Doxorubicin-Loaded Mixed Micelles Using Degradable Graft and Diblock Copolymers to Enhance Anticancer Sensitivity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.2. Synthesis and Characterization of mPEG-b-PLA
2.3. Synthesis and Characterization of P(HPMA-Lac-co-His)-g-PLA
2.4. Preparation and Characterization of Polymeric Mixed Micelles
2.5. Drug Loading and Characterization
- Drug content (%w/w) = (weight of Dox)/(weight of Dox-mixed micelle) × 100%;
- Encapsulation efficiency (%w/w) = (weight of Dox)/(weight of infeed Dox) × 100%.
2.6. Drug Release Profiles of Dox-Mixed Micelles
2.7. Cytotoxicity Assessment
2.8. Observation of the Intracellular Drug-Releasing Behaviors in Cancer Cells and Internalization
2.9. Biodistributions and Tumor Accumulation
2.10. In Vivo Antitumor Activity and Toxicity Assessment
3. Results and Discussion
3.1. Polymer Characterizations
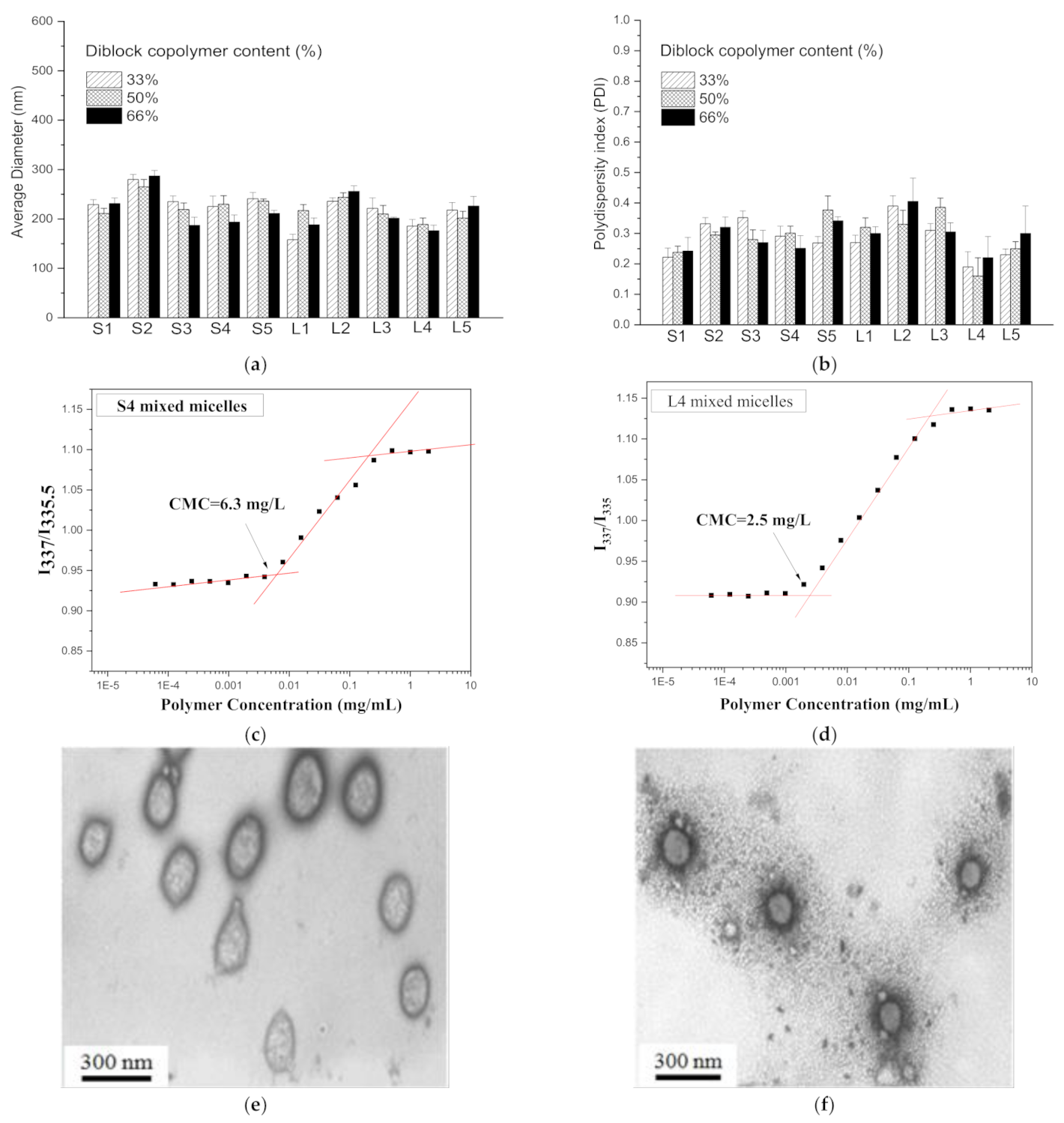
3.2. Preparation and Characteristics of Mixed Micelles
3.3. Stability and pH-Responsiveness
3.4. Cytotoxic Assessment
3.5. Observation of the Intracellular Drug-Releasing Behaviors in Cancer Cells and Internalization
3.6. In Vivo Bio-Distribution and Tumor Accumulation
3.7. In Vivo Antitumor Activity
3.8. Tumor Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Attia, A.B.E.; Ong, Z.Y.; Hedrick, J.L.; Lee, P.P.; Ee, P.L.R.; Hammond, P.T.; Yang, Y.-Y. Mixed micelles self-assembled from block copolymers for drug delivery. Curr. Opin. Colloid Interface Sci. 2011, 16, 182–194. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lo, C.L.; Hsiue, G.H. Multifunctional nanomicellar systems for delivering anticancer drugs. J. Biomed. Mater. Res. Part. A 2014, 102, 2024–2038. [Google Scholar] [CrossRef]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Kwon, G.S.; Kataoka, K. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 2012, 64, 237–245. [Google Scholar] [CrossRef]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer: Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Gerweck, L.E.; Seetharaman, K. Cellular pH gradient in tumor versus normal tissue: Potential exploitation for the treatment of cancer. Cancer Res. 1996, 56, 1194–1198. [Google Scholar]
- Zhang, X.; Lin, Y.; Gillies, R.J. Tumor pH and its measurement. J. Nucl. Med. 2010, 51, 1167–1170. [Google Scholar] [CrossRef] [Green Version]
- Gruenberg, J.; Howell, K.E. Membrane traffic in endocytosis: Insights from cell-free assays. Annu. Rev. Cell Biol. 1989, 5, 453–481. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, L.; Torchilin, V.P. pH-sensitive poly (histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials 2013, 34, 1213–1222. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Lee, E.S.; Kim, D.; Lee, K.H.; Oh, K.T.; Bae, Y.H. Physicochemical characteristics of pH-sensitive poly (L-histidine)-b-poly (ethylene glycol)/poly (l-lactide)-b-poly (ethylene glycol) mixed micelles. J. Control. Release 2008, 126, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, K.T.; Lee, E.S.; Kim, D.; Bae, Y.H. L-histidine-based pH-sensitive anticancer drug carrier micelle: Reconstitution and brief evaluation of its systemic toxicity. Int. J. Pharm. 2008, 358, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Kopecek, J.; Kopecková, P.; Minko, T.; Lu, Z.R.; Peterson, C.M. Water soluble polymers in tumor targeted delivery. J. Control. Release 2001, 74, 147–158. [Google Scholar] [CrossRef]
- Kopeček, J.; Kopečková, P. HPMA copolymers: Origins, early developments, present, and future. Adv. Drug Deliv. Rev. 2010, 62, 122–149. [Google Scholar] [CrossRef] [Green Version]
- Soga, O.; van Nostrum, C.F.; Ramzi, A.; Visser, T.; Soulimani, F.; Frederik, P.M.; Bomans, P.H.H.; Hennink, W.E. Physicochemical Characterization of Degradable Thermosensitive Polymeric Micelles. Langmuir 2004, 20, 9388–9395. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Liao, L.-C.; Lu, P.-L.; Lo, C.-L.; Tsai, H.-C.; Huang, C.-Y.; Wei, K.-C.; Yen, T.-C.; Hsiue, G.-H. The accumulation of dual pH and temperature responsive micelles in tumors. Biomaterials 2012, 33, 4576–4588. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lo, C.-L.; Lin, Y.-F.; Hsiue, G.-H. Rapamycin encapsulated in dual-responsive micelles for cancer therapy. Biomaterials 2013, 34, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Soga, O.; van Nostrum, C.F.; Fens, M.; Rijcken, C.J.F.; Schiffelers, R.M.; Storm, G.; Hennink, W.E. Thermosensitive and biodegradable polymeric micelles for paclitaxel delivery. J. Control. Release 2005, 103, 341–353. [Google Scholar] [CrossRef]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef] [Green Version]
- Papisov, M.I. Theoretical considerations of RES-avoiding liposomes: Molecular mechanics and chemistry of liposome interactions. Adv. Drug Deliv. Rev. 1998, 32, 119–138. [Google Scholar] [CrossRef]
- Gref, R.; Domb, A.; Quellec, P.; Blunk, T.; Müller, R.H.; Verbavatz, J.M.; Langer, R. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv. Drug Deliv. Rev. 1995, 16, 215–233. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.-L.; Chen, Y.-C.; Ou, T.-W.; Chen, H.-H.; Tsai, H.-C.; Wen, C.-J.; Lo, C.-L.; Wey, S.-P.; Lin, K.-J.; Yen, T.-C.; et al. Multifunctional hollow nanoparticles based on graft-diblock copolymers for doxorubicin delivery. Biomaterials 2011, 32, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-L.; Huang, C.-K.; Lin, K.-M.; Hsiue, G.-H. Mixed micelles formed from graft and diblock copolymers for application in intracellular drug delivery. Biomaterials 2007, 28, 1225–1235. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Chang, W.-H.; Lo, C.-L.; Tsai, C.-H.; Chang, C.-H.; Ou, T.-W.; Yen, T.-C.; Hsiue, G.-H. Graft and diblock copolymer multifunctional micelles for cancer chemotherapy and imaging. Biomaterials 2010, 31, 2293–2301. [Google Scholar] [CrossRef]
- Neradovic, D.; van Nostrum, C.F.; Hennink, W.E. Thermoresponsive Polymeric Micelles with Controlled Instability Based on Hydrolytically Sensitive N-Isopropylacrylamide Copolymers. Macromolecules 2001, 34, 7589–7591. [Google Scholar] [CrossRef]
- Soga, O.; van Nostrum, C.F.; Hennink, W.E. Poly(N-(2-hydroxypropyl) Methacrylamide Mono/Di Lactate): A New Class of Biodegradable Polymers with Tuneable Thermosensitivity. Biomacromolecules 2004, 5, 818–821. [Google Scholar] [CrossRef]
- Lee, E.S.; Na, K.; Bae, Y.H. Super pH-Sensitive Multifunctional Polymeric Micelle. Nano Lett. 2005, 5, 325–329. [Google Scholar] [CrossRef]
- Lo, C.-L.; Lin, S.-J.; Tsai, H.-C.; Chan, W.-H.; Tsai, C.-H.; Cheng, C.-H.D.; Hsiue, G.-H. Mixed micelle systems formed from critical micelle concentration and temperature-sensitive diblock copolymers for doxorubicin delivery. Biomaterials 2009, 30, 3961–3970. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Hsu, W.-H.; Lo, J.-M.; Tsai, H.-C.; Hsiue, G.-H. Novel geometry type of nanocarriers mitigated the phagocytosis for drug delivery. J. Control. Release 2011, 154, 84–92. [Google Scholar] [CrossRef]
- Ray, G.B.; Chakraborty, I.; Moulik, S.P. Pyrene absorption can be a convenient method for probing critical micellar concentration (cmc) and indexing micellar polarity. J. Colloid Interface Sci. 2006, 294, 248–254. [Google Scholar]
- Opanasopit, P.; Yokoyama, M.; Watanabe, M.; Kawano, K.; Maitani, Y.; Okano, T. Influence of serum and albumins from different species on stability of camptothecin-loaded micelles. J. Control. Release 2005, 104, 313–321. [Google Scholar] [CrossRef]
- Kumari, P.; Muddineti, O.S.; Rompicharla, S.V.K.; Ghanta, P.; BBN, A.K.; Ghosh, B.; Biswas, S. Cholesterol-conjugated poly (d,l-lactide)-based micelles as a nanocarrier system for effective delivery of curcumin in cancer therapy. Drug Deliv. 2017, 24, 209–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Haag, R.; Kratz, F. Polymer Therapeutics: Concepts and Applications. Angew. Chem. Int. Ed. 2006, 45, 1198–1215. [Google Scholar] [CrossRef]
- Tannock, I.F.; Rotin, D. Acid pH in Tumors and Its Potential for Therapeutic Exploitation. Cancer Res. 1989, 49, 4373–4384. [Google Scholar]
- Barraud, L.; Merle, P.; Soma, E.; Lefrançois, L.; Guerret, S.; Chevallier, M.; Dubernet, C.; Couvreur, P.; Trépo, C.; Vitvitski, L. Increase of doxorubicin sensitivity by doxorubicin-loading into nanoparticles for hepatocellular carcinoma cells in vitro and in vivo. J. Hepatol. 2005, 42, 736–743. [Google Scholar] [CrossRef]
- Melguizo, C.; Cabeza, L.; Prados, J.; Ortiz, R.; Caba, O.; Rama, A.R.; Delgado, Á.V.; Arias, J.L. Enhanced antitumoral activity of doxorubicin against lung cancer cells using biodegradable poly (butylcyanoacrylate) nanoparticles. Drug Des. Dev. Ther. 2015, 9, 6433. [Google Scholar]
- Yoshimoto, Y.; Kawada, M.; Ikeda, D.; Ishizuka, M. Involvement of doxorubicin-induced Fas expression in the antitumor effect of doxorubicin on Lewis lung carcinoma in vivo. Int. Immunopharmacol. 2005, 5, 281–288. [Google Scholar] [CrossRef]
- He, Y.; Su, Z.; Xue, L.; Xu, H.; Zhang, C. Co-delivery of erlotinib and doxorubicin by pH-sensitive charge conversion nanocarrier for synergistic therapy. J. Control. Release 2016, 229, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.H.; Lewis, A.D.; Ehsan, M.N.; Sikic, B.I. Multifactorial mechanisms associated with broad cross-resistance of ovarian carcinoma cells selected by cyanomorpholino doxorubicin. Cancer Res. 1991, 51, 5181–5187. [Google Scholar]
- Goren, D.; Horowitz, A.T.; Tzemach, D.; Tarshish, M.; Zalipsky, S.; Gabizon, A. Nuclear Delivery of Doxorubicin via Folate-targeted Liposomes with Bypass of Multidrug-resistance Efflux Pump. Clin. Cancer. Res. 2000, 6, 1949–1957. [Google Scholar]
- Li, Y.-L.; Zhu, L.; Liu, Z.; Cheng, R.; Meng, F.; Cui, J.-H.; Ji, S.-J.; Zhong, Z. Reversibly Stabilized Multifunctional Dextran Nanoparticles Efficiently Deliver Doxorubicin into the Nuclei of Cancer Cells. Angew. Chem. Int. Ed. 2009, 48, 9914–9918. [Google Scholar] [CrossRef]
- Odom, A.L.; Hatwig, C.A.; Stanley, J.S.; Benson, A.M. Biochemical determinants of adriamycin® toxicity in mouse liver, heart and intestine. Biochem. Pharmacol. 1992, 43, 831–836. [Google Scholar] [CrossRef]
- Bae, Y.; Kataoka, K. Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers. Adv. Drug Deliv. Rev. 2009, 61, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Bertram, J.S.; Janik, P. Establishment of a cloned line of Lewis lung carcinoma cells adapted to cell culture. Cancer Lett. 1980, 11, 63–73. [Google Scholar] [CrossRef]
- Wilmanns, C.; Fan, D.; O’Brian, C.A.; Bucana, C.D.; Fidler, I.J. Orthotopic and ectopic organ environments differentially influence the sensitivity of murine colon carcinoma cells to doxorubicin and 5-fluorouracil. Int. J. Cancer 1992, 52, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Feleszko, W.; Młynarczuk, I.; Bałkowiec-Iskra, E.Z.; Czajka, A.; Świtaj, T.; Stokłosa, T.; Giermasz, A.; Jakóbisiak, M. Lovastatin potentiates antitumor activity and attenuates cardiotoxicity of doxorubicin in three tumor models in mice. Clin. Cancer Res. 2000, 6, 2044–2052. [Google Scholar] [PubMed]
- Hirai, K.; Sasahira, T.; Ohmori, H.; Fujii, K.; Kuniyasu, H. Inhibition of heme oxygenase-1 by zinc protoporphyrin IX reduces tumor growth of LL/2 lung cancer in C57BL mice. Int. J. Cancer 2007, 120, 500–505. [Google Scholar] [CrossRef]
- Cabeza, L.; Ortiz, R.; Prados, J.; Delgado, Á.V.; Martín-Villena, M.J.; Clares, B.; Perazzoli, G.; Entrena, J.M.; Melguizo, C.; Arias, J.L. Improved antitumor activity and reduced toxicity of doxorubicin encapsulated in poly (ε-caprolactone) nanoparticles in lung and breast cancer treatment: An in vitro and in vivo study. Eur. J. Pharm. Sci. 2017, 102, 24–34. [Google Scholar] [CrossRef]
- Moodley, T.; Singh, M. Current stimuli-responsive mesoporous silica nanoparticles for cancer therapy. Pharmaceutics 2021, 13, 71. [Google Scholar] [CrossRef]
- Rijcken, C.J.; Snel, C.J.; Schiffelers, R.M.; van Nostrum, C.F.; Hennink, W.E. Hydrolysable core-crosslinked thermosensitive polymeric micelles: Synthesis, characterisation and in vivo studies. Biomaterials 2007, 28, 5581–5593. [Google Scholar] [CrossRef]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia Enables Tumor-specific Nanoparticle Delivery: Effect of Particle Size. Cancer Res. 2000, 60, 4440–4445. [Google Scholar] [PubMed]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Del. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, W.; Lock, L.L.; Tang, J.; Sui, M.; Sun, M.; Cui, H.; Xu, D.; Shen, Y. The role of micelle size in tumor accumu-lation, penetration, and treatment. ACS Nano 2015, 9, 7195–7206. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-Selective Delivery of Macromolecular Drugs via the EPR Effect: Background and Future Prospects. Bioconjugate Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Chain Length Recognition: Core-Shell Supramolecular Assembly from Oppositely Charged Block Copolymers. Science 1999, 283, 65–67. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef]
- Manchun, S.; Dass, C.R.; Sriamornsak, P. Targeted therapy for cancer using pH-responsive nanocarrier systems. Life Sci. 2012, 90, 381–387. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Tsai, C.-H.; Lin, S.-Y.; Jhang, C.-R.; Chiang, Y.-S.; Hsiue, G.-H. Stimulated release of photosensitizers from graft and diblock micelles for photodynamic therapy. Biomaterials 2012, 33, 1827–1837. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Chang, C.-J.; Hsiue, G.-H.; Chiang, Y.-T. Doxorubicin-Loaded Mixed Micelles Using Degradable Graft and Diblock Copolymers to Enhance Anticancer Sensitivity. Cancers 2021, 13, 3816. https://doi.org/10.3390/cancers13153816
Chen Y-C, Chang C-J, Hsiue G-H, Chiang Y-T. Doxorubicin-Loaded Mixed Micelles Using Degradable Graft and Diblock Copolymers to Enhance Anticancer Sensitivity. Cancers. 2021; 13(15):3816. https://doi.org/10.3390/cancers13153816
Chicago/Turabian StyleChen, Yi-Chun, Chang-Jung Chang, Ging-Ho Hsiue, and Yi-Ting Chiang. 2021. "Doxorubicin-Loaded Mixed Micelles Using Degradable Graft and Diblock Copolymers to Enhance Anticancer Sensitivity" Cancers 13, no. 15: 3816. https://doi.org/10.3390/cancers13153816
APA StyleChen, Y. -C., Chang, C. -J., Hsiue, G. -H., & Chiang, Y. -T. (2021). Doxorubicin-Loaded Mixed Micelles Using Degradable Graft and Diblock Copolymers to Enhance Anticancer Sensitivity. Cancers, 13(15), 3816. https://doi.org/10.3390/cancers13153816







