From Distress Screening to Uptake: An Italian Multicenter Study of Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
3. Materials and Methods
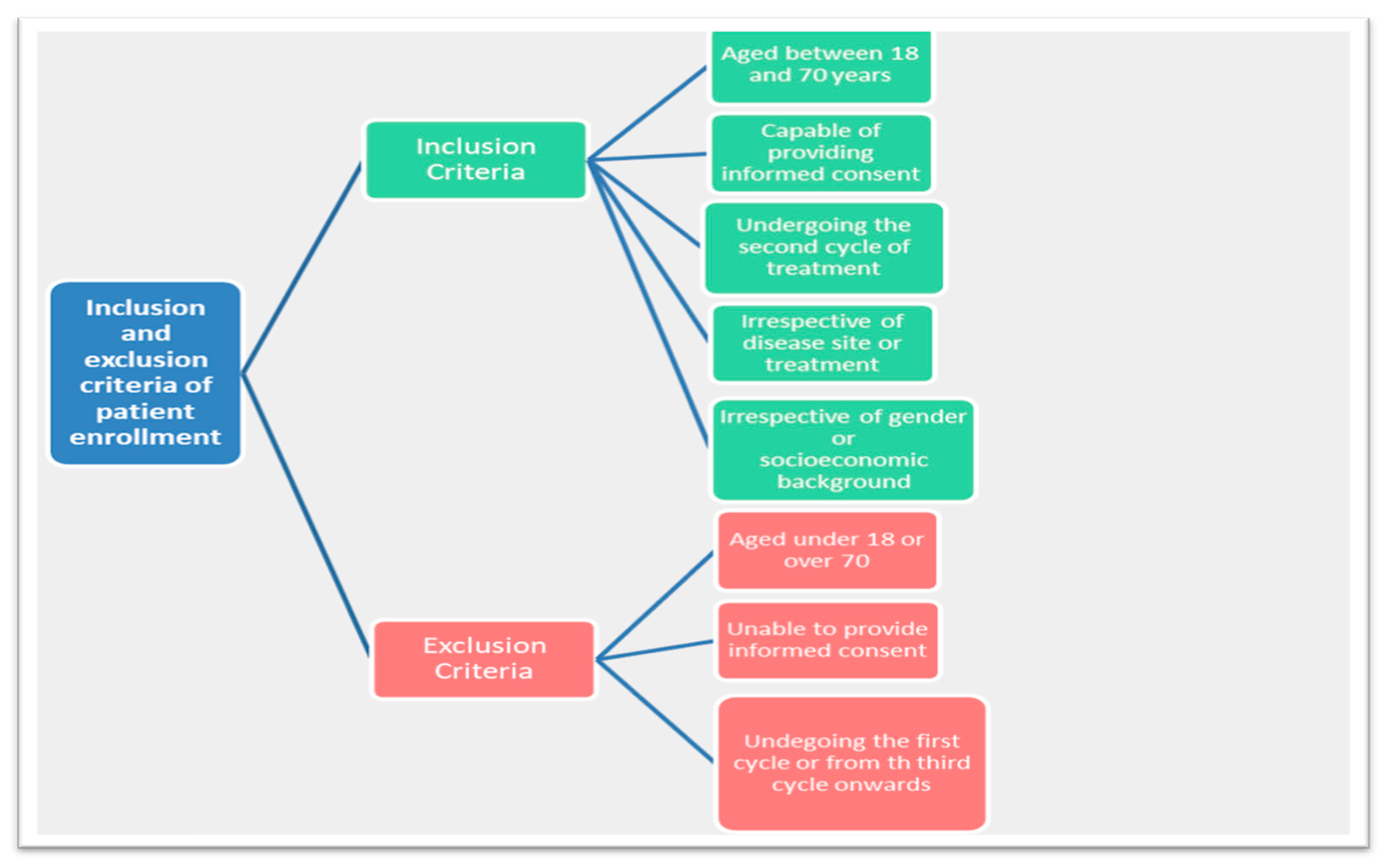
3.1. Design and Participants
3.2. Measurement
3.3. Definition of Measures
3.4. Procedures
3.5. Data Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loscalzo, M.; Clark, K.L.; Holland, J. Successful strategies for implementing biopsychosocial screening. Psychooncology 2011, 20, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, P.B.; Wagner, L.I. A new quality standard: The integration of psychosocial care into routine cancer care. J. Clin. Oncol. 2012, 30, 1154–1159. [Google Scholar] [CrossRef]
- Hammerlid, E.; Persson, L.O.; Sullivan, M.; Westin, T. Quality of life effects of psychosocial intervention in patients with head and neck cancer. Otolaryngol. Head Neck Surg. 1999, 120, 507–516. [Google Scholar] [CrossRef]
- Boyes, A.; Newell, S.; Girgis, A.; McElduf, P.; Sanson-Fisher, R. Does routine assessment and real-timefeedback improve cancer patients′ psychosocial well-being? Eur. J. Cancer Care 2006, 15, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Artherholt, S.B.; Fann, J.R. Psychosocial care in cancer. Curr. Psychiatry Rep. 2012, 14, 23–29. [Google Scholar] [CrossRef]
- Kennard, B.D.; Smith, S.M.; Olvera, R.; Bawdon, R.E.; Oh Ailin, A.; Lewis, C.P.; Winick, N. Non adherence in adolescent oncology patients: Preliminary data on psychological risk factors and relationships to outcome. J. Clin. Psychol. Med. Settings 2004, 11, 30–39. [Google Scholar] [CrossRef]
- Steel, J.L.; Geller, D.A.; Gamblin, T.C.; Olek, M.C.; Carr, B.I. Depression, immunity, and survival in patients with hepatobiliary carcinoma. J. Clin. Oncol. 2007, 25, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Ashbury, F.D.; Findlay, H.; Reynolds, B.; McKerracher, K. A Canadian survey of cancer patients′ experiences: Are their needs being met? J. Pain Symptom Manag. 1998, 16, 298–306. [Google Scholar] [CrossRef]
- White, C. Meaning and its measurement in psychosocial oncology. Psychooncology 2004, 13, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Snowden, A.; White, C.A.; Christie, Z.; Murray, E.; McGowan, G.; Scott, R. The clinical utility of the distress thermometer: A review. Br. J. Nurs. 2011, 20, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Indelli, M.; Marzola, M.; Maestri, A.; Santini, A.; Piva, E.; Boccalon, M. Depressive symptoms and quality of life in home-care-assisted cancer patients. J. Pain Symptom Manag. 1996, 12, 300–307. [Google Scholar] [CrossRef]
- Bultz, B.D.; Johansen, C. Screening for distress, the 6th vital sign: Where are we, and where are we going? Psycho Oncol. 2011, 20, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Dapueto, J.J.; Servente, L.; Francolino, C.; Hahn, E.A. Determinants of quality of life in patients with cancer. Cancer 2005, 103, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Johansen, C.; Annunziata, M.A.; Capovilla, E.; Costantini, A.; Gritti, P.; Torta, R.; Bellani, M.; Italian Society of Psycho-Oncology Distress Thermometer Study Group. Screening for distress in cancer patients: A multicenter, nationwide study in Italy. Cancer 2013, 119, 1714–1721. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Chan, M.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011, 12, 160–174. [Google Scholar] [CrossRef]
- Zabora, J.; Brintzenhofeszoc, K.; Curbow, B.; Hooker, C.; Piantadosi, S. The prevalence of psychological distress by cancer site. Psychooncology 2001, 10, 19–28. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN), Clinical Practice Guidelines in Oncology: Distress Management. Version 3.2012, 2012, Fort Washington, PA, USA. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 6 November 2017).
- Bulli, F.; Miccinesi, G.; Maruelli, A.; Katz, M.; Paci, E. The measure of psychological distress in cancer patients: The use of distress thermometer in the Oncological Rehabilitation Center of Florence. Support. Care Cancer 2009, 17, 771–779. [Google Scholar] [CrossRef]
- Hill, J.; Holcombe, C.; Clark, L.; Boothby, M.R.; Hincks, A.; Fisher, J.; Tufail, S.; Salmon, P. Predictors of onset of depression and anxiety in the year after diagnosis of breast cancer. Psychol. Med. 2011, 41, 1429–1436. [Google Scholar] [CrossRef]
- Kadan-Lottick, N.S.; Vanderwerker, L.C.; Block, S.D.; Zhang, B.; Prigerson, H.G. Psychiatric disorders and mental health service use in patients with advanced cancer: A report from the coping with cancer study. Cancer 2005, 104, 2872–2881. [Google Scholar] [CrossRef]
- Holland, J.; Watson, M.; Dunn, J. The IPOS new International Standard of Quality Cancer Care: Integrating the psychosocial domain into routine care. Psychooncology 2011, 20, 677–680. [Google Scholar] [CrossRef]
- Bultz, B.D.; Carlson, L.E. Emotional distress: The sixth vital sign-future directions in cancer care. Psychooncology 2006, 15, 93–95. [Google Scholar] [CrossRef]
- Bultz, B.D.; Groff, S.L.; Fitch, M.; Blasi, M.C.; Howes, J.; Levy, K.; Mayer, C. Implementing screening for distress, the 6th vital sign: A canadian strategy for changing practice. Psychooncology 2011, 20, 463–469. [Google Scholar] [CrossRef]
- Roth, A.J.; Kornblith, A.B.; Batel-Copel, L.; Peabody, E.; Scher, H.I.; Holland, J.C. Rapid screening for psychologic distress in men with prostate Carcinoma: A pilot study. Cancer 1998, 82, 1904–1908. [Google Scholar] [CrossRef]
- Hegel, M.T.; Collins, E.D.; Kearing, S.; Gillock, K.L.; Moore, C.P.; Ahles, T.A. Sensitivity and specificity of the distress thermomether for depression in newly diagnosed breast cancer patients. Psychooncology 2008, 17, 556–560. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Guidelines for Supportive Care/Distress Management. Version 2.2013, Jenkintown, PA, USA. 2013. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx#supportive (accessed on 22 May 2018).
- Pirl, W.F.; Fann, J.R.; Greer, J.A.; Braum, I.; Deshields, T.; Fulcher, C.; Harvey, E.; Holland, J.; Kennedy, V.; Lazenby, M.; et al. Recommendations for the implementation of distress screening programs in cancer centers: Report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer 2014, 120, 2946–2954. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.C. If we build it, they will come: Rethinking some assumptions about screening and intervening for distress. J. Natl. Compr. Cancer Netw. 2019, 17, 1017–1018. [Google Scholar] [CrossRef] [PubMed]
- Riba, M.B.; Donovan, K.A.; Anderson, B.; Braun, I.; Breitbart, W.S.; Brewer, B.W.; Buchmann, L.O.; Clark, M.M.; Collins, M.; Corbett, C.; et al. Distress Management. Version 3.2019. NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 1229–1249. [Google Scholar] [CrossRef] [PubMed]
- Philip, E.J.; Merluzzi, T.V. Psychosocial issues in post-treatment cancer survivors: Desire for support and challenges in identifying individuals in need. J. Psychosoc. Oncol. 2016, 34, 223–239. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, L.A.; Blauch, A.N.; Pucci, D.A.; DeMichele, A.; Palmer, S.C. Does screening for anxiety and depression efficiently identify patients who want help? J. Clin. Oncol. 2017, 35 (Suppl. S15), e21604. [Google Scholar] [CrossRef]
- Funk, R.; Cisneros, C.; Williams, R.C.; Kendall, J.; Hamann, H.A. What happens after distress screening? Patterns of supportive care service utilization among oncology patients identified through a systematic screening protocol. Support. Care Cancer 2016, 24, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- Meggiolaro, E.; Berardi, M.A.; Andritsch, E.; Nanni, M.G.; Sirgo, A.; Samorì, E.; Farkas, C.; Ruffilli, F.; Caruso, R.; Bellé, M.; et al. Cancer patients′ emotional distress, coping styles and perception of doctor-patient interaction in European cancer settings. Palliat. Support. Care 2016, 14, 204–211. [Google Scholar] [CrossRef] [PubMed]
- NCCN practice guidelines for the management of psychosocial distress. National Comprehensive Cancer Network. Oncology 1999, 13, 113–147. [Google Scholar]
- Donovan, K.A.; Grassi, L.; McGinty, H.L.; Jacobsen, P.B. Validation of the distress thermometer worldwide: State of the science. Psychooncology 2014, 23, 241–250. [Google Scholar] [CrossRef]
- Holland, J.C.; Andersen, B.; Breitbart, W.S.; Buchmann, L.O.; Compas, B.; Deshields, T.L.; Dudley, M.M.; Fleishman, S.; Fulcher, C.D.; Greenberg, D.B.; et al. Distress management. J. Natl. Compre. Cancer Netw. 2010, 8, 448–485. [Google Scholar] [CrossRef]
- Cutillo, A.; O′Hea, E.; Person, S.; Lessard, D.; Harralson, T.; Boudreaux, E. NCCN Distress Thermometer: Cut off points and clinical utility. Oncol. Nurs. Forum 2017, 44, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, P.B.; Donovan, K.A.; Trask, P.C.; Fleishman, S.B.; Zabora, J.; Baker, F.; Holland, J.C. Screening for psychological distress in ambulatory cancer patients. Cancer 2005, 103, 1494–1502. [Google Scholar] [CrossRef]
- Mansourabadi, A.; Moogooei, M.; Nozari, S. Evaluation of distress and stress in cancer patients in AMIR Oncology Hospital in Shiraz. Iran. J. Ped. Hematol. Oncol. 2014, 4, 131–140. [Google Scholar]
- Martinez, P.; Galdon, M.J.; Andreu, Y.; Ibanez, E. The Distress Thermometer in Spanish cancer patients: Convergent validity and diagnostic accuracy. Support. Care Cancer 2013, 21, 3095–3102. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Rossi, E.; Caruso, R.; Nanni, M.G.; Pedrazzi, S.; Sofritti, S.; Sabato, S. Educational intervention in cancer outpatient clinics on routine screening for emotional distress: An observational study. Psychooncology 2011, 20, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Hollingworth, W.; Metcalfe, C.; Mancero, S.; Harris, S.; Campbell, R.; Biddle, L.; McKell-Redwood, D.; Brennan, J. Are needs assessments cost effective in reducing distress among patients with cancer? A randomized controlled trial using the distress thermometer and problem list. J. Clin. Oncol. 2013, 31, 3631–3638. [Google Scholar] [CrossRef]
- Graves, K.D.; Arnold, S.M.; Love, C.L.; Kirsh, K.L.; Moore, P.G.; Passik, S.D. Distress screening in a multidisciplinary lung cancer clinic: Prevalence and predictors of clinically significant distress. Lung Cancer 2007, 55, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, A.J.; Vahabzadeh, A.; Magnude, K. Screening for distress and depression in cancer settings: 10 lessons from 40 years of primary-care research. Psychoncology 2011, 20, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Pincus, H.A.; Patel, S.R. Barriers to the delivery of psychosocial care for cancer patients: Bridging mind and body. J. Clin. Oncol. 2009, 27, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, S.; Davis, K.; Bluethmann, S.M.; Quintiliani, L.M.; Kendall, J.; Ratwani, R.M.; Diefenbach, M.A.; Graves, K.D. Screening for psychosocial distress among patients with cancer: Implications for clinical practice, healthcare policy, and dissemination to enhance cancer survivorship. Transl. Behav. Med. 2019, 9, 282–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clover, K.A.; Mitchell, A.J.; Britton, B.; Carter, G. Why do oncology outpatients who report emotional distress decline help? Psychooncology 2015, 24, 812–818. [Google Scholar] [CrossRef]
- Tondorf, T.; Grossert, A.; Rothschild, S.I.; Koller, M.T.; Rochlitz, C.; Kiss, A.; Schaefert, R.; Meinlschmidt, G.; Hunziker, S.; Zwahlen, D. Focusing on cancer patients’ intentions to use psychooncological support: A longitudinal, mixed-methods study. Psychooncology 2018, 27, 1656–1663. [Google Scholar] [CrossRef]
- Söllner, W.; DeVries, A.; Steixner, E.; Lukas, P.; Sprinzl, G.; Rumpold, G.; Maislinger, S. How successful are oncologists in identifying patient distress, perceived social support, and need for psychosocial counseling? Br. J. Cancer 2001, 84, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, L.E.; Angen, M.; Cullum, J.; Goodey, E.; Koopmans, J.; Lamont, L.; MacRae, J.H.; Martin, M.; Pelletier, G.; Robinson, J.; et al. High levels of untreated distress and fatigue in cancer patients. Br. J. Cancer 2004, 90, 2297–2304. [Google Scholar] [CrossRef]
- Carlson, L.E.; Waller, A.; Mitchell, A.J. Screening for distress and unmet needs in patients with cancer: Review and recommendations. J. Clin. Oncol. 2012, 30, 1160–1177. [Google Scholar] [CrossRef] [PubMed]
- Bauwens, S.; Baillon, C.; Distelmans, W.; Theuns, P. Systematic screening for distress in oncology practice using the Distress Barometer: The impact on referrals topsychosocial care. Psychooncology 2014, 23, 804–811. [Google Scholar] [CrossRef]
- Carlson, L.E.; Bultz, B.D. Efficacy and medical costs offset of psychosocial interventions in cancer care: Making the case for economic analyses. Psychooncology 2004, 13, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, M.; Asaria, M.; Walker, S.; Lewin, R.; Sowden, A.J.; Ritchie, G. The Effects of Psychosocial Interventions in Cancer and Heart Disease: A Review of Systematic Reviews; Centre for Reviews and Dissemination, University of York: York, UK, 2005. [Google Scholar]
- Die Trill, M. Psychological aspects of depression in cancer patients: An update. Ann. Oncol. 2012, 23 (Suppl. S10), 302–305. [Google Scholar] [CrossRef] [PubMed]
- Bergerot, C.D.; Cavalcanti Ferreira de Araujo, T.C. Assessment of distress and quality of life of cancer patients over the course of chemotherapy. Invest. Educ. Enferm. 2014, 32, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Donovan, K.A.; Grassi, L.; Deshields, T.L.; Corbett, C.; Riba, M.B. Advancing the science of distress screening and management in cancer care. Epidemiol. Psychiatr. Sci. 2019, 29, e85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, L.; Caruso, R.; Sabato, S.; Massarenti, S.; Nanni, M.G. The UniFe Psychiatry Working Group Coauthors Psychosocial screening and assessment in oncology and palliative care settings. Front. Psychol. 2015, 5, 1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | No DT Questionnaire | DT Questionnaire | Total | p-Value # |
|---|---|---|---|---|
| Total no. patients | 112 | 294 | 406 | - |
| Median age, years (range) | 63 (36–73) | 59.5 (21–79) | 60.5 (21–79) | 0.0174 |
| Gender | ||||
| Male | 43 (38.4) | 113 (38.4) | 156 | 0.9937 |
| Female | 69 (61.6) | 181 (61.6) | 250 | |
| Children (n) | 11 (15.9) | 42 (17.5) | 53 | 0.5858 |
| None | 19 (27.5) | 79 (32.9) | 98 | |
| One | 39 (56.5) | 119 (49.6) | 158 | |
| Two or more | 43 | 54 | 97 | |
| Occupation | ||||
| Worker | 26 (36.6) | 124 (45.1) | 150 | 0.0080 |
| Retired | 37 (52.1) | 91 (33.1) | 128 | |
| Other (housewife, unemployed, student) | 8 (11.3) | 60 (21.8) | 68 | |
| Unknown | 41 | 19 | 60 | |
| Primary site of disease | ||||
| Breast | 29 (25.9) | 95 (32.3) | 124 | 0.3227 |
| Gastrointestinal tract | 24 (21.4) | 70 (23.8) | 94 | |
| Lung | 13 (11.6) | 29 (9.9) | 42 | |
| Urogenital tract | 12 (10.7) | 40 (13.6) | 52 | |
| Hematologic malignancy | 14 (12.5) | 28 (9.5) | 42 | |
| Other location | 20 (17.9) | 32 (10.9) | 52 | |
| Treatment setting | 79 (76.0) | 179 (63.7) | 258 | 0.0231 |
| Advanced | 25 (24.0) | 102 (36.3) | 127 | |
| Neoadjuvant/adjuvant | 8 | 13 | 21 | |
| Previous psychological contact | ||||
| No | 107 (96.4) | 249 (85.6) | 356 | 0.0014 |
| Yes | 4 (3.6) | 42 (14.4) | 46 | |
| Unknown | 1 | 3 |
| PL | DT 0–3 (n = 138) | DT 4–5 (n = 56) | DT ≥ 6 (n = 68) | Total (n = 262) | p-Value # |
|---|---|---|---|---|---|
| Practical problems | |||||
| No.(%) patients with ≥1 practical problem | 22 (15.9) | 21 (37.5) | 33 (48.5) | 76 (29.0) | <0.0001 |
| Median no. practical problems (range) | 1 (1–2) | 1 (1–3) | 1 (1–4) | 1 (1–4) | - |
| Relational problems | |||||
| No.(%) patients with ≥1 relationship problem | 12 (8.7) | 9 (16.1) | 23 (33.8) | 44 (16.8) | <0.0001 |
| Median no. relationship problems (range) | 1 (1–2) | 1 (1–2) | 1 (1–3) | 1 (1–3) | - |
| Emotional problems | |||||
| No.(%) patients with ≥1 emotional problem | 82 (59.4) | 48 (85.7) | 62 (91.2) | 192 (73.3) | <0.0001 |
| Median no. emotional problems (range) | 2 (1–6) | 3 (1–6) | 4 (1–6) | 2 (1–6) | - |
| Spiritual aspects | |||||
| No.(%) patients with spiritual problems | 2 (1.4) | 1 (1.8) | 10 (14.7) | 13 (5.0) | 0.0001 |
| Physical problems | |||||
| No.(%) of patients with ≥1 physical problem | 122 (88.4) | 49 (87.5) | 64 (94.1) | 235 (89.7) | 0.2500 |
| Median no. of physical problems (range) | 3 (1–12) | 6 (1–15) | 7 (1–15) | 5 (1–15) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meggiolaro, E.; De Padova, S.; Ruffilli, F.; Bertelli, T.; Bragagni, M.; Prati, S.; Pisotti, L.; Massa, I.; Foca, F.; Tamberi, S.; et al. From Distress Screening to Uptake: An Italian Multicenter Study of Cancer Patients. Cancers 2021, 13, 3761. https://doi.org/10.3390/cancers13153761
Meggiolaro E, De Padova S, Ruffilli F, Bertelli T, Bragagni M, Prati S, Pisotti L, Massa I, Foca F, Tamberi S, et al. From Distress Screening to Uptake: An Italian Multicenter Study of Cancer Patients. Cancers. 2021; 13(15):3761. https://doi.org/10.3390/cancers13153761
Chicago/Turabian StyleMeggiolaro, Elena, Silvia De Padova, Federica Ruffilli, Tatiana Bertelli, Marina Bragagni, Sabrina Prati, Lidia Pisotti, Ilaria Massa, Flavia Foca, Stefano Tamberi, and et al. 2021. "From Distress Screening to Uptake: An Italian Multicenter Study of Cancer Patients" Cancers 13, no. 15: 3761. https://doi.org/10.3390/cancers13153761







