Mouse Models in Meningioma Research: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Articles Selection
3.2. Meningioma Cell Lines
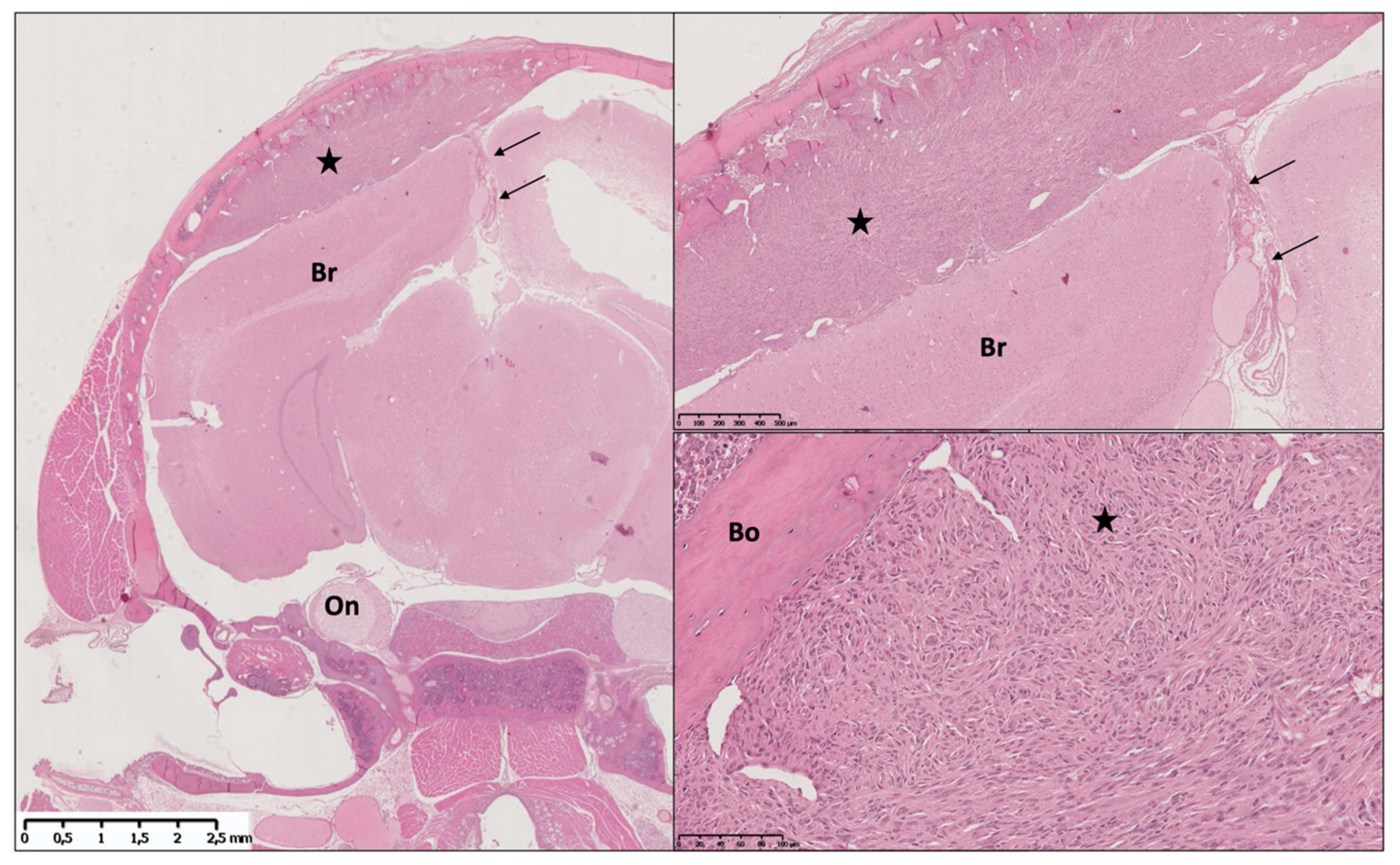
3.3. Mouse Xenograft Models
3.3.1. Heterotopic Models
3.3.2. Orthotopic Models
Injection Technique
Tumor Take and Meningioma Phenotype Results
Tumor Growth Monitoring and Mouse Imaging
Intraoperative Fluorescent Tumor Visualization
Limits of Xenografts Models
3.4. Genetically Engineered Mouse Models (GEMM)
3.4.1. Cre-loxP System
3.4.2. RCAS-TVA System
3.4.3. Limits of GEMMs
3.5. Future Directions
3.5.1. Next-Generation Mouse Modeling of Cancer with CRISPR/Cas9 Technology
3.5.2. Extending GEMMs Models to Study Other Mutational Events and New Meningeal Promotors
3.5.3. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22, iv1–iv96. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, T.J.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, D.C.; Wen, P.Y.; Vogelbaum, M.A. Meningiomas: Knowledge Base, Treatment Outcomes, and Uncertainties. A RANO Review. J. Neurosurg. 2015, 122, 4–23. [Google Scholar] [CrossRef] [Green Version]
- Kaley, T.; Barani, I.; Chamberlain, M.; McDermott, M.; Panageas, K.; Raizer, J.; Rogers, L.; Schiff, D.; Vogelbaum, M.; Weber, D.; et al. Historical Benchmarks for Medical Therapy Trials in Surgery- and Radiation-Refractory Meningioma: A RANO Review. Neuro Oncol. 2014, 16, 829–840. [Google Scholar] [CrossRef] [Green Version]
- Youngblood, M.W.; Duran, D.; Montejo, J.D.; Li, C.; Omay, S.B.; Özduman, K.; Sheth, A.H.; Zhao, A.Y.; Tyrtova, E.; Miyagishima, D.F.; et al. Correlations between Genomic Subgroup and Clinical Features in a Cohort of More than 3000 Meningiomas. J. Neurosurg. 2019, 133, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Honda, T.; Yamada, H.; Wake, N.; Barrett, J.C.; Oshimura, M. Evidence for Multiple Pathways to Cellular Senescence. Cancer Res. 1994, 54, 6090–6093. [Google Scholar] [PubMed]
- Simon, M.; Park, T.W.; Leuenroth, S.; Hans, V.H.; Löning, T.; Schramm, J. Telomerase Activity and Expression of the Telomerase Catalytic Subunit, HTERT, in Meningioma Progression. J. Neurosurg. 2000, 92, 832–840. [Google Scholar] [CrossRef]
- Maes, L.; Lippens, E.; Kalala, J.P.O.; de Ridder, L. The HTERT-Protein and Ki-67 Labelling Index in Recurrent and Non-Recurrent Meningiomas. Cell Prolif. 2005, 38, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Püttmann, S.; Senner, V.; Braune, S.; Hillmann, B.; Exeler, R.; Rickert, C.H.; Paulus, W. Establishment of a Benign Meningioma Cell Line by HTERT-Mediated Immortalization. Lab. Invest. 2005, 85, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Baia, G.S.; Slocum, A.L.; Hyer, J.D.; Misra, A.; Sehati, N.; VandenBerg, S.R.; Feuerstein, B.G.; Deen, D.F.; McDermott, M.W.; Lal, A. A Genetic Strategy to Overcome the Senescence of Primary Meningioma Cell Cultures. J. Neurooncol. 2006, 78, 113–121. [Google Scholar] [CrossRef]
- Cargioli, T.G.; Ugur, H.C.; Ramakrishna, N.; Chan, J.; Black, P.M.; Carroll, R.S. Establishment of an in Vivo Meningioma Model with Human Telomerase Reverse Transcriptase. Neurosurgery 2007, 60, 750–759; discussion 759–760. [Google Scholar] [CrossRef]
- Mei, Y.; Bi, W.L.; Greenwald, N.F.; Agar, N.Y.; Beroukhim, R.; Dunn, G.P.; Dunn, I.F. Genomic Profile of Human Meningioma Cell Lines. PLoS ONE 2017, 12, e0178322. [Google Scholar] [CrossRef]
- Striedinger, K.; VandenBerg, S.R.; Baia, G.S.; McDermott, M.W.; Gutmann, D.H.; Lal, A. The Neurofibromatosis 2 Tumor Suppressor Gene Product, Merlin, Regulates Human Meningioma Cell Growth by Signaling through YAP. Neoplasia 2008, 10, 1204–1212. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.H. Characterization of a Newly Established Malignant Meningioma Cell Line of the Human Brain: IOMM-Lee. Neurosurgery 1990, 27, 389–395; discussion 396. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.C.; Goldman, C.K.; Gillespie, G.Y. Vascular Endothelial Growth Factor in Human Glioma Cell Lines: Induced Secretion by EGF, PDGF-BB, and BFGF. J. Neurosurg. 1995, 82, 864–873. [Google Scholar] [CrossRef]
- Tanaka, K.; Sato, C.; Maeda, Y.; Koike, M.; Matsutani, M.; Yamada, K.; Miyaki, M. Establishment of a Human Malignant Meningioma Cell Line with Amplified C-Myc Oncogene. Cancer 1989, 64, 2243–2249. [Google Scholar] [CrossRef]
- Ragel, B.T.; Elam, I.L.; Gillespie, D.L.; Flynn, J.R.; Kelly, D.A.; Mabey, D.; Feng, H.; Couldwell, W.T.; Jensen, R.L. A Novel Model of Intracranial Meningioma in Mice Using Luciferase-Expressing Meningioma Cells. Laboratory Investigation. J. Neurosurg. 2008, 108, 304–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldt, N.; Kesseler, C.; Fala, P.; John, P.; Kirches, E.; Angenstein, F.; Mawrin, C. Crispr/Cas-Based Modeling of NF2 Loss in Meningioma Cells. J. Neurosci. Methods 2021, 356, 109141. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.W.; Pinkerton, H.; Thornton, H.; Nagy, D. Heterotransplantation of Human Glioblastoma Multiforme and Meningioma to Nude Mice. Proc. Soc. Exp. Biol. Med. 1977, 155, 85–88. [Google Scholar] [CrossRef]
- Ragel, B.T.; Couldwell, W.T.; Gillespie, D.L.; Wendland, M.M.; Whang, K.; Jensen, R.L. A Comparison of the Cell Lines Used in Meningioma Research. Surg. Neurol. 2008, 70, 295–307; discussion 307. [Google Scholar] [CrossRef]
- Jensen, R.L.; Leppla, D.; Rokosz, N.; Wurster, R.D. Matrigel Augments Xenograft Transplantation of Meningioma Cells into Athymic Mice. Neurosurgery 1998, 42, 130–135; discussion 135–136. [Google Scholar] [CrossRef]
- Jensen, R.L.; Wurster, R.D. Calcium Channel Antagonists Inhibit Growth of Subcutaneous Xenograft Meningiomas in Nude Mice. Surg. Neurol. 2001, 55, 275–283. [Google Scholar] [CrossRef]
- McCutcheon, I.E.; Flyvbjerg, A.; Hill, H.; Li, J.; Bennett, W.F.; Scarlett, J.A.; Friend, K.E. Antitumor Activity of the Growth Hormone Receptor Antagonist Pegvisomant against Human Meningiomas in Nude Mice. J. Neurosurg. 2001, 94, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Olson, J.J.; Beck, D.W.; Schlechte, J.A.; Loh, P.M. Effect of the Antiprogesterone RU-38486 on Meningioma Implanted into Nude Mice. J. Neurosurg. 1987, 66, 584–587. [Google Scholar] [CrossRef]
- Gupta, V.; Su, Y.S.; Samuelson, C.G.; Liebes, L.F.; Chamberlain, M.C.; Hofman, F.M.; Schönthal, A.H.; Chen, T.C. Irinotecan: A Potential New Chemotherapeutic Agent for Atypical or Malignant Meningiomas. J. Neurosurg. 2007, 106, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Salhia, B.; Rutka, J.T.; Lingwood, C.; Nutikka, A.; Van Furth, W.R. The Treatment of Malignant Meningioma with Verotoxin. Neoplasia 2002, 4, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, H.; Wang, H. Experimental Study on the Inhibitory Effects of Verapamil on the Proliferation of Meningiomas Cells. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2007, 27, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Hua, L.; Han, T.; Tian, M.; Wang, D.; Tang, H.; Sun, S.; Chen, H.; Cheng, H.; Zhang, T.; et al. The CREB-Binding Protein Inhibitor ICG-001: A Promising Therapeutic Strategy in Sporadic Meningioma with NF2 Mutations. Neurooncol. Adv. 2020, 2, vdz055. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Samuleson, C.G.; Su, S.; Chen, T.C. Nelfinavir Potentiation of Imatinib Cytotoxicity in Meningioma Cells via Survivin Inhibition. Neurosurg. Focus 2007, 23, E9. [Google Scholar] [CrossRef] [PubMed]
- Haase, D.; Schmidl, S.; Ewald, C.; Kalff, R.; Huebner, C.; Firsching, R.; Keilhoff, G.; Evert, M.; Paulus, W.; Gutmann, D.H.; et al. Fatty Acid Synthase as a Novel Target for Meningioma Therapy. Neuro Oncol. 2010, 12, 844–854. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Song, T.; Li, J.; Ao, F.; Gong, X.; Lu, Y.; Zhang, C.; Chen, L.; Liu, Y.; He, H.; et al. RAS Promotes Proliferation and Resistances to Apoptosis in Meningioma. Mol. Neurobiol. 2017, 54, 779–787. [Google Scholar] [CrossRef]
- Kim, H.; Park, K.-J.; Ryu, B.-K.; Park, D.-H.; Kong, D.-S.; Chong, K.; Chae, Y.-S.; Chung, Y.-G.; Park, S.I.; Kang, S.-H. Forkhead Box M1 (FOXM1) Transcription Factor Is a Key Oncogenic Driver of Aggressive Human Meningioma Progression. Neuropathol. Appl. Neurobiol. 2020, 46, 125–141. [Google Scholar] [CrossRef]
- Matsuda, Y.; Kawamoto, K.; Kiya, K.; Kurisu, K.; Sugiyama, K.; Uozumi, T. Antitumor Effects of Antiprogesterones on Human Meningioma Cells in Vitro and in Vivo. J. Neurosurg. 1994, 80, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Ragel, B.T.; Jensen, R.L.; Gillespie, D.L.; Prescott, S.M.; Couldwell, W.T. Celecoxib Inhibits Meningioma Tumor Growth in a Mouse Xenograft Model. Cancer 2007, 109, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Nigim, F.; Esaki, S.-I.; Hood, M.; Lelic, N.; James, M.F.; Ramesh, V.; Stemmer-Rachamimov, A.; Cahill, D.P.; Brastianos, P.K.; Rabkin, S.D.; et al. A New Patient-Derived Orthotopic Malignant Meningioma Model Treated with Oncolytic Herpes Simplex Virus. Neuro Oncol. 2016, 18, 1278–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baia, G.S.; Dinca, E.B.; Ozawa, T.; Kimura, E.T.; McDermott, M.W.; James, C.D.; VandenBerg, S.R.; Lal, A. An Orthotopic Skull Base Model of Malignant Meningioma. Brain Pathol. 2008, 18, 172–179. [Google Scholar] [CrossRef]
- Iwami, K.; Natsume, A.; Ohno, M.; Ikeda, H.; Mineno, J.; Nukaya, I.; Okamoto, S.; Fujiwara, H.; Yasukawa, M.; Shiku, H.; et al. Adoptive Transfer of Genetically Modified Wilms’ Tumor 1–Specific T Cells in a Novel Malignant Skull Base Meningioma Model. Neuro Oncol. 2013, 15, 747–758. [Google Scholar] [CrossRef] [Green Version]
- McCutcheon, I.E.; Friend, K.E.; Gerdes, T.M.; Zhang, B.M.; Wildrick, D.M.; Fuller, G.N. Intracranial Injection of Human Meningioma Cells in Athymic Mice: An Orthotopic Model for Meningioma Growth. J. Neurosurg. 2000, 92, 306–314. [Google Scholar] [CrossRef]
- Kondraganti, S.; Gondi, C.S.; McCutcheon, I.; Dinh, D.H.; Gujrati, M.; Rao, J.S.; Olivero, W.C. RNAi-Mediated Downregulation of Urokinase Plasminogen Activator and Its Receptor in Human Meningioma Cells Inhibits Tumor Invasion and Growth. Int. J. Oncol. 2006, 28, 1353–1360. [Google Scholar] [CrossRef] [Green Version]
- Ho, W.S.; Sizdahkhani, S.; Hao, S.; Song, H.; Seldomridge, A.; Tandle, A.; Maric, D.; Kramp, T.; Lu, R.; Heiss, J.D.; et al. LB-100, a Novel Protein Phosphatase 2A (PP2A) Inhibitor, Sensitizes Malignant Meningioma Cells to the Therapeutic Effects of Radiation. Cancer Lett. 2018, 415, 217–226. [Google Scholar] [CrossRef]
- Petermann, A.; Haase, D.; Wetzel, A.; Balavenkatraman, K.K.; Tenev, T.; Gührs, K.-H.; Friedrich, S.; Nakamura, M.; Mawrin, C.; Böhmer, F.-D. Loss of the Protein-Tyrosine Phosphatase DEP-1/PTPRJ Drives Meningioma Cell Motility. Brain Pathol. 2011, 21, 405–418. [Google Scholar] [CrossRef]
- Friedrich, S.; Schwabe, K.; Grote, M.; Krauss, J.K.; Nakamura, M. Effect of Systemic Celecoxib on Human Meningioma after Intracranial Transplantation into Nude Mice. Acta Neurochir. 2013, 155, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, S.; Schwabe, K.; Klein, R.; Krusche, C.A.; Krauss, J.K.; Nakamura, M. Comparative Morphological and Immunohistochemical Study of Human Meningioma after Intracranial Transplantation into Nude Mice. J. Neurosci. Methods 2012, 205, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wilisch-Neumann, A.; Kliese, N.; Pachow, D.; Schneider, T.; Warnke, J.-P.; Braunsdorf, W.E.; Böhmer, F.-D.; Hass, P.; Pasemann, D.; Helbing, C.; et al. The Integrin Inhibitor Cilengitide Affects Meningioma Cell Motility and Invasion. Clin. Cancer Res. 2013, 19, 5402–5412. [Google Scholar] [CrossRef] [Green Version]
- Tuchen, M.; Wilisch-Neumann, A.; Daniel, E.A.; Baldauf, L.; Pachow, D.; Scholz, J.; Angenstein, F.; Stork, O.; Kirches, E.; Mawrin, C. Receptor Tyrosine Kinase Inhibition by Regorafenib/Sorafenib Inhibits Growth and Invasion of Meningioma Cells. Eur. J. Cancer 2017, 73, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Pachow, D.; Andrae, N.; Kliese, N.; Angenstein, F.; Stork, O.; Wilisch-Neumann, A.; Kirches, E.; Mawrin, C. MTORC1 Inhibitors Suppress Meningioma Growth in Mouse Models. Clin. Cancer Res. 2013, 19, 1180–1189. [Google Scholar] [CrossRef] [Green Version]
- Burns, S.S.; Akhmametyeva, E.M.; Oblinger, J.L.; Bush, M.L.; Huang, J.; Senner, V.; Chen, C.-S.; Jacob, A.; Welling, D.B.; Chang, L.-S. Histone Deacetylase Inhibitor AR-42 Differentially Affects Cell-Cycle Transit in Meningeal and Meningioma Cells, Potently Inhibiting NF2-Deficient Meningioma Growth. Cancer Res. 2013, 73, 792–803. [Google Scholar] [CrossRef] [Green Version]
- Chow, H.-Y.; Dong, B.; Duron, S.G.; Campbell, D.A.; Ong, C.C.; Hoeflich, K.P.; Chang, L.-S.; Welling, D.B.; Yang, Z.-J.; Chernoff, J. Group I Paks as Therapeutic Targets in NF2-Deficient Meningioma. Oncotarget 2015, 6, 1981–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelhaugh, S.K.; Guastella, A.R.; Varadarajan, K.; Klinger, N.V.; Parajuli, P.; Ahmad, A.; Sethi, S.; Aboukameel, A.; Kiousis, S.; Zitron, I.M.; et al. Development of Patient-Derived Xenograft Models from a Spontaneously Immortal Low-Grade Meningioma Cell Line, KCI-MENG1. J. Transl. Med. 2015, 13, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigim, F.; Kiyokawa, J.; Gurtner, A.; Kawamura, Y.; Hua, L.; Kasper, E.M.; Brastianos, P.K.; Cahill, D.P.; Rabkin, S.D.; Martuza, R.L.; et al. A Monoclonal Antibody Against Β1 Integrin Inhibits Proliferation and Increases Survival in an Orthotopic Model of High-Grade Meningioma. Target. Oncol. 2019, 14, 479–489. [Google Scholar] [CrossRef]
- Karsy, M.; Hoang, N.; Barth, T.; Burt, L.; Dunson, W.; Gillespie, D.L.; Jensen, R.L. Combined Hydroxyurea and Verapamil in the Clinical Treatment of Refractory Meningioma: Human and Orthotopic Xenograft Studies. World Neurosurg. 2016, 86, 210–219. [Google Scholar] [CrossRef]
- La Cava, F.; Fringuello Mingo, A.; Irrera, P.; Di Vito, A.; Cordaro, A.; Brioschi, C.; Colombo Serra, S.; Cabella, C.; Terreno, E.; Miragoli, L. Orthotopic Induction of CH157MN Convexity and Skull Base Meningiomas into Nude Mice Using Stereotactic Surgery and MRI Characterization. Animal Model. Exp. Med. 2019, 2, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Giles, A.J.; Hao, S.; Padget, M.; Song, H.; Zhang, W.; Lynes, J.; Sanchez, V.; Liu, Y.; Jung, J.; Cao, X.; et al. Efficient ADCC Killing of Meningioma by Avelumab and a High-Affinity Natural Killer Cell Line, HaNK. JCI Insight 2019, 4, e130688. [Google Scholar] [CrossRef] [Green Version]
- Skibinski, C.G.; Williamson, T.; Riggins, G.J. Mebendazole and Radiation in Combination Increase Survival through Anticancer Mechanisms in an Intracranial Rodent Model of Malignant Meningioma. J. Neurooncol. 2018, 140, 529–538. [Google Scholar] [CrossRef]
- Das, A.; Alshareef, M.; Henderson, F.; Martinez Santos, J.L.; Vandergrift, W.A.; Lindhorst, S.M.; Varma, A.K.; Infinger, L.; Patel, S.J.; Cachia, D. Ganoderic Acid A/DM-Induced NDRG2 over-Expression Suppresses High-Grade Meningioma Growth. Clin. Transl. Oncol. 2020, 22, 1138–1145. [Google Scholar] [CrossRef]
- Das, A.; Alshareef, M.; Martinez Santos, J.L.; Porto, G.B.F.; McDonald, D.G.; Infinger, L.K.; Vandergrift, W.A.; Lindhorst, S.M.; Varma, A.K.; Patel, S.J.; et al. Evaluating Anti-Tumor Activity of Palbociclib plus Radiation in Anaplastic and Radiation-Induced Meningiomas: Pre-Clinical Investigations. Clin. Transl. Oncol. 2020, 22, 2017–2025. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, L.; Du, Y.; Huang, L.F.; Braun, F.K.; Kogiso, M.; Zhao, Y.; Li, C.; Lindsay, H.; Zhao, S.; et al. Patient-Derived Orthotopic Xenograft (PDOX) Mouse Models of Primary and Recurrent Meningioma. Cancers 2020, 12, 1478. [Google Scholar] [CrossRef] [PubMed]
- Angus, S.P.; Oblinger, J.L.; Stuhlmiller, T.J.; DeSouza, P.A.; Beauchamp, R.L.; Witt, L.; Chen, X.; Jordan, J.T.; Gilbert, T.S.K.; Stemmer-Rachamimov, A.; et al. EPH Receptor Signaling as a Novel Therapeutic Target in NF2-Deficient Meningioma. Neuro Oncol. 2018, 20, 1185–1196. [Google Scholar] [CrossRef] [Green Version]
- Peyre, M.; Stemmer-Rachamimov, A.; Clermont-Taranchon, E.; Quentin, S.; El-Taraya, N.; Walczak, C.; Volk, A.; Niwa-Kawakita, M.; Karboul, N.; Giovannini, M.; et al. Meningioma Progression in Mice Triggered by Nf2 and Cdkn2ab Inactivation. Oncogene 2013, 32, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Van Furth, W.R.; Laughlin, S.; Taylor, M.D.; Salhia, B.; Mainprize, T.; Henkelman, M.; Cusimano, M.D.; Ackerley, C.; Rutka, J.T. Imaging of Murine Brain Tumors Using a 1.5 Tesla Clinical MRI System. Can. J. Neurol. Sci. 2003, 30, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cal-Gonzalez, J.; Vaquero, J.J.; Herraiz, J.L.; Pérez-Liva, M.; Soto-Montenegro, M.L.; Peña-Zalbidea, S.; Desco, M.; Udías, J.M. Improving PET Quantification of Small Animal [68Ga]DOTA-Labeled PET/CT Studies by Using a CT-Based Positron Range Correction. Mol. Imaging Biol. 2018, 20, 584–593. [Google Scholar] [CrossRef] [Green Version]
- Soto-Montenegro, M.L.; Peña-Zalbidea, S.; Mateos-Pérez, J.M.; Oteo, M.; Romero, E.; Morcillo, M.Á.; Desco, M. Meningiomas: A Comparative Study of 68Ga-DOTATOC, 68Ga-DOTANOC and 68Ga-DOTATATE for Molecular Imaging in Mice. PLoS ONE 2014, 9, e111624. [Google Scholar] [CrossRef] [Green Version]
- Linsler, S.; Ketter, R.; Oertel, J.; Urbschat, S. Fluorescence Imaging of Meningioma Cells with Somatostatin Receptor Ligands: An in Vitro Study. Acta Neurochir. 2019, 161, 1017–1024. [Google Scholar] [CrossRef]
- Linsler, S.; Müller, S.J.; Müller, A.; Senger, S.; Oertel, J.M. Fluorescence Image-Guided Resection of Intracranial Meningioma: An Experimental in Vivo Study on Nude Mice. Ann. Anat. 2021, 237, 151752. [Google Scholar] [CrossRef]
- Castle, K.D.; Chen, M.; Wisdom, A.J.; Kirsch, D.G. Genetically Engineered Mouse Models for Studying Radiation Biology. Transl. Cancer Res. 2017, 6, S900–S913. [Google Scholar] [CrossRef] [PubMed]
- Kalamarides, M.; Niwa-Kawakita, M.; Leblois, H.; Abramowski, V.; Perricaudet, M.; Janin, A.; Thomas, G.; Gutmann, D.H.; Giovannini, M. Nf2 Gene Inactivation in Arachnoidal Cells Is Rate-Limiting for Meningioma Development in the Mouse. Genes Dev. 2002, 16, 1060–1065. [Google Scholar] [CrossRef] [Green Version]
- Kalamarides, M.; Stemmer-Rachamimov, A.O.; Takahashi, M.; Han, Z.-Y.; Chareyre, F.; Niwa-Kawakita, M.; Black, P.M.; Carroll, R.S.; Giovannini, M. Natural History of Meningioma Development in Mice Reveals: A Synergy of Nf2 and P16(Ink4a) Mutations. Brain Pathol. 2008, 18, 62–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalamarides, M.; Stemmer-Rachamimov, A.O.; Niwa-Kawakita, M.; Chareyre, F.; Taranchon, E.; Han, Z.-Y.; Martinelli, C.; Lusis, E.A.; Hegedus, B.; Gutmann, D.H.; et al. Identification of a Progenitor Cell of Origin Capable of Generating Diverse Meningioma Histological Subtypes. Oncogene 2011, 30, 2333–2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyre, M.; Salaud, C.; Clermont-Taranchon, E.; Niwa-Kawakita, M.; Goutagny, S.; Mawrin, C.; Giovannini, M.; Kalamarides, M. PDGF Activation in PGDS-Positive Arachnoid Cells Induces Meningioma Formation in Mice Promoting Tumor Progression in Combination with Nf2 and Cdkn2ab Loss. Oncotarget 2015, 6, 32713–32722. [Google Scholar] [CrossRef]
- Boetto, J.; Apra, C.; Bielle, F.; Peyre, M.; Kalamarides, M. Selective Vulnerability of the Primitive Meningeal Layer to Prenatal Smo Activation for Skull Base Meningothelial Meningioma Formation. Oncogene 2018, 37, 4955–4963. [Google Scholar] [CrossRef]
- McClatchey, A.I.; Saotome, I.; Mercer, K.; Crowley, D.; Gusella, J.F.; Bronson, R.T.; Jacks, T. Mice Heterozygous for a Mutation at the Nf2 Tumor Suppressor Locus Develop a Range of Highly Metastatic Tumors. Genes Dev. 1998, 12, 1121–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boström, J.; Meyer-Puttlitz, B.; Wolter, M.; Blaschke, B.; Weber, R.G.; Lichter, P.; Ichimura, K.; Collins, V.P.; Reifenberger, G. Alterations of the Tumor Suppressor Genes CDKN2A (P16(INK4a)), P14(ARF), CDKN2B (P15(INK4b)), and CDKN2C (P18(INK4c)) in Atypical and Anaplastic Meningiomas. Am. J. Pathol. 2001, 159, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Goutagny, S.; Yang, H.W.; Zucman-Rossi, J.; Chan, J.; Dreyfuss, J.M.; Park, P.J.; Black, P.M.; Giovannini, M.; Carroll, R.S.; Kalamarides, M. Genomic Profiling Reveals Alternative Genetic Pathways of Meningioma Malignant Progression Dependent on the Underlying NF2 Status. Clin. Cancer Res. 2010, 16, 4155–4164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, A.; Banerjee, R.; Lohse, C.M.; Kleinschmidt-DeMasters, B.K.; Scheithauer, B.W. A Role for Chromosome 9p21 Deletions in the Malignant Progression of Meningiomas and the Prognosis of Anaplastic Meningiomas. Brain Pathol. 2002, 12, 183–190. [Google Scholar] [CrossRef]
- Yeung, J.; Yaghoobi, V.; Miyagishima, D.; Vesely, M.D.; Zhang, T.; Badri, T.; Nassar, A.; Han, X.; Sanmamed, M.F.; Youngblood, M.; et al. Targeting the CSF1/CSF1R Axis Is a Potential Treatment Strategy for Malignant Meningiomas. Neuro Oncol. 2021. [Google Scholar] [CrossRef]
- Kawashima, M.; Suzuki, S.O.; Yamashima, T.; Fukui, M.; Iwaki, T. Prostaglandin D Synthase (Beta-Trace) in Meningeal Hemangiopericytoma. Mod. Pathol. 2001, 14, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Urade, Y.; Kitahama, K.; Ohishi, H.; Kaneko, T.; Mizuno, N.; Hayaishi, O. Dominant Expression of MRNA for Prostaglandin D Synthase in Leptomeninges, Choroid Plexus, and Oligodendrocytes of the Adult Rat Brain. Proc. Natl. Acad. Sci. USA 1993, 90, 9070–9074. [Google Scholar] [CrossRef] [Green Version]
- Yamashima, T.; Sakuda, K.; Tohma, Y.; Yamashita, J.; Oda, H.; Irikura, D.; Eguchi, N.; Beuckmann, C.T.; Kanaoka, Y.; Urade, Y.; et al. Prostaglandin D Synthase (Beta-Trace) in Human Arachnoid and Meningioma Cells: Roles as a Cell Marker or in Cerebrospinal Fluid Absorption, Tumorigenesis, and Calcification Process. J. Neurosci. 1997, 17, 2376–2382. [Google Scholar] [CrossRef] [PubMed]
- Von Werder, A.; Seidler, B.; Schmid, R.M.; Schneider, G.; Saur, D. Production of Avian Retroviruses and Tissue-Specific Somatic Retroviral Gene Transfer in Vivo Using the RCAS/TVA System. Nat. Protoc. 2012, 7, 1167–1183. [Google Scholar] [CrossRef]
- Figarella-Branger, D.; Vagner-Capodano, A.M.; Bouillot, P.; Graziani, N.; Gambarelli, D.; Devictor, B.; Zattara-Cannoni, H.; Bianco, N.; Grisoli, F.; Pellissier, J.F. Platelet-Derived Growth Factor (PDGF) and Receptor (PDGFR) Expression in Human Meningiomas: Correlations with Clinicopathological Features and Cytogenetic Analysis. Neuropathol. Appl. Neurobiol. 1994, 20, 439–447. [Google Scholar] [CrossRef]
- Mawrin, C.; Sasse, T.; Kirches, E.; Kropf, S.; Schneider, T.; Grimm, C.; Pambor, C.; Vorwerk, C.K.; Firsching, R.; Lendeckel, U.; et al. Different Activation of Mitogen-Activated Protein Kinase and Akt Signaling Is Associated with Aggressive Phenotype of Human Meningiomas. Clin. Cancer Res. 2005, 11, 4074–4082. [Google Scholar] [CrossRef] [Green Version]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [Green Version]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-Guided Human Genome Engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef] [Green Version]
- Prager, B.C.; Vasudevan, H.N.; Dixit, D.; Bernatchez, J.A.; Wu, Q.; Wallace, L.C.; Bhargava, S.; Lee, D.; King, B.H.; Morton, A.R.; et al. The Meningioma Enhancer Landscape Delineates Novel Subgroups and Drives Druggable Dependencies. Cancer Discov. 2020, 10, 1722–1741. [Google Scholar] [CrossRef] [PubMed]
- DeSisto, J.; O’Rourke, R.; Jones, H.E.; Pawlikowski, B.; Malek, A.D.; Bonney, S.; Guimiot, F.; Jones, K.L.; Siegenthaler, J.A. Single-Cell Transcriptomic Analyses of the Developing Meninges Reveal Meningeal Fibroblast Diversity and Function. Dev. Cell 2020, 54, 43–59.e4. [Google Scholar] [CrossRef] [PubMed]

| Name | Phenotype | Immortalization | Genetics | Year | Ref. |
|---|---|---|---|---|---|
| HBL-52 | Grade I, Meningothelial | - | TRAF7 mutant | 2008 | [12] |
| Me3TSC | Grade I, Meningothelial | hTERT + SV40 | - | 2007 | [11] |
| BenMen1 | Grade I, Meningothelial | hTERT | 22q loss NF2 mutant | 2005 | [9] |
| SF4433 | Grade I, Meningothelial | hTERT + HPV E6/E7 | No 22q loss | 2006 | [10] |
| MENII-1 | Grade II | hTERT + HPV E6/E7 | 22q loss | 2008 | [13] |
| IOMM-Lee | Grade III | - | No 22q loss | 1990 | [14] |
| CH 157 MN | Grade III | - | 22q loss NF2 mutant | 1995 | [15] |
| KT 21 | Grade III | - | 22q loss C-myc | 1989 | [16] |
| Mouse Strain/Age of Injection (Weeks) | Injected Cell Types (WHO Grade)/Numbers/Volume (uL) | Site of Injection | Tumor Take (%) | Treatment | Clinical Results | Year, Reference |
|---|---|---|---|---|---|---|
| Athymic/6 | IOMM-Lee (III); human tumor/106/10 | WM/floor of TF | 85–100 | - | - | 2000, [38] |
| Athymic/6–8 | IOMM-Lee (III)/106/3 | Floor of TF | 100 | Verotoxin | Inhibition of TG | 2002, [26] |
| Athymic/6 | BenMenI (I) | Convexity | 100 | - | - | 2005 [9] |
| Athymic/6–8 | IOMM-Lee (III)/5.105 | Brain | 100 | siRNA | Inhibition of TG | 2006 [39] |
| Athymic/4 | Me3TSC (I)-Me10T (I)/106/5 | Convexity (SDS) | 100 | - | - | 2007, [11] |
| Athymic/3 | CH-157-MN (III); IOMM-Lee (III)/104–106/3 | Floor of TF/SDS convexity | 90 | Lb100 + RT | Increased survival compared to RT alone | 2008, [17] 2018, [40] |
| Athymic/5–6 | IOMM-Lee (III)/5.104/0.5 | Skull base | 100 | Temozolomide | Inhibition of TG Increased survival | 2008, [36] |
| Athymic/5 | KT21-DEP1 loss (III) | Convexity | 100 | - | - | 2010, [41] |
| Athymic/5 | PD grade I/106/10 | Convexity (prefrontal cortex) | 90–100 | Celecoxib | No effect | 2012, [42,43] |
| Athymic/8–10 | IOMM-Lee (III)/2.5 × 105/5 uL | Convexity | 100 | Cliengitide + Radiotherapy Sorafenib Temsirolimus | Inhibition of TG | 2013, [44,45,46] |
| Athymic/6–8 | BenMen1(I)/106/3 | Skull base | 100 | Histone deacetylase inhibitor AR 42 | Affect cell cycle progression Inhibition of TG | 2013, [47] |
| NOD/SCID/gamma null mouse/8 | IOMM-Lee (III)/5.104/3 | Skull Base (Pgi) | 100 | Peripheral blood mononuclear cells | Inhibition of TG | 2013, [37] |
| Athymic/6–8 | BenMenI (I)- KT21-MG1 (III)/106/5 | Skull base | 100 | Group 1 Pak inhibitor | Inhibition of TG | 2014, [48] |
| Athymic/NA | PD grade I/106/10 | Convexity | 100 | - | - | 2015, [49] |
| Athymic/5–6 | Primary malignant meningioma NF2-mutant MN3/tumorosphere 50000/3–5 | Convexity | 100 | Oncolytic HSV OS2966 | Increased survival Increased Survival | 2016, [35] 2019, [50] |
| Athymic/NA | CH-157MN (III) | Convexity | 100 | Hydroxyurea + verapamil | No effect | 2016, [51] |
| Athymic/5–6 | CH-157 MN (III)/5.104/5 uL | Convexity/Skull base | 55–80 | - | - | 2019, [52] |
| Athymic/6–8 | IOMM-Lee (III)/104 | Skull base | 100 | - | - | 2019, [53] |
| Athymic/6 | KT21-MG1/50000/ | Convexity | 100 | Mebendazole + /− Radiotherapy | Inhibition of TG Increased survival | 2019, [54] |
| SCID mice/4–6 | IOMM-Lee (III)/106/3–10 | Skull base | 100 | Ganoderic Acid DM | Inhibition of TG Increased survival | 2019, [55] |
| SCID mice/4–6 | IOMM-Lee (III)- BenMen1 (I) | Skull base | 100 | Palbiciblib + RT | Inhibition of TG Increased survival | 2020, [56] |
| Construction | Genetics | Temporal Window of Activation | Phenotype (Grade) | Meningioma Prevalence | Year, Reference |
|---|---|---|---|---|---|
| AdCre; Nf2flox/flox | Nf2 loss | PN2-PN3 | M/F (I) | 29% (TO) 19% (SD) | 2002, [66] |
| AdCre; Nf2flox/flox; Ink4a*/* | Nf2 loss + homozygous P16Ink4a mutation | PN2-PN3 | M/F/T (I) | 38% (TO) 36 % (SD) | 2008, [67] |
| AdCre; Nf2flox/flox; Ink4ab−/− | Nf2 + CDKN2AB loss | PN2-PN3 | 66% (I) 31% (II) 3% (III) | 72% | 2013, [59] |
| PGDSCre; Nf2flox:flox | Nf2 loss in PGDS + cells | E12.5-PN2 | M (I) F (I) | 38% 38% | 2011, [68] |
| PGDSCre; Nf2flox/flox; p16ink4a/− | Nf2 loss + P16ink4a mutation in PGDS + cells | E12.5-PN2 | M (I) F (I) | 50% 50% | 2011, [68] |
| PGDSCre; Nf2flox/flox; p53flox/flox | Nf2 loss + p53 nullizygosity in PGDS + cells | E12.5-PN2 | F (I) | 43% | 2011, [68] |
| PGDStv-a; PDGF-B | PDGF overexpression in PGDS + cells | E12.5-PN2 | (I) | 27% | 2015, [69] |
| PGDStv-a; PDGF-B; AdCre; Nf2flox/flox | PDGF overexpression + Nf2 loss in PGDS + cells | E12.5-PN7 | Grade I (60%) Grade II (40%) | 52% | 2015, [69] |
| PGDStv-a; PDGF-B; AdCre; Nf2flox/flox; Cdkn2ab−/− | PDGF Overexpression + nf2 loss + Cdkn2ab loss | E12.5-PN7 | Grade I (33%) Grade II (47%) Grade III (20%) | 79% | 2015, [69] |
| PDGSCre; SmoM2 | Activating mutation of Smo in PGDS + cells | E12.5-PN2 | M (I) | 21% | 2017, [70] |
| Phenotype | Description |
|---|---|
| Meningothelial proliferation (early tumor formation) | Microscopic lesions composed of meningothelial cells |
| Grade I meningiomas | No mitotic figures, mild cytologic atypia, no brain invasion |
| Grade II meningiomas | One or two mitoses/HPF, true brain invasion |
| Grade III meningiomas | Marked cellular atypia (giant nuclei, pleomorphic nuclei, nuclei with marked chromatin clearing), brisk mitotic activity (three or more mitoses/HPF) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boetto, J.; Peyre, M.; Kalamarides, M. Mouse Models in Meningioma Research: A Systematic Review. Cancers 2021, 13, 3712. https://doi.org/10.3390/cancers13153712
Boetto J, Peyre M, Kalamarides M. Mouse Models in Meningioma Research: A Systematic Review. Cancers. 2021; 13(15):3712. https://doi.org/10.3390/cancers13153712
Chicago/Turabian StyleBoetto, Julien, Matthieu Peyre, and Michel Kalamarides. 2021. "Mouse Models in Meningioma Research: A Systematic Review" Cancers 13, no. 15: 3712. https://doi.org/10.3390/cancers13153712
APA StyleBoetto, J., Peyre, M., & Kalamarides, M. (2021). Mouse Models in Meningioma Research: A Systematic Review. Cancers, 13(15), 3712. https://doi.org/10.3390/cancers13153712






