The Microbiome as a Potential Target for Therapeutic Manipulation in Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Therapeutic Challenges in Treating Patients with Pancreatic Cancer
2.1. Surgery and Chemotherapy in Patients with Pancreas Cancer
2.2. Immunotherapy Use in Pancreatic Cancer and the Associated Challenges
3. What Is Microbiome?
4. The Microbiome and Its Relation to Anatomical Site
4.1. Oral Microbiome and Its Potential Association with PDAC
4.2. Gut Microbiome and Its Potential Association with PDAC
4.3. Intratumoral Microbiome and Its Potential Association with PDAC
5. Potential Mechanism of Microbiota in Carcinogenesis
5.1. Inflammation
5.2. Modulation of the Immune System
5.3. Microbial Metabolite and the Regulation of Metabolism
6. Potential Novel Treatment Approaches for Patients with Pancreatic Ductal Adenocarcinoma
6.1. Antibiotic Use and its Association with PDAC
6.2. Probiotic Use and its Association with PDAC
6.3. Prebiotic Use and its Association with PDAC
6.4. Synbiotics and Postbiotics
6.5. Faecal Microbiota Transplantation
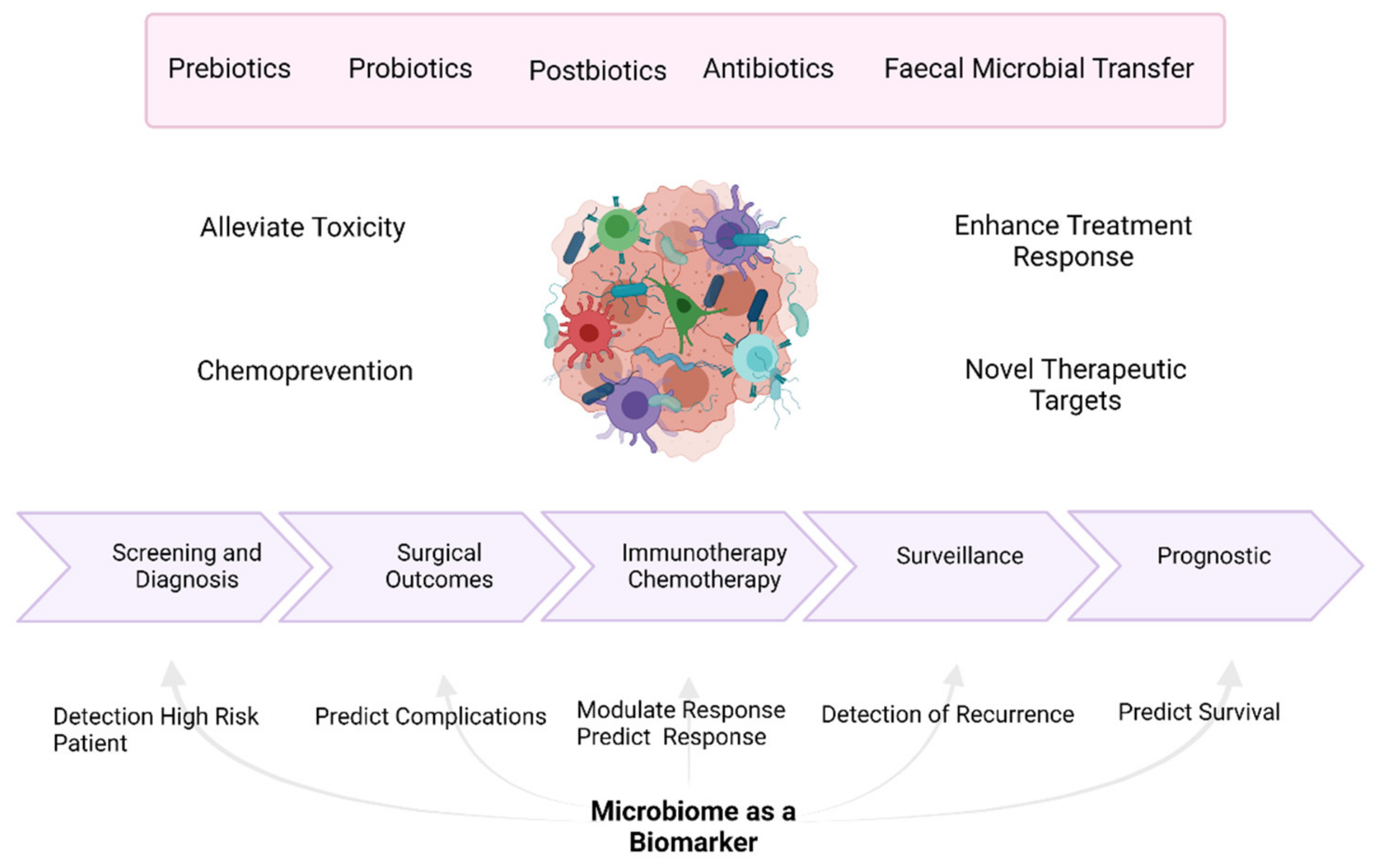
7. The Wider Implications of the Microbiome in PDAC
7.1. Cancer Prevention, including PDAC
7.2. The Microbiome and its Potential to Potentiate Efficacy of Anti-Cancer Therapy
7.3. Alleviating Treatment Side Effects Using the Microbiome
7.4. The Microbiome as a Biomarker in PDAC
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Lowenfels, A.B.; Maisonneuve, P.; Cavallini, G.; Ammann, R.W.; Lankisch, P.G.; Andersen, J.R.; Dimagno, E.P.; Andren-Sandberg, A.; Domellof, L. Pancreatitis and the Risk of Pancreatic Cancer. N. Engl. J. Med. 1993, 328, 1433–1437. [Google Scholar] [CrossRef]
- De Sousa Cavalcante, L.; Monteiro, G. Gemcitabine: Metabolism and Molecular Mechanisms of Action, Sensitivity and Chemoresistance in Pancreatic Cancer. Eur. J. Pharmacol. 2014, 741, 8–16. [Google Scholar] [CrossRef]
- Thomas, D.; Radhakrishnan, P. Tumor-Stromal Crosstalk in Pancreatic Cancer and Tissue Fibrosis. Mol. Cancer 2019, 18, 1–15. [Google Scholar] [CrossRef]
- De Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global Burden of Cancers Attributable to Infections in 2008: A Review and Synthetic Analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Goodman, B.; Gardner, H. The Microbiome and Cancer. J. Pathol. 2018, 244, 667–676. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Bulle, A.; Lim, K.H. Beyond Just a Tight Fortress: Contribution of Stroma to Epithelial-Mesenchymal Transition in Pancreatic Cancer. Signal Transduct. Target. Ther. 2020, 5, 1–12. [Google Scholar] [CrossRef]
- Martinez-Bosch, N.; Vinaixa, J.; Navarro, P. Immune Evasion in Pancreatic Cancer: From Mechanisms to Therapy. Cancers 2018, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Kunovsky, L.; Tesarikova, P.; Kala, Z.; Kroupa, R.; Kysela, P.; Dolina, J.; Trna, J. The Use of Biomarkers in Early Diagnostics of Pancreatic Cancer. Can. J. Gastroenterol. Hepatol. 2018, 2018. [Google Scholar] [CrossRef]
- Meleady, P.; Abdul Rahman, R.; Henry, M.; Moriarty, M.; Clynes, M. Proteomic Analysis of Pancreatic Ductal Adenocarcinoma. Expert Rev. Proteom. 2020, 17, 453–467. [Google Scholar] [CrossRef]
- Montemagno, C.; Cassim, S.; De Leiris, N.; Durivault, J.; Faraggi, M.; Pagès, G. Pancreatic Ductal Adenocarcinoma: The Dawn of the Era of Nuclear Medicine? Int. J. Mol. Sci. 2021, 22, 6413. [Google Scholar] [CrossRef]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic Cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Tzeng, C.-W.D.; Tran Cao, H.S.; Lee, J.E.; Pisters, P.W.T.; Varadhachary, G.R.; Wolff, R.A.; Abbruzzese, J.L.; Crane, C.H.; Evans, D.B.; Wang, H.; et al. Treatment Sequencing for Resectable Pancreatic Cancer: Influence of Early Metastases and Surgical Complications on Multimodality Therapy Completion and Survival. J. Gastrointest. Surg. 2014, 18, 16–24, discussion 24–25. [Google Scholar] [CrossRef] [PubMed]
- Riall, T.S.; Lillemoe, K.D. Underutilization of Surgical Resection in Patients with Localized Pancreatic Cancer. Ann. Surg. 2007, 246, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Abdelghani, M.B.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with Nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 Trial of Single Agent Ipilimumab (Anti-CTLA-4) for Locally Advanced or Metastatic Pancreatic Adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Oh, D.Y.; Dhani, N.; Renouf, D.J.; Lee, M.A.; Sun, W.; Fisher, G.; Hezel, A.; Chang, S.C.; Vlahovic, G.; et al. Durvalumab with or Without Tremelimumab for Patients with Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1431–1438. [Google Scholar] [CrossRef]
- Emens, L.A.; Middleton, G. The Interplay of Immunotherapy and Chemotherapy: Harnessing Potential Synergies. Cancer Immunol. Res. 2015, 3, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Renouf, D.J.; Dhani, N.C.; Kavan, P.; Jonker, D.J.; Wei, A.C.; Hsu, T.; Tang, P.A.; Graham, B.; Gallinaro, L.; Hasan, T.; et al. The Canadian Cancer Trials Group PA.7 Trial: Results from the Safety Run in of a Randomized Phase II Study of Gemcitabine (GEM) and Nab-Paclitaxel (Nab-P) versus GEM, Nab-P, Durvalumab (D), and Tremelimumab (T) as First-Line Therapy in Metastatic Pancreatic Ductal Adenocarcinoma (MPDAC). J. Clin. Oncol. 2018, 36 (Suppl. 4), 349. [Google Scholar] [CrossRef]
- Renouf, D.J.; Knox, J.J.; Kavan, P.; Jonker, D.; Welch, S.; Couture, F.; Lemay, F.; Tehfe, M.; Harb, M.; Aucoin, N.; et al. LBA65 The Canadian Cancer Trials Group PA.7 Trial: Results of a Randomized Phase II Study of Gemcitabine (GEM) and Nab-Paclitaxel (Nab-P) vs GEM, Nab-P, Durvalumab (D) and Tremelimumab (T) as First Line Therapy in Metastatic Pancreatic Ductal Adenocarcinoma (MPDAC). Ann. Oncol. 2020, 31, S1195. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; de Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/ Mismatch Repair–Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Luchini, C.; Brosens, L.A.A.; Wood, L.D.; Chatterjee, D.; Shin, J.I.; Sciammarella, C.; Fiadone, G.; Malleo, G.; Salvia, R.; Kryklyva, V.; et al. Comprehensive Characterisation of Pancreatic Ductal Adenocarcinoma with Microsatellite Instability: Histology, Molecular Pathology and Clinical Implications. Gut 2021, 70, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Jaffee, E.M.; Hruban, R.H.; Biedrzycki, B.; Laheru, D.; Schepers, K.; Sauter, P.R.; Goemann, M.; Coleman, J.; Grochow, L.; Donehower, R.C.; et al. Novel Allogeneic Granulocyte-Macrophage Colony-Stimulating Factor-Secreting Tumor Vaccine for Pancreatic Cancer: A Phase I Trial of Safety and Immune Activation. J. Clin. Oncol. 2001, 19, 145–156. [Google Scholar] [CrossRef]
- Laheru, D.; Lutz, E.; Burke, J.; Biedrzycki, B.; Solt, S.; Onners, B.; Tartakovsky, I.; Nemunaitis, J.; Le, D.; Sugar, E.; et al. Allogeneic Granulocyte Macrophage Colony-Stimulating Factor-Secreting Tumor Immunotherapy Alone or in Sequence with Cyclophosphamide for Metastatic Pancreatic Cancer: A Pilot Study of Safety, Feasibility, and Immune Activation. Clin. Cancer Res. 2008, 14, 1455–1463. [Google Scholar] [CrossRef]
- Le, D.T.; Lutz, E.; Uram, J.N.; Sugar, E.A.; Onners, B.; Solt, S.; Zheng, L.; Diaz, L.A.; Donehower, R.C.; Jaffee, E.M.; et al. Evaluation of Ipilimumab in Combination with Allogeneic Pancreatic Tumor Cells Transfected with a GM-CSF Gene in Previously Treated Pancreatic Cancer. J. Immunother. 2013, 36, 382–389. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Shelomi, M.; Danchin, E.G.J.; Heckel, D.; Wipfler, B.; Bradler, S.; Zhou, X.; Pauchet, Y. Horizontal Gene Transfer of Pectinases from Bacteria Preceded the Diversification of Stick and Leaf Insects. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the Human Infant Intestinal Microbiota. PLoS Biol. 2007, 5, 1556–1573. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery Mode Shapes the Acquisition and Structure of the Initial Microbiota across Multiple Body Habitats in Newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science (80-) 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.B.; Bornet, F.; Bouley, C.; Cummings, J.H. Colonic Microflora: Nutrition and Health0. Summary and Conclusions of an International Life Sciences Institute (ILSI) [Europe] Workshop Held in Barcelona, Spain. Nutr. Rev. 2009, 53, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate Mediates a Microbiome-Brain-β-Cell Axis to Promote Metabolic Syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef]
- Cani, P.D.; Dewever, C.; Delzenne, N.M. Inulin-Type Fructans Modulate Gastrointestinal Peptides Involved in Appetite Regulation (Glucagon-like Peptide-1 and Ghrelin) in Rats. Br. J. Nutr. 2004, 92, 521–526. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The Microbiome and Cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic Bacteria Direct Expression of an Intestinal Bactericidal Lectin. Science (80-) 2006, 313, 1126–1130. [Google Scholar] [CrossRef]
- Schauber, J.; Svanholm, C.; Termén, S.; Iffland, K.; Menzel, T.; Scheppach, W.; Melcher, R.; Agerberth, B.; Lührs, H.; Gudmundsson, G.H. Expression of the Cathelicidin LL-37 Is Modulated by Short Chain Fatty Acids in Colonocytes: Relevance of Signalling Pathways. Gut 2003, 52, 735–741. [Google Scholar] [CrossRef]
- Bouskra, D.; Brézillon, C.; Bérard, M.; Werts, C.; Varona, R.; Boneca, I.G.; Eberl, G. Lymphoid Tissue Genesis Induced by Commensals through NOD1 Regulates Intestinal Homeostasis. Nature 2008, 456, 507–510. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Rajpoot, M.; Sharma, A.K.; Sharma, A.; Gupta, G.K. Understanding the Microbiome: Emerging Biomarkers for Exploiting the Microbiota for Personalized Medicine against Cancer. Semin. Cancer Biol. 2018, 52, 1–8. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, E. The Role of Gut Microbiota in Immune Homeostasis and Autoimmunity. Gut Microbes 2012, 3, 4. [Google Scholar] [CrossRef]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of Gut Microbiota with Bile Acid Metabolism and Its Influence on Disease States. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Shi, J.; Mehwish, H.M.; Zhu, J.; Li, Q.; Shao, D.; Huang, Q.; Yang, H. Interaction between Diet Composition and Gut Microbiota and Its Impact on Gastrointestinal Tract Health. Food Sci. Hum. Wellness 2017, 6, 121–130. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining Dysbiosis and Its Influence on Host Immunity and Disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial Community Variation in Human Body Habitats across Space and Time. Science (80-) 2009, 326, 1694–1697. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Fraher, M.H.; O’Toole, P.W.; Quigley, E.M.M. Techniques Used to Characterize the Gut Microbiota: A Guide for the Clinician. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 312–322. [Google Scholar] [CrossRef]
- Heikkilä, P.; But, A.; Sorsa, T.; Haukka, J. Periodontitis and Cancer Mortality: Register-Based Cohort Study of 68,273 Adults in 10-Year Follow-Up. Int. J. Cancer 2018, 142, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Joshipura, K.; Giovannucci, E.; Fuchs, C.S. A Prospective Study of Periodontal Disease and Pancreatic Cancer in US Male Health Professionals. J. Natl. Cancer Inst. 2007, 99, 171–175. [Google Scholar] [CrossRef]
- Ahn, J.; Segers, S.; Hayes, R.B. Periodontal Disease, Porphyromonas Gingivalis Serum Antibody Levels and Orodigestive Cancer Mortality. Carcinogenesis 2012, 33, 1055–1058. [Google Scholar] [CrossRef]
- Meyer, M.S.; Joshipura, K.; Giovannucci, E.; Michaud, D.S. A Review of the Relationship between Tooth Loss, Periodontal Disease, and Cancer. Cancer Causes Control 2008, 19, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.H.; Grote, V.A.; Tjønneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma Antibodies to Oral Bacteria and Risk of Pancreatic Cancer in a Large European Prospective Cohort Study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Rafiei, M.; Kiani, F.; Sayehmiri, F.; Sayehmiri, K.; Sheikhi, A.; Zamanian Azodi, M. Study of Porphyromonas Gingivalis in Periodontal Diseases: A Systematic Review and Meta-Analysis. Med. J. Islam. Repub. Iran 2017, 31, 355–362. [Google Scholar] [CrossRef]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human Oral Microbiome and Prospective Risk for Pancreatic Cancer: A Population-Based Nested Case-Control Study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T.W. Variations of Oral Microbiota Are Associated with Pancreatic Diseases Including Pancreatic Cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the Adult Digestive Tract Bacterial Microbiome Based on Seven Mouth Surfaces, Tonsils, Throat and Stool Samples. Genome Biol. 2012, 13, R42. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; et al. An Integrated Catalog of Reference Genes in the Human Gut Microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef]
- Raderer, M.; Wrba, F.; Kornek, G.; Maca, T.; Koller, D.Y.; Weinlaender, G.; Hejna, M.; Scheithauer, W. Association between Helicobacter Pylori Infection and Pancreatic Cancer. Oncology 1997, 55, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberg-Solomon, R.Z.; Blaser, M.J.; Limburg, P.J.; Perez-Perez, G.; Taylor, P.R.; Virtamo, J.; Albanes, D. Helicobacter Pylori Seropositivity as a Risk Factor for Pancreatic Cancer. J. Natl. Cancer Inst. 2001, 93, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Lindkvist, B.; Johansen, D.; Borgström, A.; Manjer, J. A Prospective Study of Helicobacter Pylori in Relation to the Risk for Pancreatic Cancer. BMC Cancer 2008, 8, 321. [Google Scholar] [CrossRef]
- Bao, Y.; Spiegelman, D.; Li, R.; Giovannucci, E.; Fuchs, C.S.; Michaud, D.S. History of Peptic Ulcer Disease and Pancreatic Cancer Risk in Men. Gastroenterology 2010, 138, 541–549. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, Y.; Gao, Y. Association between Helicobacter Pylori Infection and Pancreatic Cancer Development: A Meta-Analysis. PLoS ONE 2013, 8, e75559. [Google Scholar] [CrossRef] [PubMed]
- Schulte, A.; Pandeya, N.; Fawcett, J.; Fritschi, L.; Risch, H.A.; Webb, P.M.; Whiteman, D.C.; Neale, R.E. Association between Helicobacter Pylori and Pancreatic Cancer Risk: A Meta-Analysis. Cancer Causes Control 2015, 26, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, M.; Inoue, M.; Sawada, N.; Saito, E.; Abe, S.K.; Hidaka, A.; Iwasaki, M.; Yamaji, T.; Shimazu, T.; Tsugane, S. Helicobacter Pylori Infection, Atrophic Gastritis, and Risk of Pancreatic Cancer: A Population-Based Cohort Study in a Large Japanese Population: The JPHC Study. Sci. Rep. 2019, 9, 6099. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.C.; Wang, Y.J. Helicobacter Pylori and Pancreatic Cancer Risk: A Meta-Analysis Based on 2049 Cases and 2861 Controls. Asian Pac. J. Cancer Prev. 2014, 15, 4449–4454. [Google Scholar] [CrossRef]
- Hoefs, J.C.; Renner, I.G.; Ashcavai, M.; Redeker, A.G. Hepatitis B Surface Antigen in Pancreatic and Biliary Secretions. Gastroenterology 1980, 79, 191–194. [Google Scholar] [CrossRef]
- Kamiza, A.B.; Su, F.H.; Wang, W.C.; Sung, F.C.; Chang, S.N.; Yeh, C.C. Chronic Hepatitis Infection Is Associated with Extrahepatic Cancer Development: A Nationwide Population-Based Study in Taiwan. BMC Cancer 2016, 16. [Google Scholar] [CrossRef]
- Kwok, R.M.; Tran, T.T. Hepatitis B and Risk of Non–Hepatocellular Carcinoma Malignancy. Clin. Liver Dis. 2016, 20, 693–702. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, X.; Zhang, Q.; Wen, L.; Su, W.; Fu, Q.; Sun, X.; Lou, Y.; Yang, J.; Zhang, J.; et al. The Hepatitis B Virus X Protein Promotes Pancreatic Cancer through Modulation of the PI3K/AKT Signaling Pathway. Cancer Lett. 2016, 380, 98–105. [Google Scholar] [CrossRef]
- Xu, J.H.; Fu, J.J.; Wang, X.L.; Zhu, J.Y.; Ye, X.H.; Chen, S.D. Hepatitis B or C Viral Infection and Risk of Pancreatic Cancer: A Meta-Analysis of Observational Studies. World J. Gastroenterol. 2013, 19, 4234–4241. [Google Scholar] [CrossRef]
- Luo, G.; Hao, N.B.; Hu, C.J.; Yong, X.; Lü, M.H.; Cheng, B.J.; Zhang, Y.; Yang, S.M. HBV Infection Increases the Risk of Pancreatic Cancer: A Meta-Analysis. Cancer Causes Control 2013, 24, 529–537. [Google Scholar] [CrossRef]
- Li, L.; Wu, B.; Yang, L.B.; Yin, G.C.; Liu, J.Y. Chronic Hepatitis B Virus Infection and Risk of Pancreatic Cancer: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2013, 14, 275–279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hassan, M.M.; Li, D.; El-Deeb, A.S.; Wolff, R.A.; Bondy, M.L.; Davila, M.; Abbruzzese, J.L. Association between Hepatitis B Virus and Pancreatic Cancer. J. Clin. Oncol. 2008, 26, 4557–4562. [Google Scholar] [CrossRef]
- Iloeje, U.H.; Yang, H.I.; Jen, C.L.; Su, J.; Wang, L.Y.; You, S.L.; Lu, S.N.; Chen, C.J. Risk of Pancreatic Cancer in Chronic Hepatitis B Virus Infection: Data from the REVEAL-HBV Cohort Study. Liver Int. 2010, 30, 423–429. [Google Scholar] [CrossRef]
- Serra, N.; Di Carlo, P.; Gulotta, G.; d’ Arpa, F.; Giammanco, A.; Colomba, C.; Melfa, G.; Fasciana, T.; Sergi, C. Bactibilia in Women Affected with Diseases of the Biliary Tract and Pancreas. A STROBE Guidelines-Adherent Cross-Sectional Study in Southern Italy. J. Med. Microbiol. 2018, 67, 1090–1095. [Google Scholar] [CrossRef]
- Di Carlo, P.; Serra, N.; D’arpa, F.; Agrusa, A.; Gulotta, G.; Fasciana, T.; Rodolico, V.; Giammanco, A.; Sergi, C. The Microbiota of the Bilio-Pancreatic System: A Cohort, STROBE-Compliant Study. Infect. Drug Resist. 2019, 12, 1513–1527. [Google Scholar] [CrossRef]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential Role of Intratumor Bacteria in Mediating Tumor Resistance to the Chemotherapeutic Drug Gemcitabine. Science (80-) 2017, 357, 1156–1160. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The Human Tumor Microbiome Is Composed of Tumor Type-Specific Intracellular Bacteria. Science (80-) 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Jenq, R.; Wargo, J.; Correspondence, F.M.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806. [Google Scholar] [CrossRef]
- Riquelme, E.; Maitra, A.; McAllister, F. Immunotherapy for Pancreatic Cancer: More than Just a Gut Feeling. Cancer Discov. 2018, 8, 386–388. [Google Scholar] [CrossRef]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A Review of Human Carcinogens—Part B: Biological Agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Scott, A.J.; Alexander, J.L.; Merrifield, C.A.; Cunningham, D.; Jobin, C.; Brown, R.; Alverdy, J.; O’Keefe, S.J.; Gaskins, H.R.; Teare, J.; et al. International Cancer Microbiome Consortium Consensus Statement on the Role of the Human Microbiome in Carcinogenesis. Gut 2019, 68, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Shacter, E.; Weitzman, S.A. Chronic Inflammation and Cancer. Oncology (Williston Park) 2002, 16, 217–226, discussion 230. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Ochi, A.; Nguyen, A.H.; Bedrosian, A.S.; Mushlin, H.M.; Zarbakhsh, S.; Barilla, R.; Zambirinis, C.P.; Fallon, N.C.; Rehman, A.; Pylayeva-Gupta, Y.; et al. MyD88 Inhibition Amplifies Dendritic Cell Capacity to Promote Pancreatic Carcinogenesis via Th2 Cells. J. Exp. Med. 2012, 209, 1671–1687. [Google Scholar] [CrossRef] [PubMed]
- Ochi, A.; Graffeo, C.S.; Zambirinis, C.P.; Rehman, A.; Hackman, M.; Fallon, N.; Barilla, R.M.; Henning, J.R.; Jamal, M.; Rao, R.; et al. Toll-like Receptor 7 Regulates Pancreatic Carcinogenesis in Mice and Humans. J. Clin. Investig. 2012, 122, 4118–4129. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Lowenfels, A.B. The Epidemiology of Pancreatitis and Pancreatic Cancer. Gastroenterology 2013, 144, 1252–1261. [Google Scholar] [CrossRef]
- Löhr, M.; Klöppel, G.; Maisonneuve, P.; Lowenfels, A.B.; Lüttges, J. Frequency of K-Ras Mutations in Pancreatic Intraductal Neoplasias Associated with Pancreatic Ductal Adenocarcinoma and Chronic Pancreatitis: A Meta-Analysis. Neoplasia 2005, 7, 17–23. [Google Scholar] [CrossRef]
- Dapito, D.H.; Mencin, A.; Gwak, G.Y.; Pradere, J.P.; Jang, M.K.; Mederacke, I.; Caviglia, J.M.; Khiabanian, H.; Adeyemi, A.; Bataller, R.; et al. Promotion of Hepatocellular Carcinoma by the Intestinal Microbiota and TLR4. Cancer Cell 2012, 21, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Gao, H.; Chen, H.; Wang, J.; Chen, M.; Li, G.; Wang, L.; Gu, J.; Tu, H. Identification and Impact of Hepatitis B Virus DNA and Antigens in Pancreatic Cancer Tissues and Adjacent Non-Cancerous Tissues. Cancer Lett. 2013, 335, 447–454. [Google Scholar] [CrossRef]
- Wang, D.-S.; Chen, D.-L.; Ren, C.; Wang, Z.-Q.; Qiu, M.-Z.; Luo, H.-Y.; Zhang, D.-S.; Wang, F.-H.; Li, Y.-H.; Xu, R.-H. ABO Blood Group, Hepatitis B Viral Infection and Risk of Pancreatic Cancer. Int. J. Cancer 2012, 131, 461–468. [Google Scholar] [CrossRef]
- Ertz-Archambault, N.; Keim, P.; Hoff, D. Von. Microbiome and Pancreatic Cancer: A Comprehensive Topic Review of Literature. World J. Gastroenterol. 2017, 23, 1899–1908. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The Intestinal Microbiota Modulates the Anticancer Immune Effects of Cyclophosphamide. Science (80-) 2013, 342, 971–976. [Google Scholar] [CrossRef]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment. Science (80-) 2013, 342, 967–970. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti-PD-L1 Efficacy. Science (80-) 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1. [Google Scholar] [CrossRef]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the Effects of Diet on Bacterial Metabolism in the Large Intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J.D. Nutrition and Colonic Health: The Critical Role of the Microbiota. Curr. Opin. Gastroenterol. 2008, 24, 51–58. [Google Scholar] [CrossRef]
- Scheppach, W. Effects of Short Chain Fatty Acids on Gut Morphology and Function. Gut 1994, 35 (Suppl. 1), S35. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, Y.; He, L.; Wu, L.; Wang, X.; Liu, Z. Effects of the Intestinal Microbial Metabolite Butyrate on the Development of Colorectal Cancer. J. Cancer 2018, 9, 2510–2517. [Google Scholar] [CrossRef]
- Windey, K.; De Preter, V.; Verbeke, K. Relevance of Protein Fermentation to Gut Health; John Wiley & Sons, Ltd.: London, UK, 2012; Volume 56, pp. 184–196. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Vítězová, M.; Rittmann, S.K.M.R. Environmental Impact of Sulfate-Reducing Bacteria, Their Role in Intestinal Bowel Diseases, and Possible Control by Bacteriophages. Appl. Sci. 2021, 11, 735. [Google Scholar] [CrossRef]
- Attene-Ramos, M.S.; Wagner, E.D.; Plewa, M.J.; Gaskins, H.R. Evidence That Hydrogen Sulfide Is a Genotoxic Agent. Mol. Cancer Res. 2006, 4, 9–14. [Google Scholar] [CrossRef]
- Eibl, G.; Rozengurt, E. KRAS, YAP, and Obesity in Pancreatic Cancer: A Signaling Network with Multiple Loops. Semin. Cancer Biol. 2019, 54, 50–62. [Google Scholar] [CrossRef]
- Schulz, M.D.; Atay, Ç.; Heringer, J.; Romrig, F.K.; Schwitalla, S.; Aydin, B.; Ziegler, P.K.; Varga, J.; Reindl, W.; Pommerenke, C.; et al. High-Fat-Diet-Mediated Dysbiosis Promotes Intestinal Carcinogenesis Independently of Obesity. Nature 2014, 514, 508–512. [Google Scholar] [CrossRef]
- Djuric, Z. Obesity-Associated Cancer Risk: The Role of Intestinal Microbiota in the Etiology of the Host Proinflammatory State. Transl. Res. 2017, 179, 155–167. [Google Scholar] [CrossRef]
- Li, Q.; Jin, M.; Liu, Y.; Jin, L. Gut Microbiota: Its Potential Roles in Pancreatic Cancer. Front. Cell. Infect. Microbiol. 2020, 10, 572492. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.J.; Kook, M.-C.; Kim, Y.-I.; Cho, S.-J.; Lee, J.Y.; Kim, C.G.; Park, B.; Nam, B.-H. Helicobacter Pylori Therapy for the Prevention of Metachronous Gastric Cancer. N. Engl. J. Med. 2018, 378, 1085–1095. [Google Scholar] [CrossRef]
- Mohindroo, C.; Rogers, J.E.; Hasanov, M.; Mizrahi, J.; Overman, M.J.; Varadhachary, G.R.; Wolff, R.A.; Javle, M.M.; Fogelman, D.R.; Pant, S.; et al. A Retrospective Analysis of Antibiotics Usage and Effect on Overall Survival and Progressive Free Survival in Patients with Metastatic Pancreatic Cancer. J. Clin. Oncol. 2019, 37 (Suppl. 15), e15781. [Google Scholar] [CrossRef]
- Hasanov, M.; Mohindroo, C.; Rogers, J.; Prakash, L.; Overman, M.J.; Varadhachary, G.R.; Wolff, R.A.; Javle, M.M.; Fogelman, D.R.; Pant, S.; et al. The Effect of Antibiotic Use on Survival of Patients with Resected Pancreatic Ductal Adenocarcinoma. J. Clin. Oncol. 2019, 37 (Suppl. 15), e15773. [Google Scholar] [CrossRef]
- Corty, R.W.; Langworthy, B.W.; Fine, J.P.; Buse, J.B.; Sanoff, H.K.; Lund, J.L. Antibacterial Use Is Associated with an Increased Risk of Hematologic and Gastrointestinal Adverse Events in Patients Treated with Gemcitabine for Stage IV Pancreatic Cancer. Oncologist 2020, 25, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Fong, W.; Li, Q.; Yu, J. Gut Microbiota Modulation: A Novel Strategy for Prevention and Treatment of Colorectal Cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef] [PubMed]
- Tojo, R.; Suárez, A.; Clemente, M.G.; De Los Reyes-Gavilán, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal Microbiota in Health and Disease: Role of Bifidobacteria in Gut Homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef]
- Zhu, Y.; Michelle Luo, T.; Jobin, C.; Young, H.A. Gut Microbiota and Probiotics in Colon Tumorigenesis. Cancer Lett. 2011, 309, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Van Minnen, L.P.; Timmerman, H.M.; Lutgendorff, F.; Verheem, A.; Harmsen, W.; Konstantinov, S.R.; Smidt, H.; Visser, M.R.; Rijkers, G.T.; Gooszen, H.G.; et al. Modification of Intestinal Flora with Multispecies Probiotics Reduces Bacterial Translocation and Improves Clinical Course in a Rat Model of Acute Pancreatitis. Surgery 2007, 141, 470–480. [Google Scholar] [CrossRef]
- Singhal, B.; Mukherjee, A.; Srivastav, S. Role of Probiotics in Pancreatic Cancer Prevention: The Prospects and Challenges. Adv. Biosci. Biotechnol. 2016, 07, 468–500. [Google Scholar] [CrossRef]
- Karen, M.; Yuksel, O.; Akyürek, N.; Ofluoǧlu, E.; Çaǧlar, K.; Şahin, T.T.; Paşaoǧlu, H.; Memiş, L.; Akyürek, N.; Bostanci, H. Probiotic Agent Saccharomyces Boulardii Reduces the Incidenceof Lung Injury in Acute Necrotizing Pancreatitis Induced Rats. J. Surg. Res. 2010, 160, 139–144. [Google Scholar] [CrossRef]
- Oláh, A.; Belágyi, T.; Issekutz, Á.; Gamal, M.E.; Bengmark, S. Randomized Clinical Trial of Specific Lactobacillus and Fibre Supplement to Early Enteral Nutrition in Patients with Acute Pancreatitis. Br. J. Surg. 2002, 89, 1103–1107. [Google Scholar] [CrossRef]
- Pala, V.; Sieri, S.; Berrino, F.; Vineis, P.; Sacerdote, C.; Palli, D.; Masala, G.; Panico, S.; Mattiello, A.; Tumino, R.; et al. Yogurt Consumption and Risk of Colorectal Cancer in the Italian European Prospective Investigation into Cancer and Nutrition Cohort. Int. J. Cancer 2011, 129, 2712–2719. [Google Scholar] [CrossRef]
- Toi, M.; Hirota, S.; Tomotaki, A.; Sato, N.; Hozumi, Y.; Anan, K.; Nagashima, T.; Tokuda, Y.; Masuda, N.; Ohsumi, S.; et al. Probiotic Beverage with Soy Isoflavone Consumption for Breast Cancer Prevention: A Case-Control Study. Curr. Nutr. Food Sci. 2013, 9, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Naito, S.; Koga, H.; Yamaguchi, A.; Fujimoto, N.; Hasui, Y.; Kuramoto, H.; Iguchi, A.; Kinukawa, N. Prevention of Recurrence with Epirubicin and Lactobacillus Casei after Transurethral Resection of Bladder Cancer. J. Urol. 2008, 179, 485–490. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Chin, J. Modulating Immune Responses with Probiotic Bacteria. Immunol. Cell Biol. 2000, 78, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Zhang, X.; Covasa, M. Emerging Roles of Lactic Acid Bacteria in Protection against Colorectal Cancer. World J. Gastroenterol. 2014, 20, 7878–7886. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion Mechanisms Mediated by Probiotics and Prebiotics and Their Potential Impact on Human Health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Shoaf, K.; Mulvey, G.L.; Armstrong, G.D.; Hutkins, R.W. Prebiotic Galactooligosaccharides Reduce Adherence of Enteropathogenic Escherichia Coli to Tissue Culture Cells. Infect. Immun. 2006, 74, 6920–6928. [Google Scholar] [CrossRef]
- Forchielli, M.L.; Walker, W.A. The Role of Gut-Associated Lymphoid Tissues and Mucosal Defence. Br. J. Nutr. 2005, 93, S41–S48. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, Prebiotics and Synbiotics- a Review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Motoori, M.; Yano, M.; Miyata, H.; Sugimura, K.; Saito, T.; Omori, T.; Fujiwara, Y.; Miyoshi, N.; Akita, H.; Gotoh, K.; et al. Randomized Study of the Effect of Synbiotics during Neoadjuvant Chemotherapy on Adverse Events in Esophageal Cancer Patients. Clin. Nutr. 2017, 36, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Rad, A.H.; Maleki, L.A.; Kafil, H.S.; Zavoshti, H.F.; Abbasi, A. Postbiotics as Promising Tools for Cancer Adjuvant Therapy. Adv. Pharm. Bull. 2021, 11, 1–5. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Lee, M.M.; Eriksen, M.K.; Mullish, B.H.; Marchesi, J.R.; Dahlerup, J.F.; Hvas, C.L. Faecal Microbiota Transplantation for Recurrent Clostridioides Difficile Infection: An Updated Systematic Review and Meta-Analysis. EClinicalMedicine 2020, 29–30. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Wu, C.Y.; Yu, J. The Role of Gut Microbiota in Cancer Treatment: Friend or Foe? Gut 2020, 69, 1867–1876. [Google Scholar] [CrossRef]
- Villéger, R.; Lopès, A.; Veziant, J.; Gagnière, J.; Barnich, N.; Billard, E.; Boucher, D.; Bonnet, M. Microbial Markers in Colorectal Cancer Detection and/or Prognosis. World J. Gastroenterol. 2018, 24, 2327–2347. [Google Scholar] [CrossRef]
- Narayanan, V.; Peppelenbosch, M.P.; Konstantinov, S.R. Human Fecal Microbiome-Based Biomarkers for Colorectal Cancer. Cancer Prev. Res. 2014, 7, 1108–1111. [Google Scholar] [CrossRef]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The Microbiome in Cancer Immunotherapy: Diagnostic Tools and Therapeutic Strategies. Science (80-) 2018, 359, 1366–1370. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science (80-) 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Alcindor, T.; Beauger, N. Oxaliplatin: A Review in the Era of Molecularly Targeted Therapy. Curr. Oncol. 2011, 18, 18–25. [Google Scholar] [CrossRef]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal Microbiome Analyses Identify Melanoma Patients at Risk for Checkpoint-Blockade-Induced Colitis. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Wiesnoski, D.H.; Helmink, B.A.; Gopalakrishnan, V.; Choi, K.; DuPont, H.L.; Jiang, Z.D.; Abu-Sbeih, H.; Sanchez, C.A.; Chang, C.C.; et al. Fecal Microbiota Transplantation for Refractory Immune Checkpoint Inhibitor-Associated Colitis. Nat. Med. 2018, 24, 1804–1808. [Google Scholar] [CrossRef]
- Chen, S.; Yueh, M.F.; Bigo, C.; Barbier, O.; Wang, K.; Karin, M.; Nguyen, N.; Tukey, R.H. Intestinal Glucuronidation Protects against Chemotherapy-Induced Toxicity by Irinotecan (CPT-11). Proc. Natl. Acad. Sci. USA 2013, 110, 19143–19148. [Google Scholar] [CrossRef]
- Ding, C.; Tang, W.; Fan, X.; Wu, G. Intestinal Microbiota: A Novel Perspective in Colorectal Cancer Biotherapeutics. Onco. Targets. Ther. 2018, 11, 4797–4810. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.; Potenza, A.; Andriulli, A.; Pazienza, V. Exploring the Microbiota to Better Understand Gastrointestinal Cancers Physiology. Clin. Chem. Lab. Med. 2018, 56, 1400–1412. [Google Scholar] [CrossRef]
- Lin, X.B.; Dieleman, L.A.; Ketabi, A.; Bibova, I.; Sawyer, M.B.; Xue, H.; Field, C.J.; Baracos, V.E.; Gänzle, M.G. Irinotecan (CPT-11) Chemotherapy Alters Intestinal Microbiota in Tumour Bearing Rats. PLoS One 2012, 7, 39764. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, D.; Li, Z.; Jiang, H.; Li, J.; Ren, R.; Gao, X.; Li, J.; Wang, X.; Wang, W.; et al. The Fecal Microbiota of Patients with Pancreatic Ductal Adenocarcinoma and Autoimmune Pancreatitis Characterized by Metagenomic Sequencing. J. Transl. Med. 2021, 19, 215. [Google Scholar] [CrossRef]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium Nucleatum Infection Is Prevalent in Human Colorectal Carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Oral Microbiome and Pancreatic Cancer - Full Text View - ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03302637 (accessed on 16 July 2021).
- The Microbiome of Pancreatic Cancer: “PANDEMIC” Study - Full Text View - ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04274972 (accessed on 16 July 2021).
- Microbiome Analysis in esoPhageal, PancreatIc and Colorectal CaNcer Patients Undergoing Gastrointestinal Surgery - Full Text View - ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04189393 (accessed on 16 July 2021).
- A Prospective Translational Tissue Collection Study in Early and Advanced Pancreatic Ductal Adenocarcinoma and Pancreatic Neuroendocrine Tumours to Enable Further Disease Characterisation and the Development of Potential Predictive and Prognostic Biomarkers - Full Text View - ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03840460 (accessed on 16 July 2021).
- A Study of Live Biotherapeutic Product MRx0518 With Hypofractionated Radiation Therapy in Resectable Pancreatic Cancer - Full Text View - ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04193904 (accessed on 16 July 2021).
- MS-20 on Gut Microbiota and Risk/Severity of Cachexia in Pancreatic Cancer Patients - Full Text View - ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04600154 (accessed on 16 July 2021).
- The Mechanism of Enhancing the Anti-tumor Effects of CAR-T on PC by Gut Microbiota Regulation - Full Text View - ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04203459 (accessed on 16 July 2021).
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1-Based Immunotherapy against Epithelial Tumors. Science (80-) 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Terrisse, S.; Derosa, L.; Iebba, V.; Ghiringhelli, F.; Vaz-Luis, I.; Kroemer, G.; Fidelle, M.; Christodoulidis, S.; Segata, N.; Thomas, A.M.; et al. Intestinal Microbiota Influences Clinical Outcome and Side Effects of Early Breast Cancer Treatment. Cell Death Differ. 2021, 10, 1–19. [Google Scholar] [CrossRef]

| Study | Title | Study Design | Patients (n) | Aim | Study Status | Ref. |
|---|---|---|---|---|---|---|
| NCT03302637 | Oral Microbiome and Pancreatic Cancer | A prospective, observational, case-control study | 732 | To examine the relationship of the oral and pancreatic microbiome and their association with pancreatic cancer risk. | Completed | [159] |
| NCT04274972 | The Microbiome of Pancreatic Cancer: “PANDEMIC” Study | A prospective, observational, cohort study | 20 | To outline the pancreatic microbiome of patients with resectable PDAC undergoing pancreaticoduodenectomy and characterise the association between microbiome and post-operative complications. | Recruiting | [160] |
| NCT04189393 | Microbiome Analysis in Oesophageal, Pancreatic and Colorectal Cancer Patients Undergoing Gastrointestinal Surgery (MA-PPING) | A prospective, observational, cohort study | 60 | The study aims to map the oral and gut microbiome of patients diagnosed with pancreatic, oesophageal or colorectal cancer during their surgical patient journey from the moment of diagnosis until full recovery (three months after surgery). | Active, not recruiting | [161] |
| NCT03840460 | A Prospective Translational Tissue Collection Study in Early and Advanced Pancreatic Ductal Adenocarcinoma and Pancreatic Neuroendocrine Tumours to Enable Further Disease Characterisation and the Development of Potential Predictive and Prognostic Biomarkers (PaC-Man) | A prospective observational cohort study | 200 | This project studies the molecular makeup of pancreatic lesions and their microenvironment at various stages (from pre-cancerous lesions through to more advanced disease) to identify the molecular subtypes, biomarkers of response and toxicity and to investigate the particular intra-pancreatic colonising microorganisms. | Recruiting | [162] |
| NCT04193904 | A Study of Live Biotherapeutic Product MRx0518 With Hypofractionated Radiation Therapy in Resectable Pancreatic Cancer | An interventional open-label phase I study | 15 | A study to evaluate the safety and preliminary efficacy of MRx0518 with preoperative hypofractionated radiation in 15 patients with resectable pancreatic cancer. | Recruiting | [163] |
| NCT04600154 | MS-20 on Gut Microbiota and Risk/Severity of Cachexia in Pancreatic Cancer Patients | An interventional, randomised study | 40 | To analyse MS-20 effects on gut microbiota and risk/severity of cachexia in pancreatic cancer patients with a combination of chemotherapy and MS-20. | Active, recruiting | [164] |
| NCT04203459 | The Mechanism of Enhancing the Anti-tumour Effects of CAR-T on PC by Gut Microbiota Regulation | A prospective observational cohort study | 80 | To study the mechanism of enhancing the antitumor effects of human chimeric antigen receptor T cells on pancreatic cancer by gut microbiota regulation. | Recruiting | [165] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Rahman, R.; Lamarca, A.; Hubner, R.A.; Valle, J.W.; McNamara, M.G. The Microbiome as a Potential Target for Therapeutic Manipulation in Pancreatic Cancer. Cancers 2021, 13, 3779. https://doi.org/10.3390/cancers13153779
Abdul Rahman R, Lamarca A, Hubner RA, Valle JW, McNamara MG. The Microbiome as a Potential Target for Therapeutic Manipulation in Pancreatic Cancer. Cancers. 2021; 13(15):3779. https://doi.org/10.3390/cancers13153779
Chicago/Turabian StyleAbdul Rahman, Rozana, Angela Lamarca, Richard A. Hubner, Juan W. Valle, and Mairéad G. McNamara. 2021. "The Microbiome as a Potential Target for Therapeutic Manipulation in Pancreatic Cancer" Cancers 13, no. 15: 3779. https://doi.org/10.3390/cancers13153779
APA StyleAbdul Rahman, R., Lamarca, A., Hubner, R. A., Valle, J. W., & McNamara, M. G. (2021). The Microbiome as a Potential Target for Therapeutic Manipulation in Pancreatic Cancer. Cancers, 13(15), 3779. https://doi.org/10.3390/cancers13153779






