Muscle-Derived Cytokines Reduce Growth, Viability and Migratory Activity of Pancreatic Cancer Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
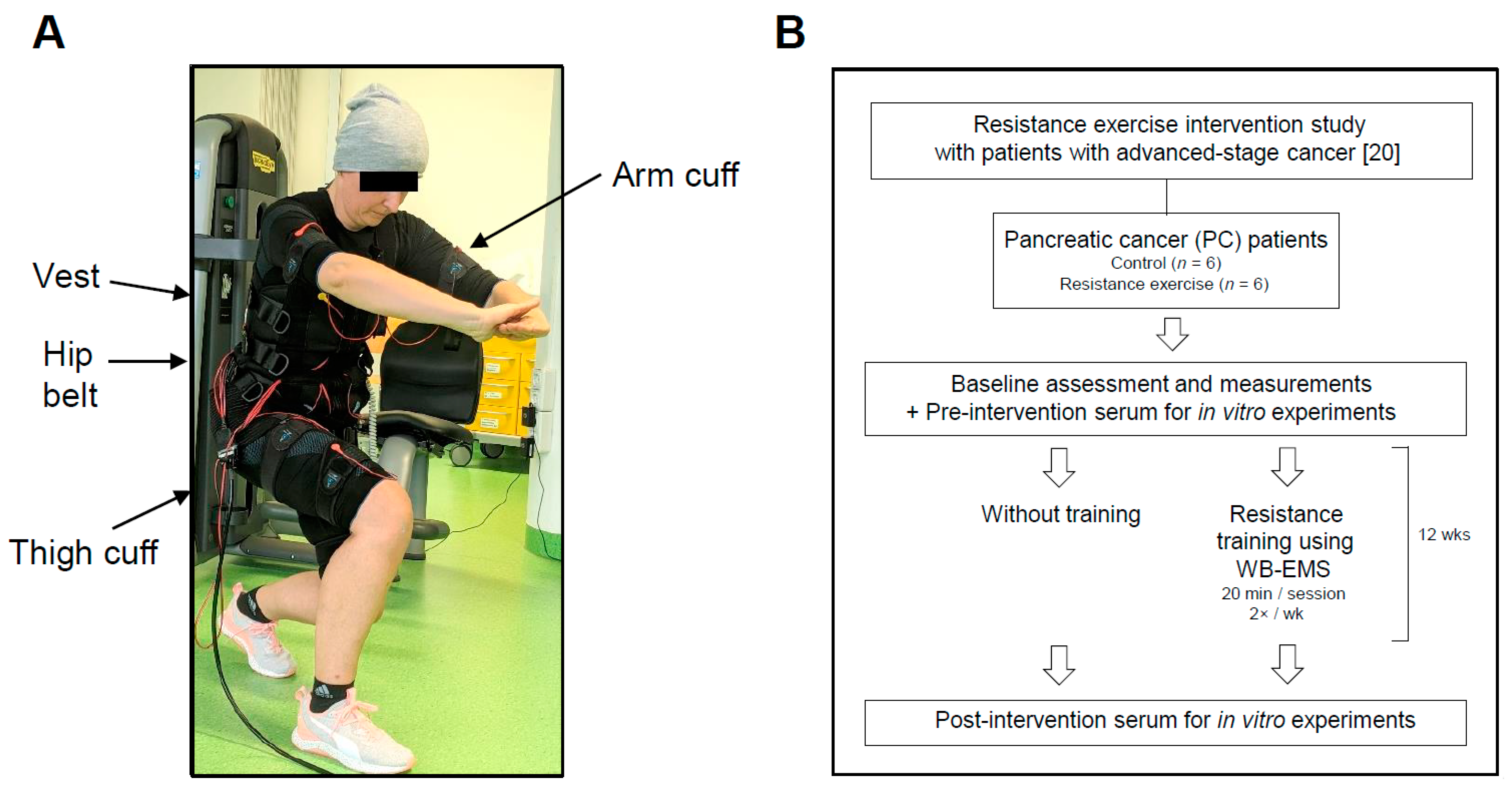
2.2. Clinical Study Design, Patient Recruitment and Exercise Intervention
2.3. Patient Serum
2.4. Cell Culture
2.5. Human Cancer Cell Growth and Apoptosis Assays
2.6. Colony Formation Assay
2.7. Scratch Wounding Assay
2.8. Electric Pulse Stimulation
2.9. Quantitative Reverse Transcription PCR (qRT-PCR)
2.10. Primer Sequences
2.11. Cytokine Array
2.12. Cytokine Enzyme-Linked Immunosorbent Assay (ELISA)
2.13. Immunoblotting
2.14. Statistical Analysis
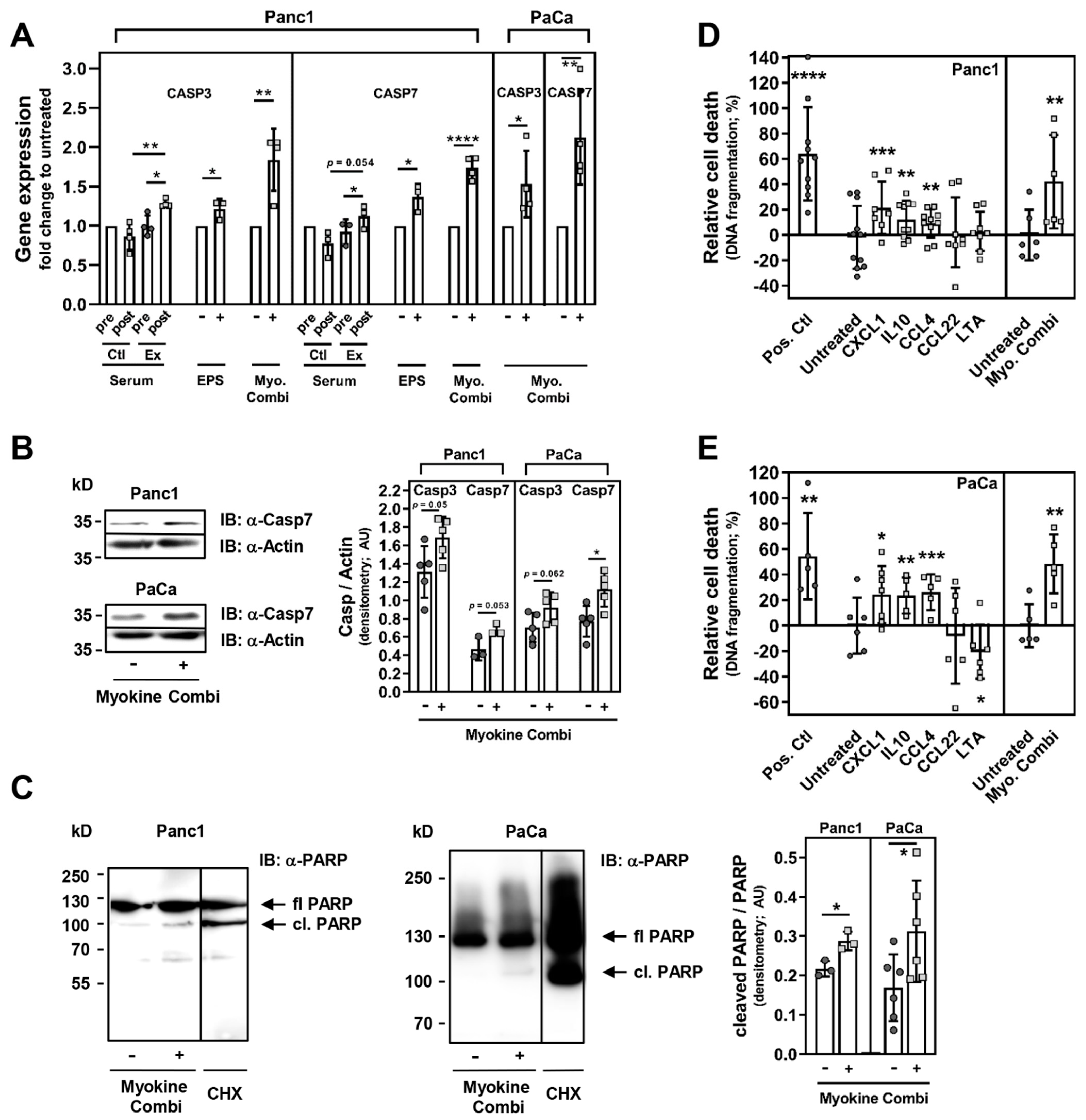
3. Results
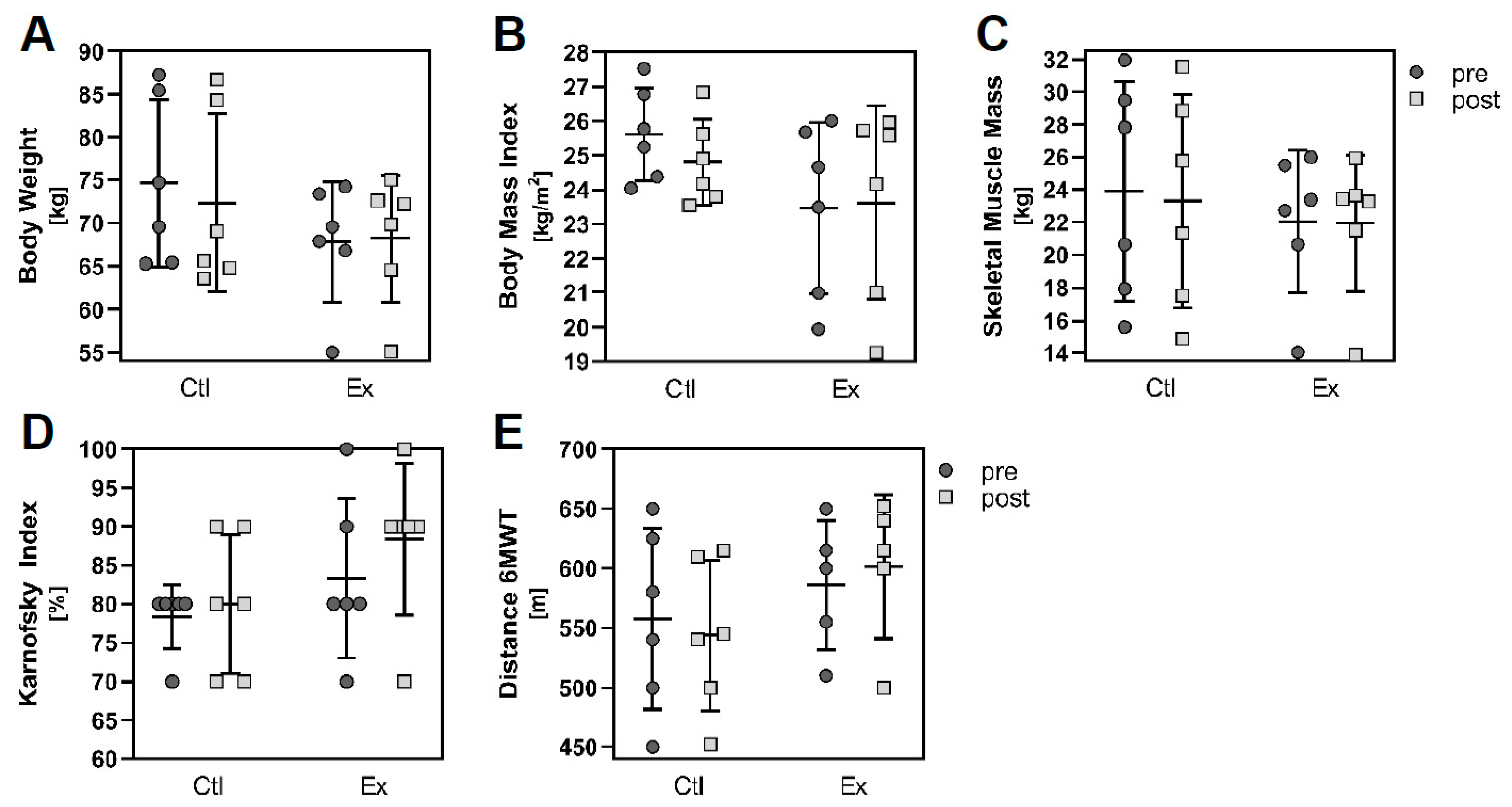
3.1. Study Design and Patient Data
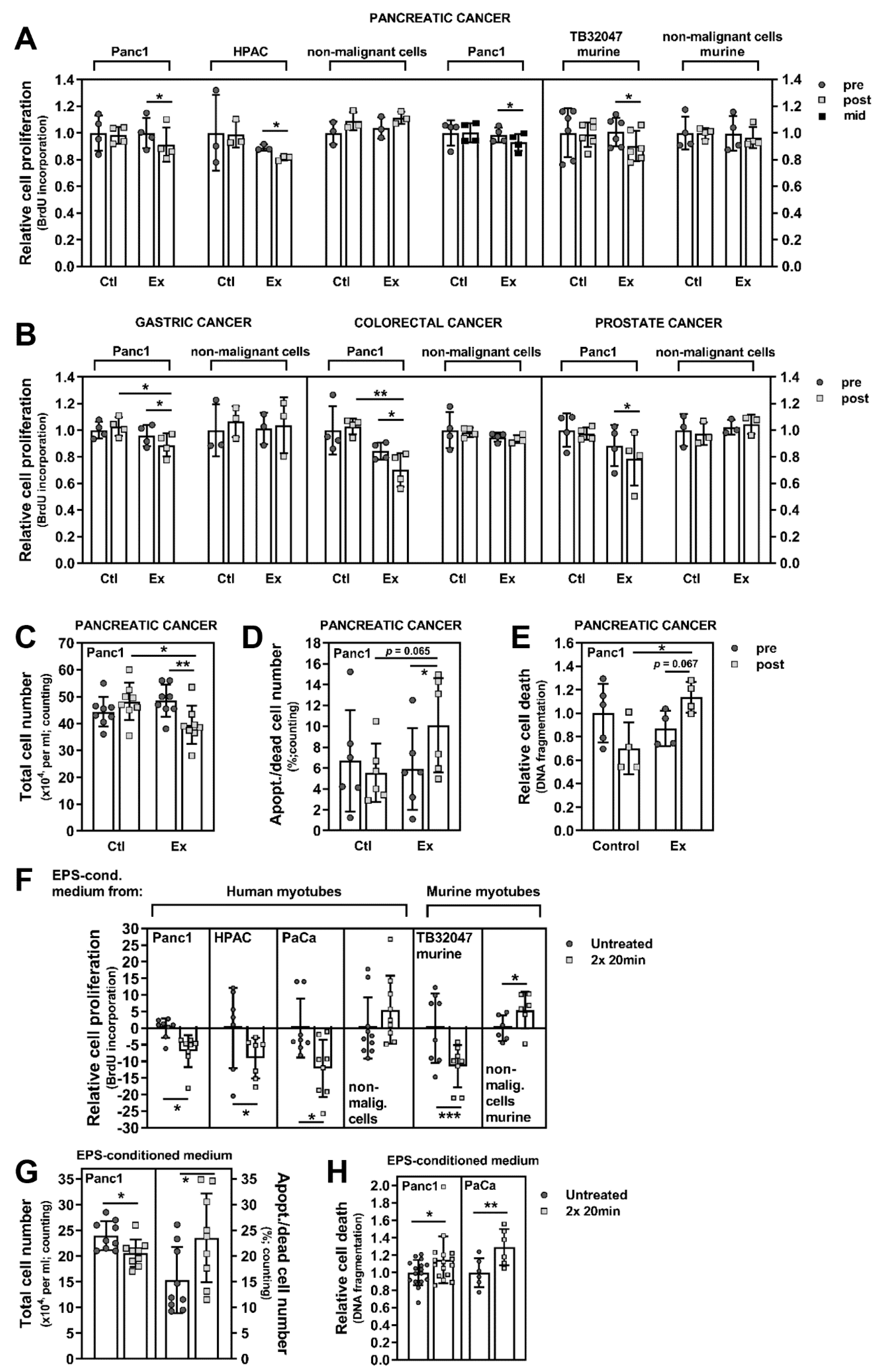
3.2. Exercise Stimuli Affect PC Cell Growth and Viability
3.3. Exercise-Induced Myokines Control PC Cell Proliferation
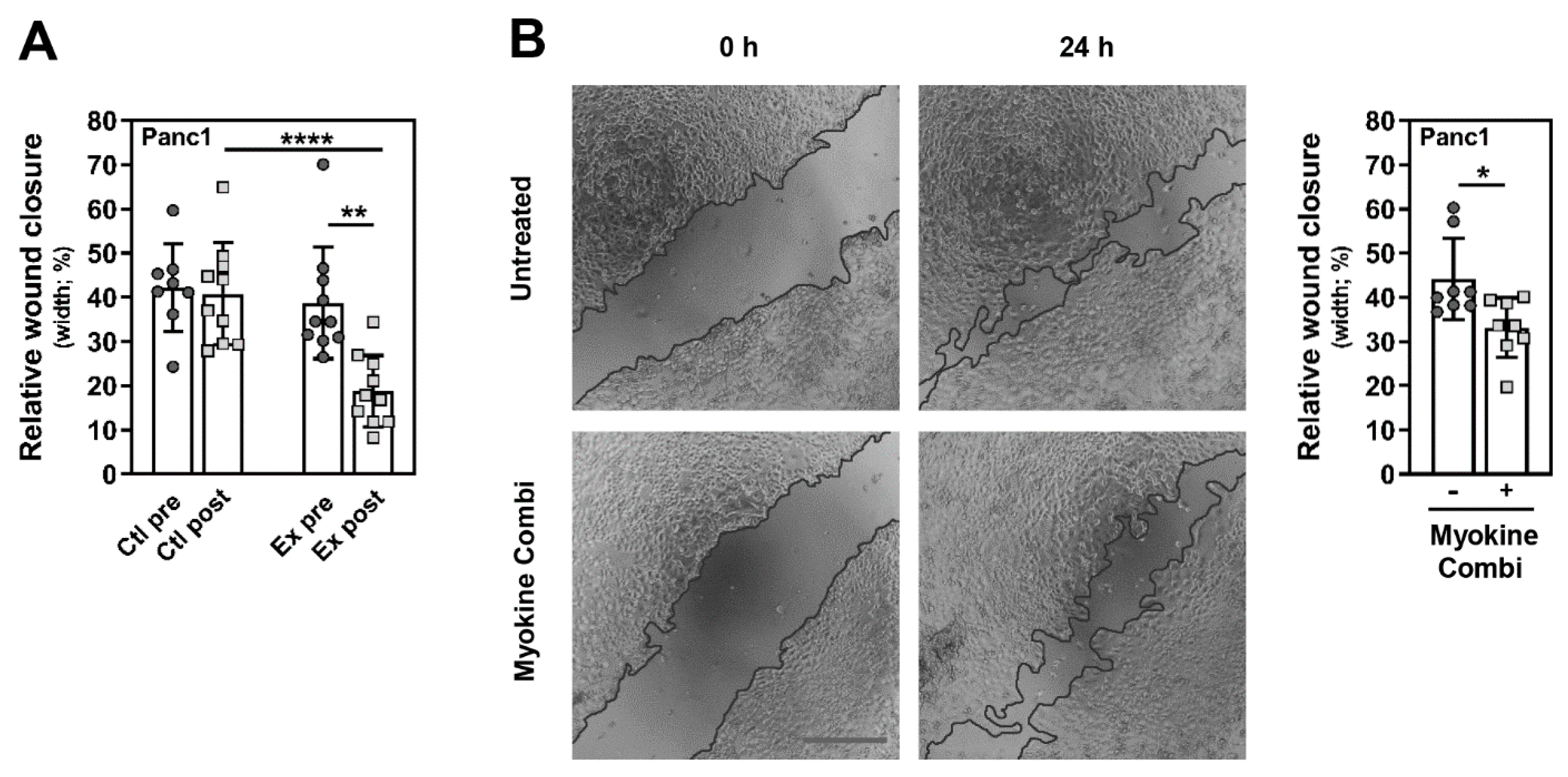
3.4. Myokine-Mediated Regulation of PC Cell Migration
3.5. CXCL1, IL10 and CCL4 Induce PC Cell Death
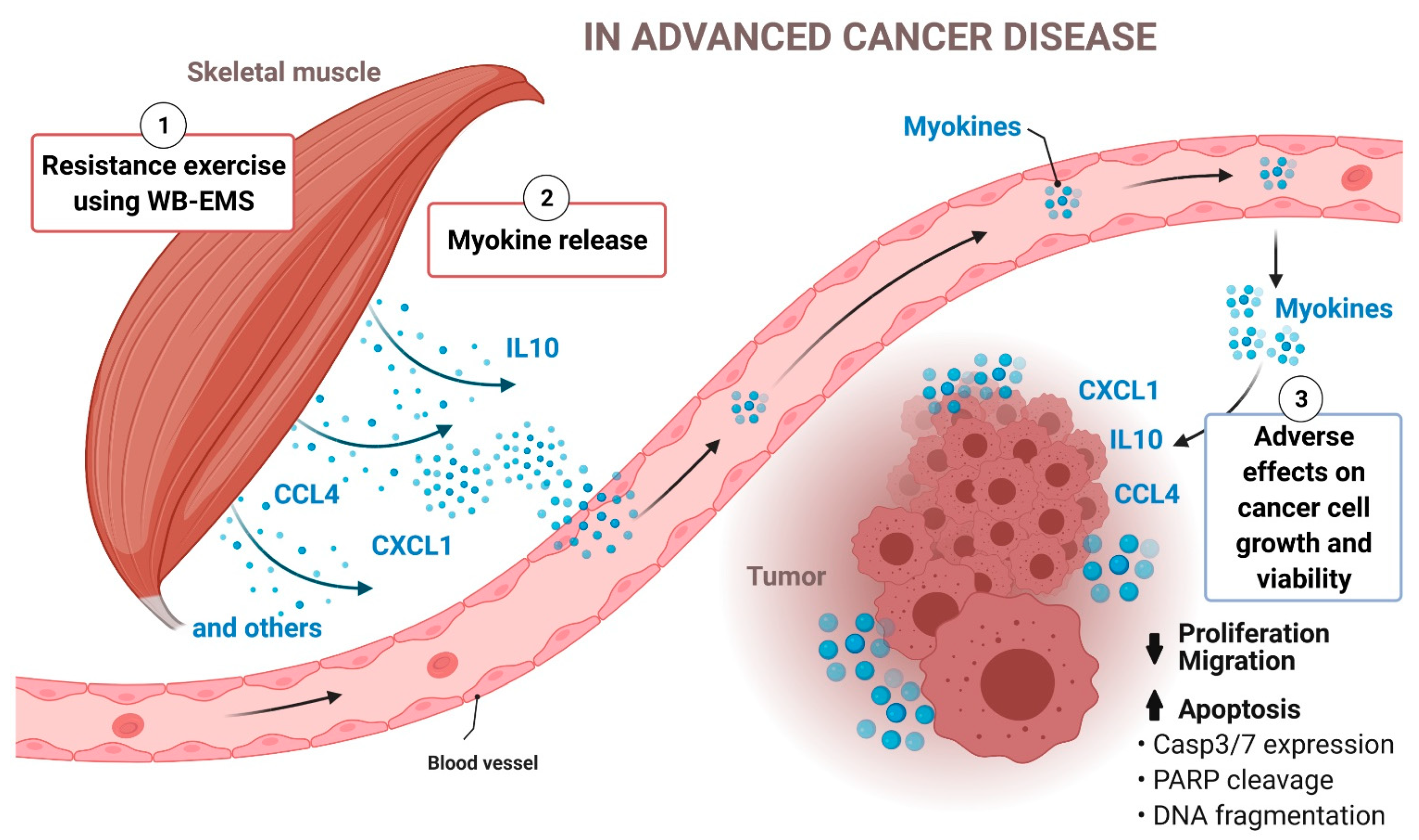
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Brown, J.C.; Gilmore, L.A. Physical Activity Reduces the Risk of Recurrence and Mortality in Cancer Patients. Exerc. Sport Sci. Rev. 2020, 48, 67–73. [Google Scholar] [CrossRef]
- Hojman, P.; Dethlefsen, C.; Brandt, C.; Hansen, J.; Pedersen, L.; Pedersen, B.K. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am. J. Physiol. Endocrinol. Metab. 2011, 301, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Chowdhury, S.; Roy, H.K. Exercise-induced myokines as emerging therapeutic agents in colorectal cancer prevention and treatment. Future Oncol. 2018, 14, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, R. Exercise-Induced Myokines with Therapeutic Potential for Muscle Wasting. Front. Physiol. 2019, 10, 287. [Google Scholar] [CrossRef]
- Karstoft, K.; Pedersen, B.K. Skeletal muscle as a gene regulatory endocrine organ. Curr. Opin. Clin. Nutr. Meta. B Care 2016, 19, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Dieli-Conwright, C.M.; Parmentier, J.H.; Sami, N.; Lee, K.; Spicer, D.; Mack, W.J.; Sattler, F.; Mittelman, S.D. Adipose tissue inflammation in breast cancer survivors: Effects of a 16-week combined aerobic and resistance exercise training intervention. Breast Cancer Res. Treat. 2018, 168, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; Keller, C.; Starkie, R.L.; Osada, T.; Febbraio, M.A.; Pedersen, B.K. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2002, 283, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Klarlund Pedersen, B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Hiscock, N.; Sacchetti, M.; Fischer, C.P.; Pedersen, B.K. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 2004, 53, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Takagi, T.; Tanimura, Y.; Takanami, Y.; Kawai, Y.; Sakuma, K.; Hang, L.P.; Mizushima, K.; Hirai, Y.; et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 2013, 62, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.H.; Barnard, R.J.; Tymchuk, C.N.; Cohen, P.; Aronson, W.J. Effect of diet and exercise on serum insulin, IGF-I, and IGFBP-1 levels and growth of LNCaP cells in vitro (United States). Cancer Causes Control. 2002, 13, 929–935. [Google Scholar] [CrossRef]
- Rundqvist, H.; Augsten, M.; Stromberg, A.; Rullman, E.; Mijwel, S.; Kharaziha, P.; Panaretakis, T.; Gustafsson, T.; Ostman, A. Effect of acute exercise on prostate cancer cell growth. PLoS ONE 2013, 8, e67579. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, Y.; Liu, Y.; Chen, Y. Irisin Enhances Doxorubicin-Induced Cell Apoptosis in Pancreatic Cancer by Inhibiting the PI3K/AKT/NF-kappaB Pathway. Med. Sci. Monit. 2019, 25, 6085–6096. [Google Scholar] [CrossRef]
- Liu, J.; Song, N.; Huang, Y.; Chen, Y. Irisin inhibits pancreatic cancer cell growth via the AMPK-mTOR pathway. Sci. Rep. 2018, 8, 15247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, P.; Li, L.; Tang, N.; Huang, F.; Kong, X.; Tan, X.; Shi, G. Irisin functions to inhibit malignant growth of human pancreatic cancer cells via downregulation of the PI3K/AKT signaling pathway. Onco. Targets. Ther. 2019, 12, 7243–7249. [Google Scholar] [CrossRef]
- Shao, L.; Li, H.; Chen, J.; Song, H.; Zhang, Y.; Wu, F.; Wang, W.; Zhang, W.; Wang, F.; Li, H.; et al. Irisin suppresses the migration, proliferation, and invasion of lung cancer cells via inhibition of epithelial-to-mesenchymal transition. Biochem. Biophys. Res. Commun. 2017, 485, 598–605. [Google Scholar] [CrossRef]
- Schwartz, A.L.; de Heer, H.D.; Bea, J.W. Initiating Exercise Interventions to Promote Wellness in Cancer Patients and Survivors. Oncology 2017, 31, 711–717. [Google Scholar]
- Schink, K.; Herrmann, H.J.; Schwappacher, R.; Meyer, J.; Orlemann, T.; Waldmann, E.; Wullich, B.; Kahlmeyer, A.; Fietkau, R.; Lubgan, D.; et al. Effects of whole-body electromyostimulation combined with individualized nutritional support on body composition in patients with advanced cancer: A controlled pilot trial. BMC Cancer 2018, 18, 886. [Google Scholar] [CrossRef]
- Schwappacher, R.; Schink, K.; Sologub, S.; Dieterich, W.; Reljic, D.; Friedrich, O.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Physical activity and advanced cancer: Evidence of exercise-sensitive genes regulating prostate cancer cell proliferation and apoptosis. J. Physiol. 2020, 598, 3871–3889. [Google Scholar] [CrossRef]
- Kemmler, W.; Engelke, K.; von Stengel, S. Effects of Whole-Body-Electromyostimulation on Sarcopenia in Lean, Elderly Sedentary Women. The TEST-III Study. Deutsche Zeitschrift fur Sportmedizin 2012, 63, 343–350. [Google Scholar] [CrossRef]
- Ruckert, F.; Aust, D.; Bohme, I.; Werner, K.; Brandt, A.; Diamandis, E.P.; Krautz, C.; Hering, S.; Saeger, H.D.; Grutzmann, R.; et al. Five primary human pancreatic adenocarcinoma cell lines established by the outgrowth method. J. Surg. Res. 2012, 172, 29–39. [Google Scholar] [CrossRef]
- Pal, K.; Pletnev, A.A.; Dutta, S.K.; Wang, E.; Zhao, R.; Baral, A.; Yadav, V.K.; Aggarwal, S.; Krishnaswamy, S.; Alkharfy, K.M.; et al. Inhibition of endoglin-GIPC interaction inhibits pancreatic cancer cell growth. Mol. Cancer Ther. 2014, 13, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Brunton, H.; Caligiuri, G.; Cunningham, R.; Upstill-Goddard, R.; Bailey, U.M.; Garner, I.M.; Nourse, C.; Dreyer, S.; Jones, M.; Moran-Jones, K.; et al. HNF4A and GATA6 Loss Reveals Therapeutically Actionable Subtypes in Pancreatic Cancer. Cell Rep. 2020, 31, 107625. [Google Scholar] [CrossRef] [PubMed]
- Hardie, R.A.; van Dam, E.; Cowley, M.; Han, T.L.; Balaban, S.; Pajic, M.; Pinese, M.; Iconomou, M.; Shearer, R.F.; McKenna, J.; et al. Mitochondrial mutations and metabolic adaptation in pancreatic cancer. Cancer Metab. 2017, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Hendifar, A.E.; Petzel, M.Q.B.; Zimmers, T.A.; Denlinger, C.S.; Matrisian, L.M.; Picozzi, V.J.; Rahib, L. Precision Promise Consortium. Pancreas Cancer-Associated Weight Loss. Oncologist 2019, 24, 691–701. [Google Scholar] [CrossRef]
- Naudin, S.; Viallon, V.; Hashim, D.; Freisling, H.; Jenab, M.; Weiderpass, E.; Perrier, F.; McKenzie, F.; Bueno-de-Mesquita, H.B.; Olsen, A.; et al. Healthy lifestyle and the risk of pancreatic cancer in the EPIC study. Eur. J. Epidemiol. 2020, 35, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, N.; Gorgens, S.W.; Thoresen, G.H.; Aas, V.; Eckel, J.; Eckardt, K. Electrical pulse stimulation of cultured skeletal muscle cells as a model for in vitro exercise—Possibilities and limitations. Acta Physiol. 2017, 220, 310–331. [Google Scholar] [CrossRef]
- Scheler, M.; Irmler, M.; Lehr, S.; Hartwig, S.; Staiger, H.; Al-Hasani, H.; Beckers, J.; de Angelis, M.H.; Haring, H.U.; Weigert, C. Cytokine response of primary human myotubes in an in vitro exercise model. Am. J. Physiol. Cell. Physiol. 2013, 305, C877–C886. [Google Scholar] [CrossRef] [PubMed]
- Pourteymour, S.; Eckardt, K.; Holen, T.; Langleite, T.; Lee, S.; Jensen, J.; Birkeland, K.I.; Drevon, C.A.; Hjorth, M. Global mRNA sequencing of human skeletal muscle: Search for novel exercise-regulated myokines. Mol. Metab. 2017, 6, 352–365. [Google Scholar] [CrossRef]
- Raschke, S.; Eckardt, K.; Bjorklund Holven, K.; Jensen, J.; Eckel, J. Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLoS ONE 2013, 8, 62008. [Google Scholar] [CrossRef]
- Pedersen, L.; Olsen, C.H.; Pedersen, B.K.; Hojman, P. Muscle-derived expression of the chemokine CXCL1 attenuates diet-induced obesity and improves fatty acid oxidation in the muscle. Am. J. Physiol. Endocrinol. Metab. 2012, 302, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Nedachi, T.; Hatakeyama, H.; Kono, T.; Sato, M.; Kanzaki, M. Characterization of contraction-inducible CXC chemokines and their roles in C2C12 myocytes. Am. J. Physiol. Endocrinol. Metab. 2009, 297, 866–878. [Google Scholar] [CrossRef]
- Tarum, J.; Folkesson, M.; Atherton, P.J.; Kadi, F. Electrical pulse stimulation: An in vitro exercise model for the induction of human skeletal muscle cell hypertrophy. A proof-of-concept study. Exp. Physiol. 2017, 102, 1405–1413. [Google Scholar] [CrossRef]
- Burch, N.; Arnold, A.S.; Item, F.; Summermatter, S.; Brochmann Santana Santos, G.; Christe, M.; Boutellier, U.; Toigo, M.; Handschin, C. Electric pulse stimulation of cultured murine muscle cells reproduces gene expression changes of trained mouse muscle. PLoS ONE 2010, 5, 10970. [Google Scholar] [CrossRef]
- Nedachi, T.; Fujita, H.; Kanzaki, M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 295, 1191–1204. [Google Scholar] [CrossRef]
- Nikolic, N.; Bakke, S.S.; Kase, E.T.; Rudberg, I.; Flo Halle, I.; Rustan, A.C.; Thoresen, G.H.; Aas, V. Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PLoS ONE 2012, 7, 33203. [Google Scholar] [CrossRef] [PubMed]
- Evers-van Gogh, I.J.; Alex, S.; Stienstra, R.; Brenkman, A.B.; Kersten, S.; Kalkhoven, E. Electric Pulse Stimulation of Myotubes as an In Vitro Exercise Model: Cell-Mediated and Non-Cell-Mediated Effects. Sci. Rep. 2015, 5, 10944. [Google Scholar] [CrossRef] [PubMed]
- Farmawati, A.; Kitajima, Y.; Nedachi, T.; Sato, M.; Kanzaki, M.; Nagatomi, R. Characterization of contraction-induced IL-6 up-regulation using contractile C2C12 myotubes. Endocr. J. 2013, 60, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Melouane, A.; Yoshioka, M.; Kanzaki, M.; St-Amand, J. Sparc, an EPS-induced gene, modulates the extracellular matrix and mitochondrial function via ILK/AMPK pathways in C2C12 cells. Life Sci. 2019, 229, 277–287. [Google Scholar] [CrossRef]
- Chang, J.S.; Kong, I.D. Irisin prevents dexamethasone-induced atrophy in C2C12 myotubes. Pflug. Arch. 2020, 472, 495–502. [Google Scholar] [CrossRef]
- Boucher, D.; Blais, V.; Denault, J.B. Caspase-7 uses an exosite to promote poly(ADP ribose) polymerase 1 proteolysis. Proc. Natl. Acad. Sci. USA 2012, 109, 5669–5674. [Google Scholar] [CrossRef] [PubMed]
- Oliver, F.J.; de la Rubia, G.; Rolli, V.; Ruiz-Ruiz, M.C.; de Murcia, G.; Murcia, J.M. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J. Biol. Chem. 1998, 273, 33533–33539. [Google Scholar] [CrossRef]
- Barnard, R.J.; Leung, P.S.; Aronson, W.J.; Cohen, P.; Golding, L.A. A mechanism to explain how regular exercise might reduce the risk for clinical prostate cancer. Eur. J. Cancer. Prev. 2007, 16, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, C.; Hansen, L.S.; Lillelund, C.; Andersen, C.; Gehl, J.; Christensen, J.F.; Pedersen, B.K.; Hojman, P. Exercise-Induced Catecholamines Activate the Hippo Tumor Suppressor Pathway to Reduce Risks of Breast Cancer Development. Cancer Res. 2017, 77, 4894–4904. [Google Scholar] [CrossRef] [PubMed]
- Devin, J.L.; Hill, M.M.; Mourtzakis, M.; Quadrilatero, J.; Jenkins, D.G.; Skinner, T.L. Acute high intensity interval exercise reduces colon cancer cell growth. J. Physiol. 2019, 597, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Ryder-Burbidge, C.; McNeil, J. Physical activity, obesity and sedentary behavior in cancer etiology: Epidemiologic evidence and biologic mechanisms. Mol. Oncol. 2021, 15, 790–800. [Google Scholar] [CrossRef]
- Ngo-Huang, A.; Parker, N.H.; Bruera, E.; Lee, R.E.; Simpson, R.; O’Connor, D.P.; Petzel, M.Q.B.; Fontillas, R.C.; Schadler, K.; Xiao, L.; et al. Home-Based Exercise Prehabilitation During Preoperative Treatment for Pancreatic Cancer Is Associated with Improvement in Physical Function and Quality of Life. Integr. Cancer 2019, 18, 1534735419894061. [Google Scholar] [CrossRef]
- Steindorf, K.; Clauss, D.; Tjaden, C.; Hackert, T.; Herbolsheimer, F.; Bruckner, T.; Schneider, L.; Ulrich, C.M.; Wiskemann, J. Quality of Life, Fatigue, and Sleep Problems in Pancreatic Cancer Patients-A Randomized Trial on the Effects of Exercise. Dtsch. Arztebl. Int. 2019, 116, 471–478. [Google Scholar]
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int. J. Cancer 2015, 136, 197–202. [Google Scholar] [CrossRef]
- Fan, G.H.; Zhu, T.Y.; Huang, J. FNDC5 promotes paclitaxel sensitivity of non-small cell lung cancers via inhibiting MDR1. Cell Signal. 2020, 72, 109665. [Google Scholar] [CrossRef]
- Kong, G.; Jiang, Y.; Sun, X.; Cao, Z.; Zhang, G.; Zhao, Z.; Zhao, Y.; Yu, Q.; Cheng, G. Irisin reverses the IL-6 induced epithelial-mesenchymal transition in osteosarcoma cell migration and invasion through the STAT3/Snail signaling pathway. Oncol. Rep. 2017, 38, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Chang, Y.H.; Lee, H.H.; Wu, J.Y.; Huang, J.X.; Chung, Y.H.; Hsu, S.T.; Chow, L.P.; Wei, K.C.; Huang, F.T. Irisin, an exercise myokine, potently suppresses tumor proliferation, invasion, and growth in glioma. FASEB J. 2020, 34, 9678–9693. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, M.; Zauli, G.; Maiorano, P.; Milani, D.; Mirandola, P.; Neri, L.M. Role of physical exercise in the regulation of epigenetic mechanisms in inflammation, cancer, neurodegenerative diseases, and aging process. J. Cell. Physiol. 2019, 234, 14852–14864. [Google Scholar] [CrossRef] [PubMed]
- Dennis, K.L.; Blatner, N.R.; Gounari, F.; Khazaie, K. Current status of interleukin-10 and regulatory T-cells in cancer. Curr. Opin. Oncol. 2013, 25, 637–645. [Google Scholar] [CrossRef]
- Goh, J.; Niksirat, N.; Campbell, K.L. Exercise training and immune crosstalk in breast cancer microenvironment: Exploring the paradigms of exercise-induced immune modulation and exercise-induced myokines. Am. J. Transl. Res. 2014, 6, 422–438. [Google Scholar]
- Steensberg, A.; Fischer, C.P.; Keller, C.; Moller, K.; Pedersen, B.K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Pilegaard, H.; Hansen, J.; Brandt, C.; Adser, H.; Hidalgo, J.; Olesen, J.; Pedersen, B.K.; Hojman, P. Exercise-induced liver chemokine CXCL-1 expression is linked to muscle-derived interleukin-6 expression. J. Physiol. 2011, 589, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Jiang, Y.; Luo, Y.; Zhang, H.; Zhan, Y. Prognostic and clinicopathological significance of CXCL1 in cancers: A systematic review and meta-analysis. Cancer Biol. Ther. 2019, 20, 1380–1388. [Google Scholar] [CrossRef]
- Do, H.T.T.; Lee, C.H.; Cho, J. Chemokines and their Receptors: Multifaceted Roles in Cancer Progression and Potential Value as Cancer Prognostic Markers. Cancers 2020, 12, 287. [Google Scholar] [CrossRef]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Chemokines are elevated in plasma after strenuous exercise in humans. Eur. J. Appl. Physiol. 2001, 84, 244–245. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Hatfield, D.L.; Comstock, B.A.; Fragala, M.S.; Davitt, P.M.; Cortis, C.; Wilson, J.M.; Lee, E.C.; Newton, R.U.; Dunn-Lewis, C.; et al. Influence of HMB supplementation and resistance training on cytokine responses to resistance exercise. J. Am. Coll. Nutr. 2014, 33, 247–255. [Google Scholar] [CrossRef]
- Mathers, J.L.; Farnfield, M.M.; Garnham, A.P.; Caldow, M.K.; Cameron-Smith, D.; Peake, J.M. Early inflammatory and myogenic responses to resistance exercise in the elderly. Muscle Nerve 2012, 46, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Jajtner, A.R.; Fragala, M.S.; Townsend, J.R.; Gonzalez, A.M.; Wells, A.J.; Fukuda, D.H.; Stout, J.R.; Hoffman, J.R. Mediators of monocyte migration in response to recovery modalities following resistance exercise. Mediat. Inflamm. 2014, 2014, 145817. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mukaida, N.; Sasaki, S.I.; Baba, T. CCL4 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1231, 23–32. [Google Scholar] [PubMed]
- Yahiaoui, L.; Gvozdic, D.; Danialou, G.; Mack, M.; Petrof, B.J. CC family chemokines directly regulate myoblast responses to skeletal muscle injury. J. Physiol. 2008, 586, 3991–4004. [Google Scholar] [CrossRef]
- Warren, G.L.; O’Farrell, L.; Summan, M.; Hulderman, T.; Mishra, D.; Luster, M.I.; Kuziel, W.A.; Simeonova, P.P. Role of CC chemokines in skeletal muscle functional restoration after injury. Am. J. Physiol. Cell. Physiol. 2004, 286, 1031–1036. [Google Scholar] [CrossRef]
- Hamacher, R.; Schmid, R.M.; Saur, D.; Schneider, G. Apoptotic pathways in pancreatic ductal adenocarcinoma. Mol. Cancer 2008, 7, 64. [Google Scholar] [CrossRef]
- Arlt, A.; Muerkoster, S.S.; Schafer, H. Targeting apoptosis pathways in pancreatic cancer. Cancer Lett. 2013, 332, 346–358. [Google Scholar] [CrossRef]
- Debatin, K.M. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol. Immunother. 2004, 53, 153–159. [Google Scholar] [CrossRef]
- Devarajan, E.; Sahin, A.A.; Chen, J.S.; Krishnamurthy, R.R.; Aggarwal, N.; Brun, A.M.; Sapino, A.; Zhang, F.; Sharma, D.; Yang, X.H.; et al. Down-regulation of caspase 3 in breast cancer: A possible mechanism for chemoresistance. Oncogene 2002, 21, 8843–8851. [Google Scholar] [CrossRef]
- Kolenko, V.; Uzzo, R.G.; Bukowski, R.; Bander, N.H.; Novick, A.C.; His, E.D.; Finke, J.H. Dead or dying: Necrosis versus apoptosis in caspase-deficient human renal cell carcinoma. Cancer Res. 1999, 59, 2838–2842. [Google Scholar]
- Palmerini, F.; Devilard, E.; Jarry, A.; Birg, F.; Xerri, L. Caspase 7 downregulation as an immunohistochemical marker of colonic carcinoma. Hum. Pathol. 2001, 32, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.N.; Kramer, A.; Borkowski, A.; Kyprianou, N. Loss of caspase-1 and caspase-3 protein expression in human prostate cancer. Cancer Res. 2001, 61, 1227–1232. [Google Scholar] [PubMed]
- Chaitanya, G.V.; Steven, A.J.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Lin, Z.Q.; Liu, B.; Wei, Y.Q. Caspase-mediated programmed cell death pathways as potential therapeutic targets in cancer. Cell Prolif. 2012, 45, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Whitham, M.; Febbraio, M.A. The ever-expanding myokinome: Discovery challenges and therapeutic implications. Nat. Rev. Drug Discov. 2016, 15, 719–729. [Google Scholar] [CrossRef]
- Hojman, P.; Gehl, J.; Christensen, J.F.; Pedersen, B.K. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018, 27, 10–21. [Google Scholar] [CrossRef]
- De Castro, G.S.; Correia-Lima, J.; Simoes, E.; Orsso, C.E.; Xiao, J.; Gama, L.R.; Gomes, S.P.; Goncalves, D.C.; Costa, R.G.F.; Radloff, K.; et al. Myokines in treatment-naive patients with cancer-associated cachexia. Clin. Nutr. 2021, 40, 2443–2455. [Google Scholar] [CrossRef]

| Parameter | Pancreatic Cancer (PC) | p | |
|---|---|---|---|
| Control (n = 6) | Resistance Exercise Using WB-EMS (n = 6) | ||
| Sex | - | ||
| Male, n (%) | 3 (50%) | 4 (60%) | |
| Female, n (%) | 3 (50%) | 2 (40%) | |
| Age (y) | 61.0 ± 8.4 | 62.7 ± 9.1 | 0.748 a |
| Tumor Stage (UICC) | - | ||
| III, n (%) | 1 (20%) | 1 (20%) | |
| IV, n (%) | 5 (80%) | 5 (80%) | |
| Oncological Therapy | - | ||
| Chemotherapy, n (%) | 6 (100%) | 6 (100%) | |
| Other therapies, n (%) | - | - | |
| Karnofsky Index (%) | 78.3 ± 4.1 | 83.3 ± 10.3 | 0.178 b |
| 6 min-Walking Distance (m) | 557.5 ± 75.9 | 586.0 ± 54.5 (n = 5) | 0.574 b |
| Body Parameters | |||
| Body Weight (kg) | 74.6 ± 9.7 | 67.8 ± 6.9 | 0.193 a |
| Weight Lossin the last 3–6 month (%) | 3.9 ± 5.1 | 9.0 ± 11.6 | 0.708 b |
| Body Mass Index (kg/m2) | 25.6 ± 1.4 | 23.5 ± 2.5 | 0.093 a |
| Skeletal Muscle Mass (kg) | 23.9 ± 6.7 | 22.0 ± 4.4 | 0.582 a |
| Blood Parameters | |||
| Albumin (g/L) | 42.5 ± 2.2 | 40.9 ± 2.1 | 0.226 a |
| C-reactive Protein (mg/L) | 19.7 ± 33.7 | 5.7 ± 3.3 | 0.335 a |
| Creatinine (mg/dL) | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.606 a |
| Hematocrit (%) | 36.0 ± 2.1 | 37.8 ± 3.4 | 0.283 a |
| Hemoglobin (g/dL) | 12.2 ± 0.8 | 12.7 ± 1.1 | 0.434 a |
| Leucocytes (×103/µL) | 5.2 ± 2.3 | 5.5 ± 3.3 | 0.851 b |
| Erythrocytes (×106/µL) | 3.9 ± 0.2 | 4.2 ± 0.3 | 0.143 b |
| Thrombocytes (×103/µL) | 234.5 ± 133.4 | 181.2 ± 69.5 | 0.818 b |
| Target | Control | Exercise | Ratio Post-Intervention | |||
|---|---|---|---|---|---|---|
| pre | post | pre | post | Ctl | Ex | |
| CXCL1 (Groα) | 1.00 | 1.05 | 1.00 | 1.88 | 1.00 | 1.59 |
| IL10 | 1.00 | 0.69 | 1.00 | 1.52 | 1.00 | 1.86 |
| CCL4 (Mip1β) | 1.00 | 0.84 | 1.00 | 1.30 | 1.00 | 1.54 |
| CCL22 (MDC) | 1.00 | 0.97 | 1.00 | 1.37 | 1.00 | 1.47 |
| LTA (TNFβ) | 1.00 | 0.74 | 1.00 | 1.23 | 1.00 | 1.36 |
| Target | Untreated | 2× 20 min EPS |
|---|---|---|
| CXCL1 (Groα) | 1.00 | 3.36 |
| IL10 | 1.00 | 1.43 |
| CCL4 (Mip1β) | 1.00 | 1.19 |
| CCL22 (MDC) | 1.00 | 0.86 |
| LTA (TNFβ) | 1.00 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwappacher, R.; Dieterich, W.; Reljic, D.; Pilarsky, C.; Mukhopadhyay, D.; Chang, D.K.; Biankin, A.V.; Siebler, J.; Herrmann, H.J.; Neurath, M.F.; et al. Muscle-Derived Cytokines Reduce Growth, Viability and Migratory Activity of Pancreatic Cancer Cells. Cancers 2021, 13, 3820. https://doi.org/10.3390/cancers13153820
Schwappacher R, Dieterich W, Reljic D, Pilarsky C, Mukhopadhyay D, Chang DK, Biankin AV, Siebler J, Herrmann HJ, Neurath MF, et al. Muscle-Derived Cytokines Reduce Growth, Viability and Migratory Activity of Pancreatic Cancer Cells. Cancers. 2021; 13(15):3820. https://doi.org/10.3390/cancers13153820
Chicago/Turabian StyleSchwappacher, Raphaela, Walburga Dieterich, Dejan Reljic, Christian Pilarsky, Debabrata Mukhopadhyay, David K. Chang, Andrew V. Biankin, Jürgen Siebler, Hans J. Herrmann, Markus F. Neurath, and et al. 2021. "Muscle-Derived Cytokines Reduce Growth, Viability and Migratory Activity of Pancreatic Cancer Cells" Cancers 13, no. 15: 3820. https://doi.org/10.3390/cancers13153820
APA StyleSchwappacher, R., Dieterich, W., Reljic, D., Pilarsky, C., Mukhopadhyay, D., Chang, D. K., Biankin, A. V., Siebler, J., Herrmann, H. J., Neurath, M. F., & Zopf, Y. (2021). Muscle-Derived Cytokines Reduce Growth, Viability and Migratory Activity of Pancreatic Cancer Cells. Cancers, 13(15), 3820. https://doi.org/10.3390/cancers13153820








