRRA mutations. Mod. Pathol. 2021, in press. [Google Scholar] [CrossRef]
- Bertucci, F.; Finetti, P.; Cervera, N.; Esterni, B.; Hermitte, F.; Viens, P.; Birnbaum, D. How basal are triple-negative breast cancers? Int. J. Cancer 2008, 123, 236–240. [Google Scholar] [CrossRef]
- Montagna, E.; Maisonneuve, P.; Rotmensz, N.; Cancello, G.; Iorfida, M.; Balduzzi, A.; Galimberti, V.; Veronesi, P.; Luini, A.; Pruneri, G.; et al. Heterogeneity of Triple-Negative Breast Cancer: Histologic Subtyping to Inform the Outcome. Clin. Breast Cancer 2013, 13, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Gluz, O.; Kolberg-Liedtke, C.; Prat, A.; Christgen, M.; Gebauer, D.; Kates, R.; Pare, L.; Grischke, E.M.; Forstbauer, H.; Braun, M.; et al. Efficacy of deescalated chemotherapy according to PAM50 subtypes, immune and proliferation genes in triple-negative early breast cancer: Primary translational analysis of the WSG-ADAPT-TN trial. Int. J. Cancer 2020, 146, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Nishizaki, T.; Chew, K.; Chu, L.; Isola, J.; Kallioniemi, A.; Weidner, N.; Waldman, F.M. Genetic alterations in lobular breast cancer by comparative genomic hybridization. Int. J. Cancer 1997, 74, 513–517. [Google Scholar] [CrossRef]
- Engstrom, M.J.; Opdahl, S.; Vatten, L.J.; Haugen, O.A.; Bofin, A.M. Invasive lobular breast cancer: The prognostic impact of histopathological grade, E-cadherin and molecular subtypes. Histopathology 2015, 66, 409–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbognin, L.; Sperduti, I.; Fabi, A.; Dieci, M.V.; Kadrija, D.; Griguolo, G.; Pilotto, S.; Guarneri, V.; Zampiva, I.; Brunelli, M.; et al. Prognostic impact of proliferation for resected early stage ‘pure’ invasive lobular breast cancer: Cut-off analysis of Ki67 according to histology and clinical validation. Breast 2017, 35, 21–26. [Google Scholar] [CrossRef]
- Narbe, U.; Bendahl, P.O.; Grabau, D.; Ryden, L.; Ingvar, C.; Ferno, M. Invasive lobular carcinoma of the breast: Long-term prognostic value of Ki67 and histological grade, alone and in combination with estrogen receptor. Springerplus 2014, 3, 70. [Google Scholar] [CrossRef] [Green Version]
- Dessauvagie, B.; Thomas, A.; Thomas, C.; Robinson, C.; Combrink, M.; Budhavaram, V.; Kunjuraman, B.; Meehan, K.; Sterrett, G.; Harvey, J. Invasive lobular carcinoma of the breast: Assessment of proliferative activity using automated Ki-67 immunostaining. Pathology 2019, 51, 681–687. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Pagan, E.; Viale, G.; Bagnardi, V.; Peruzzotti, G.; De Pas, T.; Bianco, N.; Graffeo, R.; Rocco, E.G.; et al. Endocrine-responsive lobular carcinoma of the breast: Features associated with risk of late distant recurrence. Breast Cancer Res. 2019, 21, 153. [Google Scholar] [CrossRef] [Green Version]
- Lobo, J.; Petronilho, S.; Newell, A.H.; Coach, J.; Harlow, G.; Cruz, A.; Lopes, P.; Antunes, L.; Bai, I.; Walker, E.; et al. E-cadherin clone 36 nuclear staining dictates adverse disease outcome in lobular breast cancer patients. Mod. Pathol. 2019, 32, 1574–1586. [Google Scholar] [CrossRef]
- Reis-Filho, J.S.; Cancela Paredes, J.; Milanezi, F.; Schmitt, F.C. Clinicopathologic implications of E-cadherin reactivity in patients with lobular carcinoma in situ of the breast. Cancer 2002, 94, 2114–2115. [Google Scholar] [CrossRef] [PubMed]
- Vyberg, M.; Nielsen, S. Proficiency testing in immunohistochemistry--experiences from Nordic Immunohistochemical Quality Control (NordiQC). Virchows Arch. 2016, 468, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.S.; Sousa, B.; Carreto, L.; Mendes, N.; Nobre, A.R.; Ricardo, S.; Albergaria, A.; Cameselle-Teijeiro, J.F.; Gerhard, R.; Soderberg, O.; et al. P-cadherin functional role is dependent on E-cadherin cellular context: A proof of concept using the breast cancer model. J. Pathol. 2013, 229, 705–718. [Google Scholar] [CrossRef]
- Turashvili, G.; McKinney, S.E.; Goktepe, O.; Leung, S.C.; Huntsman, D.G.; Gelmon, K.A.; Los, G.; Rejto, P.A.; Aparicio, S.A. P-cadherin expression as a prognostic biomarker in a 3992 case tissue microarray series of breast cancer. Mod. Pathol. 2011, 24, 64–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tot, T. The role of cytokeratins 20 and 7 and estrogen receptor analysis in separation of metastatic lobular carcinoma of the breast and metastatic signet ring cell carcinoma of the gastrointestinal tract. Apmis 2000, 108, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Lehr, H.A.; Folpe, A.; Yaziji, H.; Kommoss, F.; Gown, A.M. Cytokeratin 8 immunostaining pattern and E-cadherin expression distinguish lobular from ductal breast carcinoma. Am. J. Clin. Pathol. 2000, 114, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Fadare, O.; Wang, S.A.; Hileeto, D. The expression of cytokeratin 5/6 in invasive lobular carcinoma of the breast: Evidence of a basal-like subset? Hum. Pathol. 2008, 39, 331–336. [Google Scholar] [CrossRef]
- Khilko, N.; Wang, J.; Wei, B.; Hicks, D.G.; Tang, P. Invasive Lobular Carcinomas Do Not Express Basal Cytokeratin Markers CK5/6, CK14 and CK17. Breast Cancer 2010, 4, 49–55. [Google Scholar] [CrossRef]
- Shao, M.M.; Chan, S.K.; Yu, A.M.; Lam, C.C.; Tsang, J.Y.; Lui, P.C.; Law, B.K.; Tan, P.H.; Tse, G.M. Keratin expression in breast cancers. Virchows Arch. 2012, 461, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; McCue, P.A.; Sarlomo-Rikala, M.; Rys, J.; Czapiewski, P.; Wazny, K.; Langfort, R.; Waloszczyk, P.; Biernat, W.; Lasota, J.; et al. GATA3: A multispecific but potentially useful marker in surgical pathology: A systematic analysis of 2500 epithelial and nonepithelial tumors. Am. J. Surg. Pathol. 2014, 38, 13–22. [Google Scholar] [CrossRef]
- Yang, M.; Nonaka, D. A study of immunohistochemical differential expression in pulmonary and mammary carcinomas. Mod. Pathol. 2010, 23, 654–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vranic, S.; Schmitt, F.; Sapino, A.; Costa, J.L.; Reddy, S.; Castro, M.; Gatalica, Z. Apocrine carcinoma of the breast: A comprehensive review. Histol. Histopathol. 2013, 28, 1393–1409. [Google Scholar] [PubMed]
- Eusebi, V.; Betts, C.; Haagensen, D.E., Jr.; Gugliotta, P.; Bussolati, G.; Azzopardi, J.G. Apocrine differentiation in lobular carcinoma of the breast: A morphologic, immunologic, and ultrastructural study. Hum. Pathol. 1984, 15, 134–140. [Google Scholar] [CrossRef]
- Yu, J.; Dabbs, D.J.; Shuai, Y.; Niemeier, L.A.; Bhargava, R. Classical-type invasive lobular carcinoma with HER2 overexpression: Clinical, histologic, and hormone receptor characteristics. Am. J. Clin. Pathol. 2011, 136, 88–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raap, M.; Gronewold, M.; Christgen, H.; Glage, S.; Bentires-Alj, M.; Koren, S.; Derksen, P.; Boelens, M.; Jonkers, J.; Lehmann, U.; et al. Lobular carcinoma in situ and invasive lobular breast cancer are characterized by enhanced expression of Transcription Factor AP 2beta (TFAP2B). Lab. Investig. 2018, 98, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Wachtel, M.; Runge, T.; Leuschner, I.; Stegmaier, S.; Koscielniak, E.; Treuner, J.; Odermatt, B.; Behnke, S.; Niggli, F.K.; Schafer, B.W. Subtype and prognostic classification of rhabdomyosarcoma by immunohistochemistry. J. Clin. Oncol. 2006, 24, 816–822. [Google Scholar] [CrossRef]
- Ebauer, M.; Wachtel, M.; Niggli, F.K.; Schafer, B.W. Comparative expression profiling identifies an in vivo target gene signature with TFAP2B as a mediator of the survival function of PAX3/FKHR. Oncogene 2007, 26, 7267–7281. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, S.; Miki, Y.; Miyashita, M.; Hata, S.; Takahashi, Y.; Rai, Y.; Sagara, Y.; Ohi, Y.; Hirakawa, H.; Tamaki, K.; et al. Tumor microenvironment in invasive lobular carcinoma: Possible therapeutic targets. Breast Cancer Res. Treat. 2016, 155, 65–75. [Google Scholar] [CrossRef]
- Kovari, B.; Rusz, O.; Schally, A.V.; Kahan, Z.; Cserni, G. Differential immunostaining of various types of breast carcinomas for growth hormone-releasing hormone receptor—Apocrine epithelium and carcinomas emerging as uniformly positive. Apmis 2014, 122, 824–831. [Google Scholar] [CrossRef]
- Kovari, B.; Vranic, S.; Marchio, C.; Sapino, A.; Cserni, G. The expression of GHRH and its receptors in breast carcinomas with apocrine differentiation-further evidence of the presence of a GHRH pathway in these tumors. Hum. Pathol. 2017, 64, 164–170. [Google Scholar] [CrossRef]
- Thompson, E.D.; Taube, J.M.; Asch-Kendrick, R.J.; Ogurtsova, A.; Xu, H.; Sharma, R.; Meeker, A.; Argani, P.; Emens, L.A.; Cimino-Mathews, A. PD-L1 expression and the immune microenvironment in primary invasive lobular carcinomas of the breast. Mod. Pathol. 2017, 30, 1551–1560. [Google Scholar] [CrossRef]
- Pereira, H.; Pinder, S.E.; Sibbering, D.M.; Galea, M.H.; Elston, C.W.; Blamey, R.W.; Robertson, J.F.; Ellis, I.O. Pathological prognostic factors in breast cancer. IV: Should you be a typer or a grader? A comparative study of two histological prognostic features in operable breast carcinoma. Histopathology 1995, 27, 219–226. [Google Scholar] [CrossRef]
- Hood, C.I.; Font, R.L.; Zimmerman, L.E. Metastatic mammary carcinoma in the eyelid with histiocytoid appearance. Cancer 1973, 31, 793–800. [Google Scholar] [CrossRef] [Green Version]
- Walford, N.; ten Velden, J. Histiocytoid breast carcinoma: An apocrine variant of lobular carcinoma. Histopathology 1989, 14, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Croitoru, C.M.; Ayala, A.G.; Sahin, A.A.; Middleton, L.P. E-cadherin immunohistochemical analysis of histiocytoid carcinoma of the breast. Ann. Diagn. Pathol. 2002, 6, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Reis-Filho, J.S.; Fulford, L.G.; Freeman, A.; Lakhani, S.R. Pathologic quiz case: A 93-year-old woman with an enlarged and tender left breast. Histiocytoid variant of lobular breast carcinoma. Arch. Pathol. Lab. Med. 2003, 127, 1626–1628. [Google Scholar] [CrossRef] [PubMed]
- Kasashima, S.; Kawashima, A.; Zen, Y.; Ozaki, S.; Kobayashi, M.; Tsujibata, A.; Minato, H. Expression of aberrant mucins in lobular carcinoma with histiocytoid feature of the breast. Virchows Arch. 2007, 450, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Harada, O.; Thike, A.A.; Tse, G.M. Histiocytoid breast carcinoma: An enigmatic lobular entity. J. Clin. Pathol. 2011, 64, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Van Bogaert, L.J.; Maldague, P. Infiltrating lobular carcinoma of the female breast. Deviations from the usual histopathologic appearance. Cancer 1980, 45, 979–984. [Google Scholar] [CrossRef]
- Rakha, E.A.; Abbas, A.; Sheeran, R. Invasive Lobular Carcinoma Mimicking Papillary Carcinoma: A Report of Three Cases. Pathobiology 2016, 83, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Christgen, M.; Bartels, S.; van Luttikhuizen, J.L.; Schieck, M.; Pertschy, S.; Kundu, S.; Lehmann, U.; Sander, B.; Pelz, E.; Langer, F.; et al. Subclonal analysis in a lobular breast cancer with classical and solid growth pattern mimicking a solid-papillary carcinoma. J. Pathol. Clin. Res. 2017, 3, 191–202. [Google Scholar] [CrossRef]
- Motanagh, S.A.; Muller, K.E. Invasive lobular carcinoma with papillary features: A newly described variant that poses a difficult histologic differential diagnosis. Breast J. 2020, 26, 1231–1233. [Google Scholar] [CrossRef]
- Tay, T.K.Y.; Tan, P.H. Papillary neoplasms of the breast -reviewing the spectrum. Mod. Pathol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.; Azzopardi, J.G. Lobular carcinoma of the breast: A special variant of mucin-secreting carcinoma. J. Clin. Pathol. 1975, 28, 711–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbrecher, J.S.; Silverberg, S.G. Signet-ring cell carcinoma of the breast. The mucinous variant of infiltrating lobular carcinoma? Cancer 1976, 37, 828–840. [Google Scholar] [CrossRef]
- Breslow, A.; Brancaccio, M.E. Intracellular mucin production by lobular breast carcinoma cells. Arch. Pathol. Lab. Med. 1976, 100, 620–621. [Google Scholar]
- Eusebi, V.; Pich, A.; Macchiorlatti, E.; Bussolati, G. Morpho-functional differentiation in lobular carcinoma of the breast. Histopathology 1977, 1, 301–314. [Google Scholar] [CrossRef]
- Fisher, E.R.; Gregorio, R.M.; Redmond, C.; Fisher, B. Tubulolobular invasive breast cancer: A variant of lobular invasive cancer. Hum. Pathol. 1977, 8, 679–683. [Google Scholar] [CrossRef]
- Dixon, J.M.; Anderson, T.J.; Page, D.L.; Lee, D.H.; Duffy, S.W. Infiltrating lobular carcinoma of the breast. Histopathology 1982, 6, 149–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, D.T.; Tai, L.H.; Bratthauer, G.L.; Waldner, D.L.; Tavassoli, F.A. Tubulolobular carcinoma of the breast: An analysis of 27 cases of a tumor with a hybrid morphology and immunoprofile. Am. J. Surg. Pathol. 2004, 28, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Tamaru, J.; Takeuchi, I.; Ohnisi, K.; Sakamoto, G.; Adachi, A.; Kaneko, K.; Itoyama, S. Expression of E-cadherin, alpha-catenin, and beta-catenin in tubulolobular carcinoma of the breast. Virchows Arch. 2006, 448, 500–505. [Google Scholar] [CrossRef]
- Esposito, N.N.; Chivukula, M.; Dabbs, D.J. The ductal phenotypic expression of the E-cadherin/catenin complex in tubulolobular carcinoma of the breast: An immunohistochemical and clinicopathologic study. Mod. Pathol. 2007, 20, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Marchio, C.; Sapino, A.; Arisio, R.; Bussolati, G. A new vision of tubular and tubulo-lobular carcinomas of the breast, as revealed by 3-D modelling. Histopathology 2006, 48, 556–562. [Google Scholar] [CrossRef]
- Asioli, S.; Marucci, G.; Ficarra, G.; Stephens, M.; Foschini, M.P.; Ellis, I.O.; Eusebi, V. Polymorphous adenocarcinoma of the breast. Report of three cases. Virchows Arch. 2006, 448, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Page, D.L.; Abnderson, T.J.; Sakamoto, G. Infiltrating lobular carcinoma. In Diagnostic Histopathology of the Breast; Page, D.L., Anderson, T.J., Eds.; Churchill Livingston: New York, NY, USA, 1987; pp. 219–226. [Google Scholar]
- Weidner, N.; Semple, J.P. Pleomorphic variant of invasive lobular carcinoma of the breast. Hum. Pathol. 1992, 23, 1167–1171. [Google Scholar] [CrossRef]
- Bentz, J.S.; Yassa, N.; Clayton, F. Pleomorphic lobular carcinoma of the breast: Clinicopathologic features of 12 cases. Mod. Pathol. 1998, 11, 814–822. [Google Scholar] [PubMed]
- Rakha, E.A.; van Deurzen, C.H.; Paish, E.C.; Macmillan, R.D.; Ellis, I.O.; Lee, A.H. Pleomorphic lobular carcinoma of the breast: Is it a prognostically significant pathological subtype independent of histological grade? Mod. Pathol. 2013, 26, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Geyer, F.C.; Natrajan, R.; Lopez-Garcia, M.A.; Ahmad, A.S.; Savage, K.; Kreike, B.; Reis-Filho, J.S. The molecular underpinning of lobular histological growth pattern: A genome-wide transcriptomic analysis of invasive lobular carcinomas and grade- and molecular subtype-matched invasive ductal carcinomas of no special type. J. Pathol. 2010, 220, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Middleton, L.P.; Palacios, D.M.; Bryant, B.R.; Krebs, P.; Otis, C.N.; Merino, M.J. Pleomorphic lobular carcinoma: Morphology, immunohistochemistry, and molecular analysis. Am. J. Surg. Pathol. 2000, 24, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Radhi, J.M. Immunohistochemical analysis of pleomorphic lobular carcinoma: Higher expression of p53 and chromogranin and lower expression of ER and PgR. Histopathology 2000, 36, 156–160. [Google Scholar] [CrossRef]
- Frolik, D.; Caduff, R.; Varga, Z. Pleomorphic lobular carcinoma of the breast: Its cell kinetics, expression of oncogenes and tumour suppressor genes compared with invasive ductal carcinomas and classical infiltrating lobular carcinomas. Histopathology 2001, 39, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Sneige, N.; Wang, J.; Baker, B.A.; Krishnamurthy, S.; Middleton, L.P. Clinical, histopathologic, and biologic features of pleomorphic lobular (ductal-lobular) carcinoma in situ of the breast: A report of 24 cases. Mod. Pathol. 2002, 15, 1044–1050. [Google Scholar] [CrossRef]
- Palacios, J.; Sarrio, D.; Garcia-Macias, M.C.; Bryant, B.; Sobel, M.E.; Merino, M.J. Frequent E-cadherin gene inactivation by loss of heterozygosity in pleomorphic lobular carcinoma of the breast. Mod. Pathol. 2003, 16, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.C.; Chen, Y.L.; Juang, Y.L.; Jeng, Y.M. Frequent alterations of HER2 through mutation, amplification, or overexpression in pleomorphic lobular carcinoma of the breast. Breast Cancer Res. Treat. 2015, 150, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Ilic, I.R.; Djordjevic, N.P.; Randjelovic, P.J.; Stojanovic, N.M.; Radulovic, N.S.; Ilic, R.S. Seven-year survey of classical and pleomorphic invasive lobular breast carcinomas in women from southeastern Serbia: Differences in clinicopathological and immunohistochemical features. J. BUON 2016, 21, 1113–1120. [Google Scholar] [PubMed]
- Liu, Y.L.; Choi, C.; Lee, S.M.; Zhong, X.; Hibshoosh, H.; Kalinsky, K.; Connolly, E.P. Invasive Lobular Breast Carcinoma: Pleomorphic Versus Classical Subtype, Associations and Prognosis. Clin. Breast Cancer 2018, 18, 114–120. [Google Scholar] [CrossRef]
- Rosa-Rosa, J.M.; Caniego-Casas, T.; Leskela, S.; Cristobal, E.; Gonzalez-Martinez, S.; Moreno-Moreno, E.; Lopez-Miranda, E.; Holgado, E.; Perez-Mies, B.; Garrido, P.; et al. High Frequency of ERBB2 Activating Mutations in Invasive Lobular Breast Carcinoma with Pleomorphic Features. Cancers 2019, 11, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedlinger, G.M.; Joshi, S.; Hirshfield, K.M.; Barnard, N.; Ganesan, S. Targetable alterations in invasive pleomorphic lobular carcinoma of the breast. Breast Cancer Res. 2021, 23, 7. [Google Scholar] [CrossRef] [PubMed]
- Ercan, C.; van Diest, P.J.; van der Ende, B.; Hinrichs, J.; Bult, P.; Buerger, H.; van der Wall, E.; Derksen, P.W. p53 mutations in classic and pleomorphic invasive lobular carcinoma of the breast. Cell Oncol. 2012, 35, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Christgen, M.; Noskowicz, M.; Heil, C.; Schipper, E.; Christgen, H.; Geffers, R.; Kreipe, H.; Lehmann, U. IPH-926 lobular breast cancer cells harbor a p53 mutant with temperature-sensitive functional activity and allow for profiling of p53-responsive genes. Lab. Investig. 2012, 92, 1635–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzger-Filho, O.; Michiels, S.; Bertucci, F.; Catteau, A.; Salgado, R.; Galant, C.; Fumagalli, D.; Singhal, S.K.; Desmedt, C.; Ignatiadis, M.; et al. Genomic grade adds prognostic value in invasive lobular carcinoma. Ann. Oncol. 2013, 24, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, I.A.; Vermeulen, J.F.; Ercan, C.; Houthuijzen, J.M.; Saig, F.A.; Vlug, E.J.; van der Wall, E.; van Diest, P.J.; Vooijs, M.; Derksen, P.W. FER kinase promotes breast cancer metastasis by regulating alpha- and beta-integrin-dependent cell adhesion and anoikis resistance. Oncogene 2013, 32, 5582–5592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moelans, C.B.; Vlug, E.J.; Ercan, C.; Bult, P.; Buerger, H.; Cserni, G.; van Diest, P.J.; Derksen, P.W. Methylation biomarkers for pleomorphic lobular breast cancer—A short report. Cell Oncol. 2015, 38, 397–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocco, E.; Javier Carmona, F.; Razavi, P.; Won, H.H.; Cai, Y.; Rossi, V.; Chan, C.; Cownie, J.; Soong, J.; Toska, E.; et al. Neratinib is effective in breast tumors bearing both amplification and mutation of ERBB2 (HER2). Sci. Signal. 2018, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanker, A.B.; Brewer, M.R.; Sheehan, J.H.; Koch, J.P.; Sliwoski, G.R.; Nagy, R.; Lanman, R.; Berger, M.F.; Hyman, D.M.; Solit, D.B.; et al. An Acquired HER2T798I Gatekeeper Mutation Induces Resistance to Neratinib in a Patient with HER2 Mutant-Driven Breast Cancer. Cancer Discov. 2017, 7, 575–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grellety, T.; Soubeyran, I.; Robert, J.; Bonnefoi, H.; Italiano, A. A clinical case of invasive lobular breast carcinoma with ERBB2 and CDH1 mutations presenting a dramatic response to anti-HER2-directed therapy. Ann. Oncol. 2016, 27, 199–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef]
- Yang, L.P.; Sun, H.F.; Zhao, Y.; Chen, M.T.; Zhang, N.; Jin, W. Clinicopathological characteristics and survival outcomes in pleomorphic lobular breast carcinoma of the breast: A SEER population-based study. Cancer Med. 2017, 6, 2867–2875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, W.; Arms, A.; Verma, V.; Hatch, S.; Brian Butler, E.; Teh, B.S. Outcomes of pleomorphic lobular carcinoma versus invasive lobular carcinoma. Breast 2019, 43, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Mohammadi, A.; Masood, S. Lobular carcinoma of the breast with extracellular mucin: New variant of mucin-producing carcinomas? Pathol. Int. 2009, 59, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Bhargava, R.; Dabbs, D.J. Invasive lobular carcinoma with extracellular mucin production and HER-2 overexpression: A case report and further case studies. Diagn. Pathol. 2011, 5, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez Macias, G.S.; Perez Saucedo, J.E.; Cardona Huerta, S.; Garza Montemayor, M.; Villarreal Garza, C.; Garcia Hernandez, I. Invasive lobular carcinoma of the breast with extracellular mucin: A case report. Int. J. Surg. Case Rep. 2016, 25, 33–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jambal, P.; Badtke, M.M.; Harrell, J.C.; Borges, V.F.; Post, M.D.; Sollender, G.E.; Spillman, M.A.; Horwitz, K.B.; Jacobsen, B.M. Estrogen switches pure mucinous breast cancer to invasive lobular carcinoma with mucinous features. Breast Cancer Res. Treat. 2012, 137, 431–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cserni, G. A cell line thought to represent mucinous breast cancer probably represents lobular carcinoma with extracellular mucin production. Virchows Arch. 2017, 471, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Boyce, R.W.; Abd El-Rehim, D.; Kurien, T.; Green, A.R.; Paish, E.C.; Robertson, J.F.; Ellis, I.O. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod. Pathol. 2005, 18, 1295–1304. [Google Scholar] [CrossRef]
- Cserni, G.; Floris, G.; Koufopoulos, N.; Kovacs, A.; Nonni, A.; Regitnig, P.; Stahls, A.; Varga, Z. Invasive lobular carcinoma with extracellular mucin production-a novel pattern of lobular carcinomas of the breast. Clinico-pathological description of eight cases. Virchows Arch. 2017, 471, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, K.; DiazGomez, B.; Wang, Y.; Ou, J.; Hansen, K. Invasive Lobular Carcinoma With Extracellular Mucin: Not All Mucinous Mammary Carcinomas Are Ductal! Int. J. Surg. Pathol. 2019, 27, 55–58. [Google Scholar] [CrossRef]
- Soong, T.R.; Dillon, D.; Wieczorek, T.; Collins, L.; Lester, S.; Schnitt, S.; Harrison, B.T. Clinico-pathologic and molecular profile of invasive lobular carcinoma with extracellular mucin (ILCEM). Mol. Biol. Cell 2018, 98 (Suppl. 2), 107–108. [Google Scholar]
- Risaliti, A.; Pizzolitto, S.; Menis, M.; Tomada, P.F.; Cinque, A.; Melchior, C.; Rocco, M. Lobular carcinoma of the breast with neuroendocrine differentiation. Study of a case. G. Ital. Oncol. 1989, 9, 100–104. [Google Scholar]
- Mohanty, S.K.; Kim, S.A.; DeLair, D.F.; Bose, S.; Laury, A.R.; Chopra, S.; Mertens, R.B.; Dhall, D. Comparison of metastatic neuroendocrine neoplasms to the breast and primary invasive mammary carcinomas with neuroendocrine differentiation. Mod. Pathol. 2016, 29, 788–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchio, C.; Geyer, F.C.; Ng, C.K.; Piscuoglio, S.; De Filippo, M.R.; Cupo, M.; Schultheis, A.M.; Lim, R.S.; Burke, K.A.; Guerini-Rocco, E.; et al. The genetic landscape of breast carcinomas with neuroendocrine differentiation. J. Pathol. 2017, 241, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, M.; Menet, E.; Tille, J.C.; Lae, M.; Fuhrmann, L.; Bonneau, C.; Deniziaut, G.; Melaabi, S.; Ng, C.C.K.; Marchio, C.; et al. Comprehensive clinical and molecular analyses of neuroendocrine carcinomas of the breast. Mod. Pathol. 2018, 31, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Tot, T. The diffuse type of invasive lobular carcinoma of the breast: Morphology and prognosis. Virchows Arch. 2003, 443, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Tot, T. Clinical relevance of the distribution of the lesions in 500 consecutive breast cancer cases documented in large-format histologic sections. Cancer 2007, 110, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Hofmeyer, S.; Pekar, G.; Gere, M.; Tarjan, M.; Hellberg, D.; Tot, T. Comparison of the subgross distribution of the lesions in invasive ductal and lobular carcinomas of the breast: A large-format histology study. Int. J. Breast Cancer 2012, 2012, 436141. [Google Scholar] [CrossRef] [Green Version]
- Schipper, K.; Seinstra, D.; Paulien Drenth, A.; van der Burg, E.; Ramovs, V.; Sonnenberg, A.; van Rheenen, J.; Nethe, M.; Jonkers, J. Rebalancing of actomyosin contractility enables mammary tumor formation upon loss of E-cadherin. Nat. Commun. 2019, 10, 3800. [Google Scholar] [CrossRef] [Green Version]
- Boussadia, O.; Kutsch, S.; Hierholzer, A.; Delmas, V.; Kemler, R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech. Dev. 2002, 115, 53–62. [Google Scholar] [CrossRef]
- Derksen, P.W.; Braumuller, T.M.; van der Burg, E.; Hornsveld, M.; Mesman, E.; Wesseling, J.; Krimpenfort, P.; Jonkers, J. Mammary-specific inactivation of E-cadherin and p53 impairs functional gland development and leads to pleomorphic invasive lobular carcinoma in mice. Dis. Model. Mech. 2011, 4, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Annunziato, S.; Kas, S.M.; Nethe, M.; Yucel, H.; Del Bravo, J.; Pritchard, C.; Bin Ali, R.; van Gerwen, B.; Siteur, B.; Drenth, A.P.; et al. Modeling invasive lobular breast carcinoma by CRISPR/Cas9-mediated somatic genome editing of the mammary gland. Genes Dev. 2016, 30, 1470–1480. [Google Scholar] [CrossRef] [Green Version]
- Boelens, M.C.; Nethe, M.; Klarenbeek, S.; de Ruiter, J.R.; Schut, E.; Bonzanni, N.; Zeeman, A.L.; Wientjens, E.; van der Burg, E.; Wessels, L.; et al. PTEN Loss in E-Cadherin-Deficient Mouse Mammary Epithelial Cells Rescues Apoptosis and Results in Development of Classical Invasive Lobular Carcinoma. Cell Rep. 2016, 16, 2087–2101. [Google Scholar] [CrossRef] [Green Version]
- An, Y.; Adams, J.R.; Hollern, D.P.; Zhao, A.; Chang, S.G.; Gams, M.S.; Chung, P.E.D.; He, X.; Jangra, R.; Shah, J.S.; et al. Cdh1 and Pik3ca Mutations Cooperate to Induce Immune-Related Invasive Lobular Carcinoma of the Breast. Cell Rep. 2018, 25, 702–714.e706. [Google Scholar] [CrossRef] [Green Version]
- Cornelissen, L.M.; Drenth, A.P.; van der Burg, E.; de Bruijn, R.; Pritchard, C.E.J.; Huijbers, I.J.; Zwart, W.; Jonkers, J. TRPS1 acts as a context-dependent regulator of mammary epithelial cell growth/differentiation and breast cancer development. Genes Dev. 2020, 34, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Christgen, M.; Derksen, P. Lobular breast cancer: Molecular basis, mouse and cellular models. Breast Cancer Res. 2015, 17, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoda, S.A. Invasive lobular carcinoma. In Rosen’s Diagnosis of Breast Pathology by Needle Core Biopsy, 4th ed.; Hoda, S.A., Brogi, E., Koerner, F., Rosen, P.P., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2017; pp. 349–363. [Google Scholar]
- Paredes, J.; Correia, A.L.; Ribeiro, A.S.; Albergaria, A.; Milanezi, F.; Schmitt, F.C. P-cadherin expression in breast cancer: A review. Breast Cancer Res. 2007, 9, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nollet, F.; Kools, P.; van Roy, F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J. Mol. Biol. 2000, 299, 551–572. [Google Scholar] [CrossRef] [PubMed]
- Gallin, W.J. Evolution of the “classical” cadherin family of cell adhesion molecules in vertebrates. Mol. Biol. Evol. 1998, 15, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Acloque, H.; Ocana, O.H.; Abad, D.; Stern, C.D.; Nieto, M.A. Snail2 and Zeb2 repress P-cadherin to define embryonic territories in the chick embryo. Development 2017, 144, 649–656. [Google Scholar]
- Wheelock, M.J.; Shintani, Y.; Maeda, M.; Fukumoto, Y.; Johnson, K.R. Cadherin switching. J. Cell Sci. 2008, 121, 727–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacios, J.; Benito, N.; Pizarro, A.; Suarez, A.; Espada, J.; Cano, A.; Gamallo, C. Anomalous expression of P-cadherin in breast carcinoma. Correlation with E-cadherin expression and pathological features. Am. J. Pathol. 1995, 146, 605–612. [Google Scholar]
- Kovacs, A.; Dhillon, J.; Walker, R.A. Expression of P-cadherin, but not E-cadherin or N-cadherin, relates to pathological and functional differentiation of breast carcinomas. Mol. Pathol. 2003, 56, 318–322. [Google Scholar] [CrossRef] [Green Version]
- Bryan, R.T.; Atherfold, P.A.; Yeo, Y.; Jones, L.J.; Harrison, R.F.; Wallace, D.M.; Jankowski, J.A. Cadherin switching dictates the biology of transitional cell carcinoma of the bladder: Ex vivo and in vitro studies. J. Pathol. 2008, 215, 184–194. [Google Scholar] [CrossRef]
- Desmedt, C.; Zoppoli, G.; Sotiriou, C.; Salgado, R. Transcriptomic and genomic features of invasive lobular breast cancer. Semin. Cancer Biol. 2017, 44, 98–105. [Google Scholar] [CrossRef] [PubMed]
- McCart Reed, A.E.; Kutasovic, J.R.; Lakhani, S.R.; Simpson, P.T. Invasive lobular carcinoma of the breast: Morphology, biomarkers and ’omics. Breast Cancer Res. 2015, 17, 12. [Google Scholar] [CrossRef]
- Westhoff, C.C.; Jank, P.; Jacke, C.O.; Albert, U.S.; Ebrahimsade, S.; Barth, P.J.; Moll, R. Prognostic relevance of the loss of stromal CD34 positive fibroblasts in invasive lobular carcinoma of the breast. Virchows Arch. 2020, 477, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Koukoulis, G.K.; Virtanen, I.; Korhonen, M.; Laitinen, L.; Quaranta, V.; Gould, V.E. Immunohistochemical localization of integrins in the normal, hyperplastic, and neoplastic breast. Correlations with their functions as receptors and cell adhesion molecules. Am. J. Pathol. 1991, 139, 787–799. [Google Scholar] [PubMed]
- Clezardin, P.; Frappart, L.; Clerget, M.; Pechoux, C.; Delmas, P.D. Expression of thrombospondin (TSP1) and its receptors (CD36 and CD51) in normal, hyperplastic, and neoplastic human breast. Cancer Res. 1993, 53, 1421–1430. [Google Scholar] [PubMed]
- Serre, C.M.; Clezardin, P.; Frappart, L.; Boivin, G.; Delmas, P.D. Distribution of thrombospondin and integrin alpha V in DCIS, invasive ductal and lobular human breast carcinomas. Analysis by electron microscopy. Virchows Arch. 1995, 427, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Taddei, I.; Faraldo, M.M.; Teuliere, J.; Deugnier, M.A.; Thiery, J.P.; Glukhova, M.A. Integrins in mammary gland development and differentiation of mammary epithelium. J. Mammary Gland Biol. Neoplasia 2003, 8, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Taraboletti, G.; Roberts, D.D.; Liotta, L.A. Thrombospondin-induced tumor cell migration: Haptotaxis and chemotaxis are mediated by different molecular domains. J. Cell Biol. 1987, 105, 2409–2415. [Google Scholar] [CrossRef]
- Sflomos, G.; Battista, L.; Aouad, P.; De Martino, F.; Scabia, V.; Stravodimou, A.; Ayyanan, A.; Ifticene-Treboux, A.; Bucher, P.; Fiche, M.; et al. Intraductal xenografts show lobular carcinoma cells rely on their own extracellular matrix and LOXL1. EMBO Mol. Med. 2021, 13, e13180. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.; Jonkers, J.; et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef]
- Droeser, R.; Zlobec, I.; Kilic, E.; Guth, U.; Heberer, M.; Spagnoli, G.; Oertli, D.; Tapia, C. Differential pattern and prognostic significance of CD4+, FOXP3+ and IL-17+ tumor infiltrating lymphocytes in ductal and lobular breast cancers. BMC Cancer 2012, 12, 134. [Google Scholar] [CrossRef] [Green Version]
- De Miguel, M.; Calvo, E. Clinical Challenges of Immune Checkpoint Inhibitors. Cancer Cell 2020, 38, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479.e410. [Google Scholar] [CrossRef] [Green Version]
- Pelon, F.; Bourachot, B.; Kieffer, Y.; Magagna, I.; Mermet-Meillon, F.; Bonnet, I.; Costa, A.; Givel, A.M.; Attieh, Y.; Barbazan, J.; et al. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat. Commun. 2020, 11, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimsade, S.; Westhoff, C.C.; Barth, P.J. CD34+ fibrocytes are preserved in most invasive lobular carcinomas of the breast. Pathol. Res. Pract. 2007, 203, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Park, C.K.; Jung, W.H.; Koo, J.S. Expression of cancer-associated fibroblast-related proteins differs between invasive lobular carcinoma and invasive ductal carcinoma. Breast Cancer Res. Treat. 2016, 159, 55–69. [Google Scholar] [CrossRef] [PubMed]
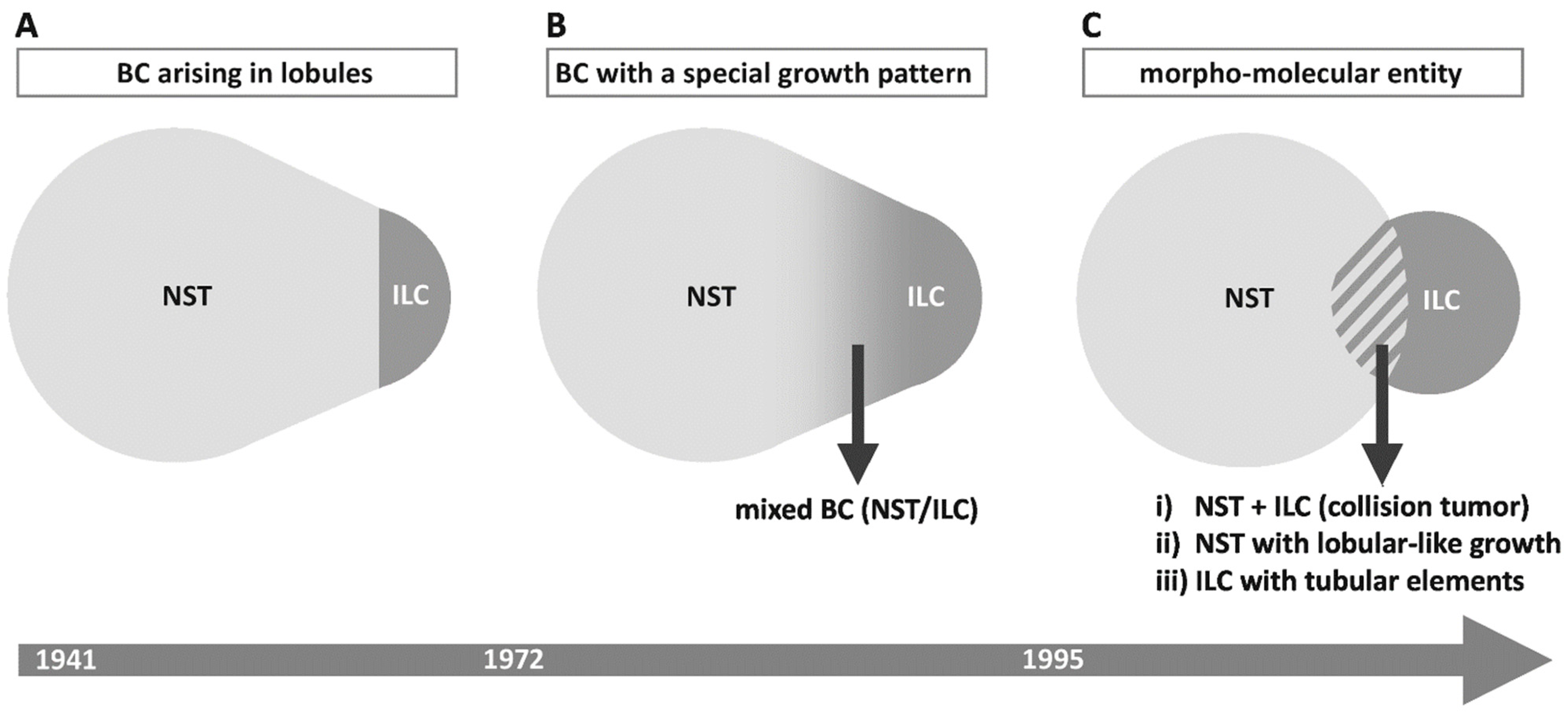

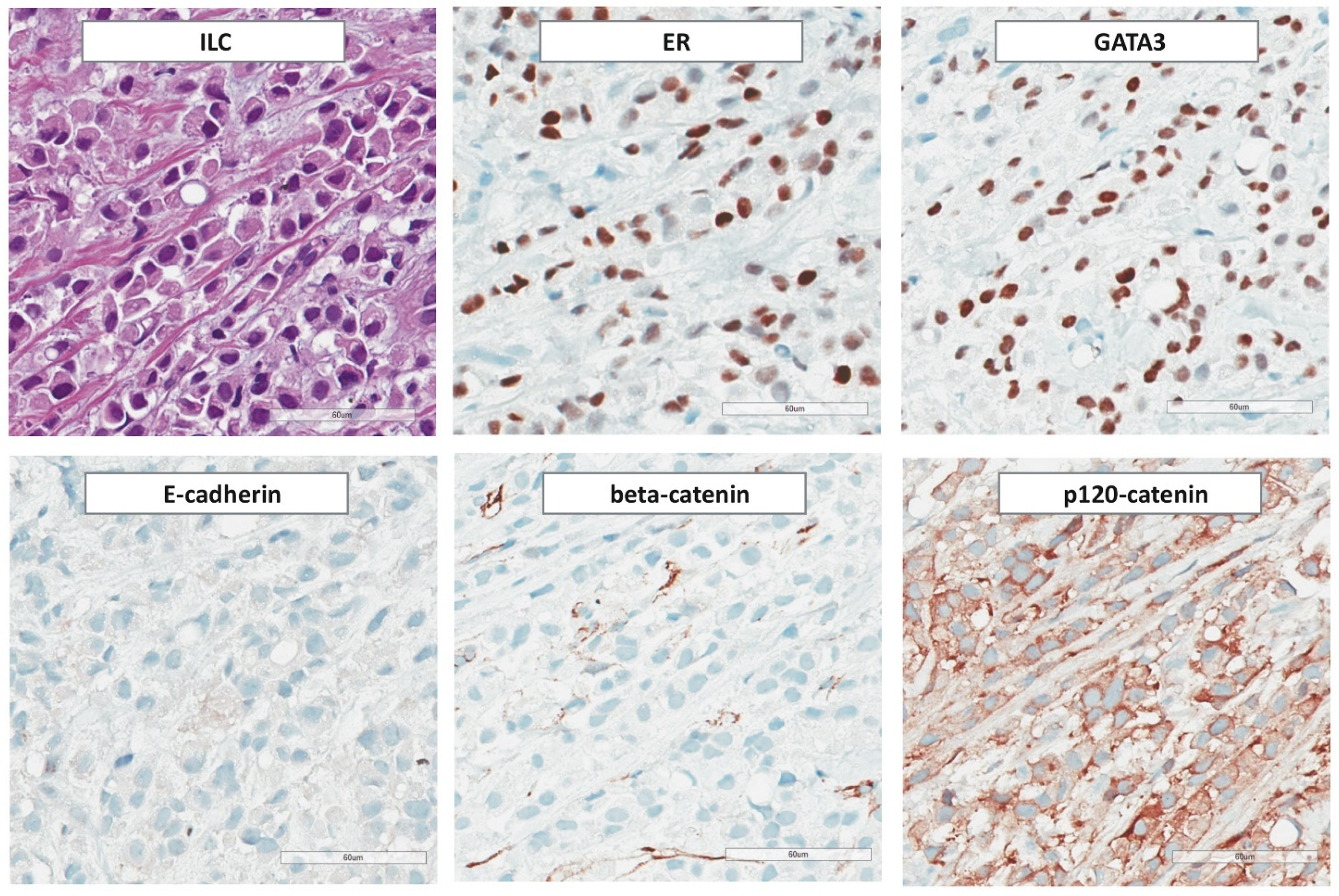

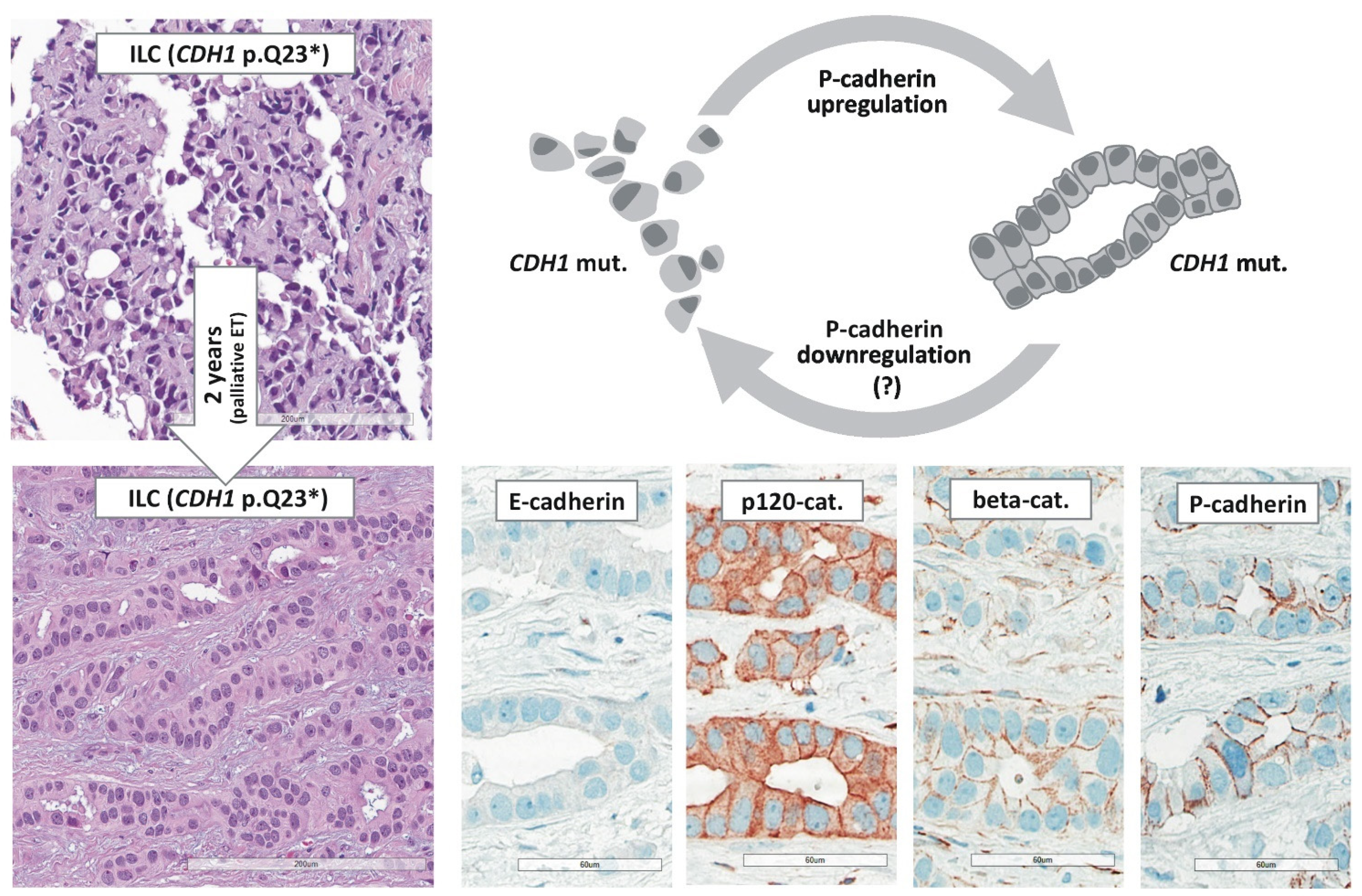
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christgen, M.; Cserni, G.; Floris, G.; Marchio, C.; Djerroudi, L.; Kreipe, H.; Derksen, P.W.B.; Vincent-Salomon, A. Lobular Breast Cancer: Histomorphology and Different Concepts of a Special Spectrum of Tumors. Cancers 2021, 13, 3695. https://doi.org/10.3390/cancers13153695
Christgen M, Cserni G, Floris G, Marchio C, Djerroudi L, Kreipe H, Derksen PWB, Vincent-Salomon A. Lobular Breast Cancer: Histomorphology and Different Concepts of a Special Spectrum of Tumors. Cancers. 2021; 13(15):3695. https://doi.org/10.3390/cancers13153695
Chicago/Turabian StyleChristgen, Matthias, Gábor Cserni, Giuseppe Floris, Caterina Marchio, Lounes Djerroudi, Hans Kreipe, Patrick W. B. Derksen, and Anne Vincent-Salomon. 2021. "Lobular Breast Cancer: Histomorphology and Different Concepts of a Special Spectrum of Tumors" Cancers 13, no. 15: 3695. https://doi.org/10.3390/cancers13153695
APA StyleChristgen, M., Cserni, G., Floris, G., Marchio, C., Djerroudi, L., Kreipe, H., Derksen, P. W. B., & Vincent-Salomon, A. (2021). Lobular Breast Cancer: Histomorphology and Different Concepts of a Special Spectrum of Tumors. Cancers, 13(15), 3695. https://doi.org/10.3390/cancers13153695







