Impact of Immune Parameters and Immune Dysfunctions on the Prognosis of Patients with Chronic Lymphocytic Leukemia
Abstract
:Simple Summary
Abstract
1. Introduction
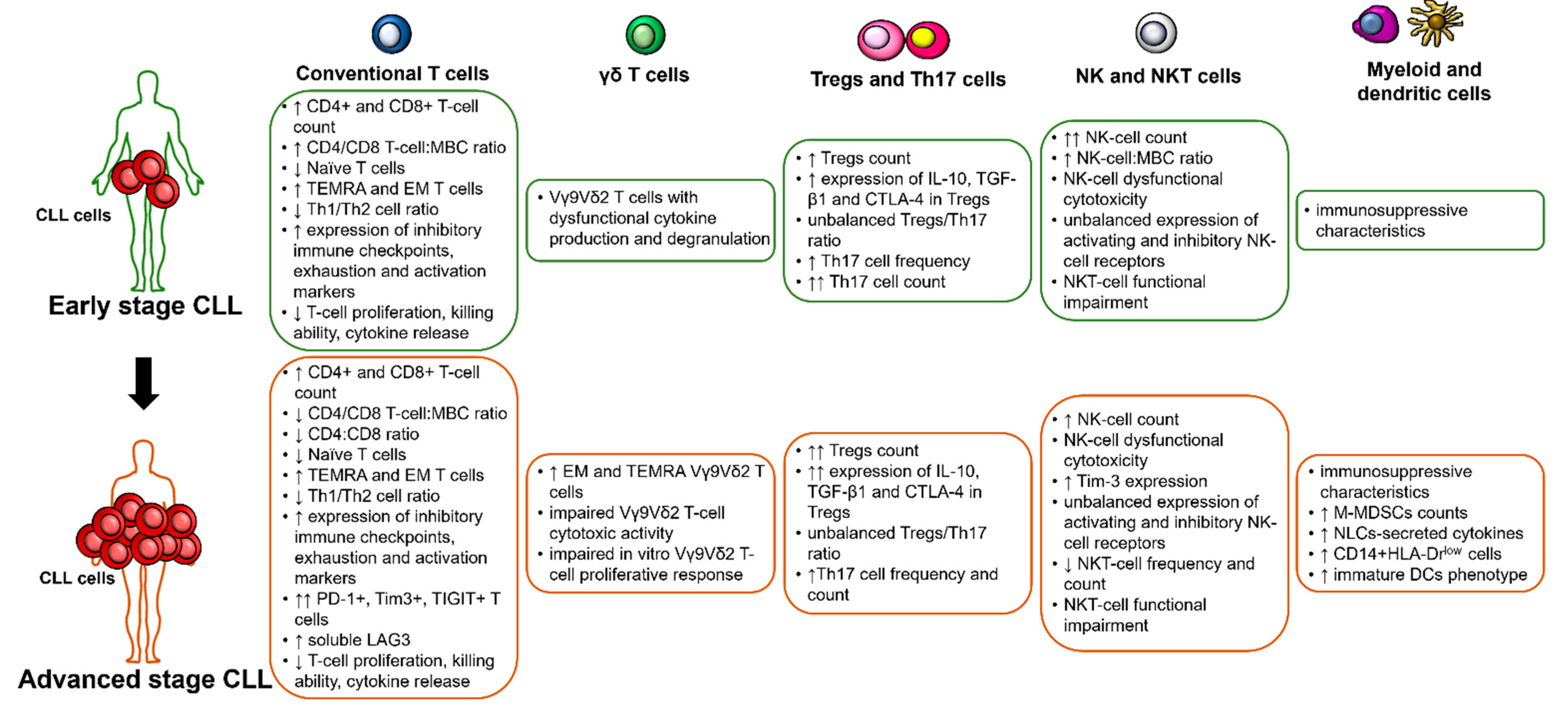
2. Specific Cellular and Humoral Immune Dysfunctions and Their Prognostic Impact in CLL
2.1. T Cells
2.1.1. Conventional T Cells
2.1.2. Gamma-Delta T Cells
2.1.3. Regulatory T Cells
2.2. Natural Killer and Natural Killer T Cells
2.2.1. Natural Killer Cells
2.2.2. Natural Killer T Cells
2.3. Normal B Cells and Hypogammaglobulinemia
2.4. Myeloid Cells
2.5. Humoral Immunity: Complement and Cytokines
3. Clinically Meaningful Immune Alterations: The Impact of Autoimmunity, Infections and Second Malignancies on the Prognosis of Patients with CLL
3.1. Autoimmune Manifestations
3.2. Infections
3.3. Second Malignancies
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CLL | chronic lymphocytic leukemia |
| MBC | monoclonal B cells |
| TEMRA | terminally differentiated memory T cells |
| EM | effector memory T cells |
| Th | T helper |
| Tregs | T regulatory cells |
| NK | natural killer |
| NKT | natural killer T |
| M-MDSCs | monocytic myeloid-derived suppressor cells |
| NLCs | nurse like cells |
| DCs | dendritic cells |
| TTFT | time-to-first treatment |
| OS | overall survival |
| PFS | progression-free survival |
| FCR | fludarabine-cyclophosphamide-rituximab |
| IGHV | immunoglobulin heavy chain variable region |
| TCR | T cell receptor |
| MBL | monoclonal B cell lymphocytosis |
| mAb | monoclonal antibody |
| ADCC | antibody-dependent cell-mediated-cytotoxicity |
| KIRs | killer Ig-like receptors |
| NCRs | natural cytokine receptors |
| Ig | immunoglobulin |
| TFS | treatment-free survival |
| TTP | time to progression |
| PMN-MDSCs | polymorphonuclear myeloid-derived suppressor cells |
| AIHA | autoimmune hemolytic anemia |
| ITP | immune thrombocytopenia |
| DAT | direct antiglobulin test |
| SIR | standardized incidence ratio |
| SEER | surveillance epidemiology and end results |
| CR | complete remission |
| LDT | lymphocyte doubling time |
| TTT | time-to-treatment |
References
- Griggio, V.; Perutelli, F.; Salvetti, C.; Boccellato, E.; Boccadoro, M.; Vitale, C.; Coscia, M. Immune Dysfunctions and Immune-Based Therapeutic Interventions in Chronic Lymphocytic Leukemia. Front. Immunol. 2020, 11, 594556. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Henley, P. Chronic lymphocytic leukaemia: The role of T cells in a B cell disease. Br. J. Haematol. 2019, 186, 220–233. [Google Scholar] [CrossRef]
- Roessner, P.M.; Seiffert, M. T-cells in chronic lymphocytic leukemia: Guardians or drivers of disease? Leukemia 2020, 34, 2012–2024. [Google Scholar] [CrossRef] [PubMed]
- Matutes, E.; Wechsler, A.; Gomez, R.; Cherchi, M.; Catovsky, D. Unusual T-Cell Phenotype in Advanced B-Chronic Lymphocytic Leukaemia. Br. J. Haematol. 1981, 49, 635–642. [Google Scholar] [CrossRef]
- Platsoucas, C.D.; Galinski, M.; Kempin, S.; Reich, L.; Clarkson, B.; Good, R.A. Abnormal T lymphocyte subpopulations in patients with B cell chronic lymphocytic leukemia: An analysis by monoclonal antibodies. J. Immunol. 1982, 129, 2305–2312. [Google Scholar] [PubMed]
- Herrmann, F.; Lochner, A.; Philippen, H.; Jauer, B.; Rühl, H. Imbalance of T cell subpopulations in patients with chronic lymphocytic leukaemia of the B cell type. Clin. Exp. Immunol. 1982, 49, 157–162. [Google Scholar]
- Dearden, C. Disease-Specific Complications of Chronic Lymphocytic Leukemia. Hematology 2008, 2008, 450–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, S.; Hanson, C.A.; Zent, C.S.; Porrata, L.F.; LaPlant, B.; Geyer, S.M.; Markovic, S.N.; Call, T.G.; Bowen, D.A.; Jelinek, D.F.; et al. Prognostic importance of T and NK-cells in a consecutive series of newly diagnosed patients with chronic lymphocytic leukaemia. Br. J. Haematol. 2008, 141, 607–614. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, A.P.; Contesti, J.; Huergo-Zapico, L.; Lopez-Soto, A.; Fernandez-Guizan, A.; Acebes-Huerta, A.; Gonzalez-Huerta, A.J.; Gonzalez, E.; Fernandez-Alvarez, C.; Gonzalez, S. Prognostic significance of CD8 and CD4 T cells in chronic lymphocytic leukemia. Leuk. Lymphoma 2010, 51, 1829–1836. [Google Scholar] [CrossRef]
- Hanna, B.S.; Roessner, P.M.; Yazdanparast, H.; Colomer, D.; Campo, E.; Kugler, S.; Yosifov, D.; Stilgenbauer, S.; Schmidt, M.; Gabriel, R.; et al. Control of chronic lymphocytic leukemia development by clonally-expanded CD8 + T-cells that undergo functional exhaustion in secondary lymphoid tissues. Leukemia 2019, 33, 625–637. [Google Scholar] [CrossRef]
- Catovsky, D.; Miliani, E.; Okos, A.; Galton, D.A.G. CLINICAL SIGNIFICANCE OF T-CELLS IN CHRONIC LYMPHOCYTIC LEUKAEMIA. Lancet 1974, 304, 751–752. [Google Scholar] [CrossRef]
- Apostolopoulos, A.; Symeonidis, A.; Zoumbos, N. Prognostic significance of immune function parameters in patients with chronic lymphocytic leukaemia. Eur. J. Haematol. 1990, 44, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, X.; Lee, E.J.; Shull, A.Y.; Pei, L.; Awan, F.; Wang, X.; Choi, J.H.; Deng, L.; Xin, H.B.; et al. Phenotypic alteration of CD8+ T cells in chronic lymphocytic leukemia is associated with epigenetic reprogramming. Oncotarget 2016, 7, 40558–40570. [Google Scholar] [CrossRef]
- Nunes, C.; Wong, R.; Mason, M.; Fegan, C.; Man, S.; Pepper, C. Expansion of a CD8+PD-1+ replicative senescence phenotype in early stage CLL patients is associated with inverted CD4:CD8 ratios and disease progression. Clin. Cancer Res. 2012, 18, 678–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elston, L.; Fegan, C.; Hills, R.; Hashimdeen, S.S.; Walsby, E.; Henley, P.; Pepper, C.; Man, S. Increased frequency of CD4+PD-1+HLA-DR+ T cells is associated with disease progression in CLL. Br. J. Haematol. 2020, 188, 872–880. [Google Scholar] [CrossRef]
- Gauthier, M.; Durrieu, F.; Martin, E.; Peres, M.; Vergez, F.; Filleron, T.; Obéric, L.; Bijou, F.; Quillet Mary, A.; Ysebaert, L. Prognostic role of CD4 T-cell depletion after frontline fludarabine, cyclophosphamide and rituximab in chronic lymphocytic leukaemia. BMC Cancer 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palma, M.; Gentilcore, G.; Heimersson, K.; Mozaffari, F.; Näsman-Glaser, B.; Young, E.; Rosenquist, R.; Hansson, L.; Österborg, A.; Mellstedt, H. T cells in chronic lymphocytic leukemia display dysregulated expression of immune checkpoints and activation markers. Haematologica 2017, 102, 562–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brusa, D.; Serra, S.; Coscia, M.; Rossi, D.; D’Arena, G.; Laurenti, L.; Jaksic, O.; Fedele, G.; Inghirami, G.; Gaidano, G.; et al. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica 2013, 98, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Podhorecka, M.; Dmoszynska, A.; Rolinski, J.; Wasik, E. T type 1/type 2 subsets balance in B-cell chronic lymphocytic leukemia—The three-color flow cytometry analysis. Leuk. Res. 2002, 26, 657–660. [Google Scholar] [CrossRef]
- Roessner, P.M.; Hanna, B.S.; Öztürk, S.; Schulz, R.; Llaó Cid, L.; Yazdanparast, H.; Scheffold, A.; Colomer, D.; Stilgenbauer, S.; Lichter, P.; et al. TBET-expressing Th1 CD4+ T cells accumulate in chronic lymphocytic leukaemia without affecting disease progression in Eµ-TCL1 mice. Br. J. Haematol. 2020, 189, 133–145. [Google Scholar] [CrossRef]
- Manna, A.; Kellett, T.; Aulakh, S.; Lewis-Tuffin, L.J.; Dutta, N.; Knutson, K.; Chini, E.; Pinilla-Ibarz, J.; Lamanna, N.; Manochakian, R.; et al. Targeting CD38 is lethal to Breg-like chronic lymphocytic leukemia cells and Tregs, but restores CD81 T-cell responses. Blood Adv. 2020, 4, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Puzzolo, M.C.; Del Giudice, I.; Peragine, N.; Mariglia, P.; De Propris, M.S.; Cappelli, L.V.; Trentin, L.; Reda, G.; Cuneo, A.; Molica, S.; et al. TH2/TH1 Shift Under Ibrutinib Treatment in Chronic Lymphocytic Leukemia. Front. Oncol. 2021, 11, 637186. [Google Scholar] [CrossRef]
- Gorczynski, R.M. IL-17 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1240, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Lad, D.P.; Varma, S.; Varma, N.; Sachdeva, M.U.S.; Bose, P.; Malhotra, P. Regulatory T-cell and T-helper 17 balance in chronic lymphocytic leukemia progression and autoimmune cytopenias. Leuk. Lymphoma 2015, 56, 2424–2428. [Google Scholar] [CrossRef]
- Jain, P.; Javdan, M.; Feger, F.K.; Chiu, P.Y.; Sison, C.; Damle, R.N.; Bhuiya, T.A.; Sen, F.; Abruzzo, L.V.; Burger, J.A.; et al. Th17 and non-th17 interleukin-17-expressing cells in chronic lymphocyticleukemia: Delineation, distribution, and clinical relevance. Haematologica 2012, 97, 599–607. [Google Scholar] [CrossRef]
- Hus, I.; Bojarska-Junak, A.; Chocholska, S.; Tomczak, W.; Woś, J.; Dmoszyńska, A.; Roliński, J. Th17/IL-17A might play a protective role in chronic lymphocytic leukemia immunity. PLoS ONE 2013, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherry, B.; Jain, P.; Chiu, P.Y.; Leung, L.; Allen, S.L.; Kolitz, J.E.; Rai, K.R.; Barrientos, J.; Liang, S.; Hawtin, R.; et al. Identification and characterization of distinct IL-17F expression patterns and signaling pathways in chronic lymphocytic leukemia and normal B lymphocytes. Immunol. Res. 2015, 63, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Jadidi-Niaragh, F.; Ghalamfarsa, G.; Memarian, A.; Asgarian-Omran, H.; Razavi, S.M.; Sarrafnejad, A.; Shokri, F. Downregulation of IL-17-producing T cells is associated with regulatory T cell expansion and disease progression in chronic lymphocytic leukemia. Tumor Biol. 2013, 34, 929–940. [Google Scholar] [CrossRef]
- Serrano, D.; Monteiro, J.; Allen, S.L.; Kolitz, J.; Schulman, P.; Lichtman, S.M.; Buchbinder, A.; Vinciguerra, V.P.; Chiorazzi, N.; Gregersen, P.K. Clonal Expansion Within the CD4+CD57+ and CD8+CD57+ T Cell Subsets in Chronic Lymphocytic Leukemia. J. Immunol. 1997, 158, 1482–1489. [Google Scholar]
- Van Den Hove, L.E.; Vandenberghe, P.; Van Gool, S.W.; Ceuppens, J.L.; Demuynck, H.; Verhoef, G.E.G.; Boogaerts, M.A. Peripheral blood lymphocyte subset shifts in patients with untreated hematological tumors: Evidence for systemic activation of the T cell compartment. Leuk. Res. 1998, 22, 175–184. [Google Scholar] [CrossRef]
- Görgün, G.; Holderried, T.A.W.; Zahrieh, D.; Neuberg, D.; Gribben, J.G. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J. Clin. Investig. 2005, 115, 1797–1805. [Google Scholar] [CrossRef]
- Wierz, M.; Janji, B.; Berchem, G.; Moussay, E.; Paggetti, J. High-dimensional mass cytometry analysis revealed microenvironment complexity in chronic lymphocytic leukemia. OncoImmunology 2018, 7, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Motta, M.; Rassenti, L.; Shelvin, B.J.; Lerner, S.; Kipps, T.J.; Keating, M.J.; Wierda, W.G. Increased expression of CD152 (CTLA-4) by normal T lymphocytes in untreated patients with B-cell chronic lymphocytic leukemia. Leukemia 2005, 19, 1788–1793. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, M.; Herishanu, Y.; Ben Zion, k.; Dezorella, N.; Sun, C.; Kay, S.; Polliack, A.; Avivi, I.; Wiestner, A.; Perry, C. Lymphocyte activation gene 3: A novel therapeutic target in chronic lymphocytic leukemia. Haematologica 2017, 102, 874–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taghiloo, S.; Allahmoradi, E.; Tehrani, M.; Hossein-Nataj, H.; Shekarriz, R.; Janbabaei, G.; Abediankenari, S.; Asgarian-Omran, H. Frequency and functional characterization of exhausted CD8+ T cells in chronic lymphocytic leukemia. Eur. J. Haematol. 2017, 98, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Catakovic, K.; Gassner, F.J.; Ratswohl, C.; Zaborsky, N.; Rebhandl, S.; Schubert, M.; Steiner, M.; Gutjahr, J.C.; Pleyer, L.; Egle, A.; et al. TIGIT expressing CD4+T cells represent a tumor-supportive T cell subset in chronic lymphocytic leukemia. OncoImmunology 2018, 7. [Google Scholar] [CrossRef]
- Riches, J.C.; Davies, J.K.; McClanahan, F.; Fatah, R.; Iqbal, S.; Agrawal, S.; Ramsay, A.G.; Gribben, J.G. T cells from CLLpatients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood 2013, 121, 1612–1621. [Google Scholar] [CrossRef]
- Jiménez, I.; Tazón-Vega, B.; Abrisqueta, P.; Nieto, J.C.; Bobillo, S.; Palacio-García, C.; Carabia, J.; Valdés-Mas, R.; Munuera, M.; Puigdefàbregas, L.; et al. Immunological and genetic kinetics from diagnosis to clinical progression in chronic lymphocytic leukemia. Biomark. Res. 2021, 9, 37. [Google Scholar] [CrossRef]
- Lowdell, M.W.; Lamb, L.; Hoyle, C.; Velardi, A.; Prentice, H.G. Non-MHC-restricted cytotoxic cells: Their roles in the control and treatment of leukaemias. Br. J. Haematol. 2001, 114, 11–24. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C. The Role of Human γδ T Cells in Anti-Tumor Immunity and Their Potential for Cancer Immunotherapy. Cells 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Castella, B.; Vitale, C.; Coscia, M.; Massaia, M. Vγ9Vδ2 T cell-based immunotherapy in hematological malignancies: From bench to bedside. Cell. Mol. Life Sci. 2011, 68, 2419–2432. [Google Scholar] [CrossRef]
- De Weerdt, I.; Hofland, T.; Lameris, R.; Endstra, S.; Jongejan, A.; Moerland, P.D.; de Bruin, R.C.G.; Remmerswaal, E.B.M.; ten Berge, I.J.M.; Liu, N.; et al. Improving CLL Vγ9Vδ2-T–cell fitness for cellular therapy by ex vivo activation and ibrutinib. Blood 2018, 132, 2260–2272. [Google Scholar] [CrossRef] [Green Version]
- Coscia, M.; Vitale, C.; Peola, S.; Foglietta, M.; Rigoni, M.; Griggio, V.; Castella, B.; Angelini, D.; Chiaretti, S.; Riganti, C.; et al. Dysfunctional Vγ9Vδ2 T cells are negative prognosticators and markers of dysregulated mevalonate pathway activity in chronic lymphocytic leukemia cells. Blood 2012, 120, 3271–3279. [Google Scholar] [CrossRef] [Green Version]
- Dees, S.; Ganesan, R.; Singh, S.; Grewal, I.S. Regulatory T cell targeting in cancer: Emerging strategies in immunotherapy. Eur. J. Immunol. 2021, 51, 280–291. [Google Scholar] [CrossRef]
- D’Arena, G.; Laurenti, L.; Minervini, M.M.; Deaglio, S.; Bonello, L.; De Martino, L.; De Padua, L.; Savino, L.; Tarnani, M.; De Feo, V.; et al. Regulatory T-cell number is increased in chronic lymphocytic leukemia patients and correlates with progressive disease. Leuk. Res. 2011, 35, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Jadidi-Niaragh, F.; Yousefi, M.; Memarian, A.; Hojjat-Farsangi, M.; Khoshnoodi, J.; Razavi, S.M.; Jeddi-Tehrani, M.; Shokri, F. Increased frequency of CD8+ and CD4+ regulatory T cells in chronic lymphocytic leukemia: Association with disease progression. Cancer Investig. 2013, 31, 121–131. [Google Scholar] [CrossRef]
- Dasgupta, A.; Mahapatra, M.; Saxena, R. A study for proposal of use of regulatory T cells as a prognostic marker and establishing an optimal threshold level for their expression in chronic lymphocytic leukemia. Leuk. Lymphoma 2015, 56, 1831–1838. [Google Scholar] [CrossRef]
- D’Arena, G.; Vitale, C.; Coscia, M.; Festa, A.; Di Minno, N.M.D.; De Feo, V.; Caraglia, M.; Calapai, G.; Laurenti, L.; Musto, P.; et al. Regulatory T Cells and Their Prognostic Relevance in Hematologic Malignancies. J. Immunol. Res. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jak, M.; Mous, R.; Remmerswaal, E.B.M.; Spijker, R.; Jaspers, A.; Yagüe, A.; Eldering, E.; Van Lier, R.A.W.; Van Oers, M.H.J. Enhanced formation and survival of CD4+ CD25hi Foxp3+ T-cells in chronic lymphocytic leukemia. Leuk. Lymphoma 2009, 50, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Mpakou, V.E.; Ioannidou, H.D.; Konsta, E.; Vikentiou, M.; Spathis, A.; Kontsioti, F.; Kontos, C.K.; Velentzas, A.D.; Papageorgiou, S.; Vasilatou, D.; et al. Quantitative and qualitative analysis of regulatory T cells in B cell chronic lymphocytic leukemia. Leuk. Res. 2017, 60, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Piper, K.P.; Karanth, M.; McLarnon, A.; Kalk, E.; Khan, N.; Murray, J.; Pratt, G.; Moss, P.A.H. Chronic lymphocytic leukaemia cells drive the global CD4+ T cell repertoire towards a regulatory phenotype and leads to the accumulation of CD4+ forkhead box P3+ T cells. Clin. Exp. Immunol. 2011, 166, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Hanna, B.S.; Roessner, P.M.; Scheffold, A.; Jebaraj, B.M.C.; Demerdash, Y.; Öztürk, S.; Lichter, P.; Stilgenbauer, S.; Seiffert, M. PI3Kδ inhibition modulates regulatory and effector T-cell differentiation and function in chronic lymphocytic leukemia. Leukemia 2019, 33, 1427–1438. [Google Scholar] [CrossRef]
- Rissiek, A.; Schulze, C.; Bacher, U.; Schieferdecker, A.; Thiele, B.; Jacholkowski, A.; Flammiger, A.; Horn, C.; Haag, F.; Tiegs, G.; et al. Multidimensional scaling analysis identifies pathological and prognostically relevant profiles of circulating T-cells in chronic lymphocytic leukemia. Int. J. Cancer 2014, 135, 2370–2379. [Google Scholar] [CrossRef] [Green Version]
- Arruga, F.; Gyau, B.B.; Iannello, A.; Vitale, N.; Vaisitti, T.; Deaglio, S. Immune Response Dysfunction in Chronic Lymphocytic Leukemia: Dissecting Molecular Mechanisms and Microenvironmental Conditions. Int. J. Mol. Sci. 2020, 21, 1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, N.; Zhang, R.; Li, J.; Zhang, Z.; Yuan, H.; Chen, G.; Zhao, F.; Wang, L.; Cao, H.; Qu, J.; et al. Increased IL-10/IL-17 ratio is aggravated along with the prognosis of patients with chronic lymphocytic leukemia. Int. Immunopharmacol. 2016, 40, 57–64. [Google Scholar] [CrossRef]
- Weiss, L.; Melchardt, T.; Egle, A.; Grabmer, C.; Greil, R.; Tinhofer, I. Regulatory T cells predict the time to initial treatment in early stage chronic lymphocytic leukemia. Cancer 2011, 117, 2163–2169. [Google Scholar] [CrossRef]
- D’Arena, G.; D’Auria, F.; Simeon, V.; Laurenti, L.; Deaglio, S.; Mansueto, G.; Del Principe, M.I.; Statuto, T.; Pietrantuono, G.; Guariglia, R.; et al. A shorter time to the first treatment may be predicted by the absolute number of regulatory T-cells in patients with Rai stage 0 chronic lymphocytic leukemia. Am. J. Hematol. 2012, 87, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, K.; Schmitt, M.; Własiuk, P.; Chen, J.; Bojarska-Junak, A.; Kowal, M.; Roliñski, J.; Dmoszyñska, A. The high frequency of T regulatory cells in patients with B-cell chronic lymphocytic leukemia is diminished through treatment with thalidomide. Leukemia 2008, 22, 222–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itchaki, G.; Brown, J.R. Lenalidomide in the treatment of chronic lymphocytic leukemia. Expert Opin. Investig. Drugs 2017, 26, 633–650. [Google Scholar] [CrossRef]
- Solman, I.G.; Blum, L.K.; Hoh, H.Y.; Kipps, T.J.; Burger, J.A.; Barrientos, J.C.; O’Brien, S.; Mulligan, S.P.; Kay, N.E.; Hillmen, P.; et al. Ibrutinib restores immune cell numbers and function in first-line and relapsed/refractory chronic lymphocytic leukemia. Leuk. Res. 2020, 97, 106432. [Google Scholar] [CrossRef]
- Beyer, M.; Kochanek, M.; Darabi, K.; Popov, A.; Jensen, M.; Endl, E.; Knolle, P.A.; Thomas, R.K.; Von Bergwelt-Baildon, M.; Debey, S.; et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood 2005, 106, 2018–2025. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Martinez, D.; Lanuza, P.M.; Gomez, N.; Muntasell, A.; Cisneros, E.; Moraru, M.; Azaceta, G.; Anel, A.; Martinez-Lostao, L.; Villalba, M.; et al. Activated Allogeneic NK Cells Preferentially Kill Poor Prognosis B-Cell Chronic Lymphocytic Leukemia Cells. Front. Immunol. 2016, 7, 454. [Google Scholar] [CrossRef]
- Hadadi, L.; Hafezi, M.; Amirzargar, A.A.; Sharifian, R.A.; Abediankenari, S.; Asgarian-Omran, H. Dysregulated Expression of Tim-3 and NKp30 Receptors on NK Cells of Patients with Chronic Lymphocytic Leukemia. Oncol. Res. Treat. 2019, 42, 202–208. [Google Scholar] [CrossRef]
- Zent, C.S. Cell-mediated immunity in chronic lymphocytic leukemia. Leuk. Lymphoma 2010, 51, 1775–1776. [Google Scholar] [CrossRef]
- Huergo-Zapico, L.; Acebes-Huerta, A.; Gonzalez-Rodriguez, A.P.; Contesti, J.; Gonzalez-Garcia, E.; Payer, A.R.; Villa-Alvarez, M.; Fernandez-Guizan, A.; Lopez-Soto, A.; Gonzalez, S. Expansion of NK cells and reduction of NKG2D expression in chronic lymphocytic leukemia. Correlation with progressive disease. PLoS ONE 2014, 9, e108326. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.T.; Zhu, H.Y.; Wu, Y.J.; Xia, Y.; Wu, J.Z.; Wu, W.; Liang, J.H.; Wang, L.; Fan, L.; Li, J.Y.; et al. Elevated absolute NK cell counts in peripheral blood predict good prognosis in chronic lymphocytic leukemia. J. Cancer Res. Clin. Oncol. 2018, 144, 449–457. [Google Scholar] [CrossRef]
- Vitale, C.; Falchi, L.; Ten Hacken, E.; Gao, H.; Shaim, H.; Van Roosbroeck, K.; Calin, G.; O’Brien, S.; Faderl, S.; Wang, X.; et al. Ofatumumab and Lenalidomide for Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia: Correlation between Responses and Immune Characteristics. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 2359–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, S.E.; Swerdlow, S.H.; Felgar, R.E. Natural killer cell subsets and natural killer-like T-cell populations in benign and neoplastic B-cell proliferations vary based on clinicopathologic features. Hum. Pathol. 2011, 42, 679–687. [Google Scholar] [CrossRef] [Green Version]
- Costello, R.T.; Knoblauch, B.; Sanchez, C.; Mercier, D.; Le Treut, T.; Sebahoun, G. Expression of natural killer cell activating receptors in patients with chronic lymphocytic leukaemia. Immunology 2012, 135, 151–157. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, A.W.t.; Jillab, M.; Smith, M.R.; Alpaugh, R.K.; Cole, M.E.; Litwin, S.; Millenson, M.M.; Al-Saleem, T.; Cohen, A.D.; Campbell, K.S. NK cell dysfunction in chronic lymphocytic leukemia is associated with loss of the mature cells expressing inhibitory killer cell Ig-like receptors. Articolo Riv. 2017, 6, e1330235. [Google Scholar] [CrossRef] [PubMed]
- Ghnewa, Y.G.; O’Reilly, V.P.; Vandenberghe, E.; Browne, P.V.; McElligott, A.M.; Doherty, D.G. Retinoic acid induction of CD1d expression primes chronic lymphocytic leukemia B cells for killing by CD8(+) invariant natural killer T cells. Clin. Immunol. 2017, 183, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Bojarska-Junak, A.; Hus, I.; Chocholska, S.; Tomczak, W.; Wos, J.; Czubak, P.; Putowski, L.; Rolinski, J. CD1d expression is higher in chronic lymphocytic leukemia patients with unfavorable prognosis. Leuk. Res. 2014, 38, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Jadidi-Niaragh, F.; Jeddi-Tehrani, M.; Ansaripour, B.; Razavi, S.M.; Sharifian, R.A.; Shokri, F. Reduced frequency of NKT-like cells in patients with progressive chronic lymphocytic leukemia. Med. Oncol. 2012, 29, 3561–3569. [Google Scholar] [CrossRef] [PubMed]
- Bojarska-Junak, A.; Hus, I.; Sieklucka, M.; Wasik-Szczepanek, E.; Mazurkiewicz, T.; Polak, P.; Dmoszynska, A.; Rolinski, J. Natural killer-like T CD3+/CD16+CD56+ cells in chronic lymphocytic leukemia: Intracellular cytokine expression and relationship with clinical outcome. Oncol. Rep. 2010, 24, 803–810. [Google Scholar] [CrossRef] [Green Version]
- Gorini, F.; Azzimonti, L.; Delfanti, G.; Scarfò, L.; Scielzo, C.; Bertilaccio, M.T.; Ranghetti, P.; Gulino, A.; Doglioni, C.; Di Napoli, A.; et al. Invariant NKT cells contribute to chronic lymphocytic leukemia surveillance and prognosis. Blood 2017, 129, 3440–3451. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.E.; Alcantara, M.B.; Minoda, Y.; Kannourakis, G.; Berzins, S.P. An emerging role for immune regulatory subsets in chronic lymphocytic leukaemia. Int. Immunopharmacol. 2015, 28, 897–900. [Google Scholar] [CrossRef]
- Foa, R.; Catovsky, D.; Brozovic, M.; Marsh, G.; Ooyirilangkumaran, T.; Cherchi, M.; Galton, D.A.G. Clinical staging and immunological findings in chronic lymphocytic leukemia. Cancer 1979, 44, 483–487. [Google Scholar] [CrossRef]
- Davey, F.R.; Kurec, A.S.; Tomar, R.H.; Smith, J.R. Serum Immunoglobulins and Lymphocyte Subsets in Chronic Lymphocytic Leukemia. Am. J. Clin. Pathol. 1987, 87, 60–65. [Google Scholar] [CrossRef]
- Rozman, C.; Montserrat, E.; Viñolas, N. Serum immunoglobulins in B-chronic lymphocytic leukemia.Natural history and prognostic significance. Cancer 1988, 61, 279–283. [Google Scholar] [CrossRef]
- Visentin, A.; Compagno, N.; Cinetto, F.; Imbergamo, S.; Zambello, R.; Piazza, F.; Semenzato, G.; Trentin, L.; Agostini, C. Clinical profile associated with infections in patients with chronic lymphocytic leukemia. Protective role of immunoglobulin replacement therapy. Haematologica 2015, 100, e515–e518. [Google Scholar] [CrossRef] [Green Version]
- Francis, S.; Karanth, M.; Pratt, G.; Starczynski, J.; Hooper, L.; Fegan, C.; Pepper, C.; Valcarcel, D.; Milligan, D.W.; Delgado, J. The effect of immunoglobulinVH gene mutation status and other prognostic factors on the incidence of major infections in patients with chronic lymphocytic leukemia. Cancer 2006, 107, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.A.; Eriksen, C.T.; Brieghel, C.; Biccler, J.L.; Cunha-Bang, C.D.; Helleberg, M.; Niemann, C.U. Incidence and predictors of infection among patients prior to treatment of chronic lymphocytic leukemia: A Danish nationwide cohort study. Haematologica 2018, 103, e300–e303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, D.; De Paoli, L.; Rossi, F.M.; Cerri, M.; Deambrogi, C.; Rasi, S.; Zucchetto, A.; Capello, D.; Gattei, V.; Gaidano, G. Early stage chronic lymphocytic leukaemia carrying unmutated IGHV genes is at risk of recurrent infections during watch and wait. Br. J. Haematol. 2008, 141, 734–736. [Google Scholar] [CrossRef]
- Hensel, M.; Kornacker, M.; Yammeni, S.; Egerer, G.; Ho, A.D. Disease activity and pretreatment, rather than hypogammaglobulinaemia, are major risk factors for infectious complications in patients with chronic lymphocytic leukaemia. Br. J. Haematol. 2003, 122, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.A.; Vojdeman, F.J.; Andersen, M.K.; Brown, P.d.N.; Geisler, C.H.; Weis Bjerrum, O.; Niemann, C.U. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia is a predictor of early death. Leuk. Lymphoma 2016, 57, 1592–1599. [Google Scholar] [CrossRef]
- Svensson, T.; Höglund, M.; Cherif, H. Clinical significance of serum immunoglobulin G subclass deficiency in patients with chronic lymphocytic leukemia. Scand. J. Infect. Dis. 2013, 45, 537–542. [Google Scholar] [CrossRef]
- Crassini, K.R.; Zhang, E.; Balendran, S.; Freeman, J.A.; Best, O.G.; Forsyth, C.J.; Mackinlay, N.J.; Han, P.; Stevenson, W.S.; Mulligan, S.P. Humoral immune failure defined by immunoglobulin class and immunoglobulin G subclass deficiency is associated with shorter treatment-free and overall survival in Chronic Lymphocytic Leukaemia. Br. J. Haematol. 2018, 181, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Parikh, S.A.; Leis, J.F.; Chaffee, K.G.; Call, T.G.; Hanson, C.A.; Ding, W.; Chanan-Khan, A.A.; Bowen, D.; Conte, M.; Schwager, S.; et al. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia: Natural history, clinical correlates, and outcomes. Cancer 2015, 121, 2883–2891. [Google Scholar] [CrossRef]
- Reda, G.; Cassin, R.; Gentile, M.; Mauro, F.R.; Giannarelli, D.; Fattizzo, B.; Barbieri, M.; Silvestris, I.; Fabris, S.; Morabito, F.; et al. IgA hypogammaglobulinemia predicts outcome in chronic lymphocytic leukemia. Articolo Riv. 2019, 33, 1519–1522. [Google Scholar] [CrossRef]
- Shvidel, L.; Tadmor, T.; Braester, A.; Bairey, O.; Rahimi-Levene, N.; Herishanu, Y.; Klepfish, A.; Ruchlemer, R.; Berrebi, A.; Polliack, A. Serum immunoglobulin levels at diagnosis have no prognostic significance in stage A chronic lymphocytic leukemia: A study of 1113 cases from the Israeli CLL Study Group. Eur. J. Haematol. 2014, 93, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Corbingi, A.; Innocenti, I.; Tomasso, A.; Pasquale, R.; Visentin, A.; Varettoni, M.; Flospergher, E.; Autore, F.; Morelli, F.; Trentin, L.; et al. Monoclonal gammopathy and serum immunoglobulin levels as prognostic factors in chronic lymphocytic leukaemia. Br. J. Haematol. 2020, 190, 901–908. [Google Scholar] [CrossRef]
- Mauro, F.R.; Morabito, F.; Vincelli, I.D.; Petrucci, L.; Campanelli, M.; Salaroli, A.; Uccello, G.; Petrungaro, A.; Ronco, F.; Raponi, S.; et al. Clinical relevance of hypogammaglobulinemia, clinical and biologic variables on the infection risk and outcome of patients with stage A chronic lymphocytic leukemia. Leuk. Res. 2017, 57, 65–71. [Google Scholar] [CrossRef]
- Ishdorj, G.; Streu, E.; Lambert, P.; Dhaliwal, H.S.; Mahmud, S.M.; Gibson, S.B.; Banerji, V.; Marshall, A.J.; Johnston, J.B. IgA levels at diagnosis predict for infections, time to treatment, and survival in chronic lymphocytic leukemia. Blood Adv. 2019, 3, 2188–2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Tian, X.; Lee, Y.S.; Gunti, S.; Lipsky, A.; Herman, S.E.M.; Salem, D.; Stetler-Stevenson, M.; Yuan, C.; Kardava, L.; et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood 2015, 126, 2213–2219. [Google Scholar] [CrossRef]
- Li, Y.; You, M.J.; Yang, Y.; Hu, D.; Tian, C. The Role of Tumor-Associated Macrophages in Leukemia. Acta Haematol. 2020, 143, 112–117. [Google Scholar] [CrossRef]
- Gustafson, M.P.; Abraham, R.S.; Lin, Y.; Wu, W.; Gastineau, D.A.; Zent, C.S.; Dietz, A.B. Association of an increased frequency of CD14+ HLA-DR lo/neg monocytes with decreased time to progression in chronic lymphocytic leukaemia (CLL). Br. J. Haematol. 2012, 156, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Zahran, A.M.; Moeen, S.M.; Thabet, A.F.; Rayan, A.; Abdel-Rahim, M.H.; Mohamed, W.M.Y.; Hetta, H.F. Monocytic myeloid-derived suppressor cells in chronic lymphocytic leukemia patients: A single center experience. Leuk. Lymphoma 2020, 61, 1645–1652. [Google Scholar] [CrossRef]
- Jitschin, R.; Braun, M.; Buttner, M.; Dettmer-Wilde, K.; Bricks, J.; Berger, J.; Eckart, M.J.; Krause, S.W.; Oefner, P.J.; Le Blanc, K.; et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood 2014, 124, 750–760. [Google Scholar] [CrossRef] [Green Version]
- Zarobkiewicz, M.; Kowalska, W.; Chocholska, S.; Tomczak, W.; Szymańska, A.; Morawska, I.; Wojciechowska, A.; Bojarska-Junak, A. High M-MDSC Percentage as a Negative Prognostic Factor in Chronic Lymphocytic Leukaemia. Cancers 2020, 12, 2614. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, G.; Jung, B.; Chiu, P.Y.; Aslam, R.; Palacios, F.; Mazzarello, A.N.; Vergani, S.; Bagnara, D.; Chen, S.S.; Yancopoulos, S.; et al. Myeloid-derived suppressor cell subtypes differentially influence T-cell function, T-helper subset differentiation, and clinical course in CLL. Leukemia 2021. [Google Scholar] [CrossRef]
- Serra, S.; Vaisitti, T.; Audrito, V.; Bologna, C.; Buonincontri, R.; Chen, S.S.; Arruga, F.; Brusa, D.; Coscia, M.; Jaksic, O.; et al. Adenosine signaling mediates hypoxic responses in the chronic lymphocytic leukemia microenvironment. Blood Adv. 2016, 1, 47–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fecteau, J.F.; Bharati, I.S.; O’Hayre, M.; Handel, T.M.; Kipps, T.J.; Messmer, D. Sorafenib-induced apoptosis of chronic lymphocytic leukemia cells is associated with downregulation of RAF and myeloid cell leukemia sequence 1 (Mcl-1). Mol. Med. (Camb. Mass.) 2012, 18, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hanna, B.S.; Öztürk, S.; Seiffert, M. Beyond bystanders: Myeloid cells in chronic lymphocytic leukemia. Mol. Immunol. 2019, 110, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Fayad, L.; Keating, M.J.; Reuben, J.M.; O’Brien, S.; Lee, B.N.; Lerner, S.; Kurzrock, R. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: Correlation with phenotypic characteristics and outcome. Blood 2001, 97, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Middleton, O.; Cosimo, E.; Dobbin, E.; Mccaig, A.M.; Clarke, C.; Brant, A.M.; Leach, M.T.; Michie, A.M.; Wheadon, H. Complement deficiencies limit CD20 monoclonal antibody treatment efficacy in CLL. Leukemia 2015, 29, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Varga, L.; Czink, E.; Miszlai, Z.; Pálóczi, K.; Bányai, A.; Szegedi, G.; Füst, G. Low activity of the classical complement pathway predicts short survival of patients with chronic lymphocytic leukaemia. Clin. Exp. Immunol. 2008, 99, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Musolino, C.; Tonacci, A.; Pioggia, G.; Casciaro, M.; Gangemi, S. Clinico-Biological Implications of Modified Levels of Cytokines in Chronic Lymphocytic Leukemia: A Possible Therapeutic Role. Cancers 2020, 12, 524. [Google Scholar] [CrossRef] [Green Version]
- Munk Pedersen, I.; Reed, J. Microenvironmental interactions and survival of CLL B-cells. Leuk. Lymphoma 2004, 45, 2365–2372. [Google Scholar] [CrossRef]
- Ghamlouch, H.; Ouled-Haddou, H.; Damaj, G.; Royer, B.; Gubler, B.; Marolleau, J.-P. A Combination of Cytokines Rescues Highly Purified Leukemic CLL B-Cells from Spontaneous Apoptosis In Vitro. PLoS ONE 2013, 8, e60370. [Google Scholar] [CrossRef]
- Karmali, R.; Paganessi, L.A.; Frank, R.R.; Jagan, S.; Larson, M.L.; Venugopal, P.; Gregory, S.A.; Christopherson, K.W. Aggressive disease defined by cytogenetics is associated with cytokine dysregulation in CLL/SLL patients. J. Leukoc. Biol. 2013, 93, 161–170. [Google Scholar] [CrossRef]
- Schröttner, P.; Leick, M.; Burger, M. The role of chemokines in B cell chronic lymphocytic leukaemia: Pathophysiological aspects and clinical impact. Ann. Hematol. 2010, 89, 437–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.-J.; Dozmorov, I.; Li, W.; Yancopoulos, S.; Sison, C.; Centola, M.; Jain, P.; Allen, S.L.; Kolitz, J.E.; Rai, K.R.; et al. Identification of outcome-correlated cytokine clusters in chronic lymphocytic leukemia. Blood 2011, 118, 5201–5210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Cooke, L.; Riley, C.; Qi, W.; Mount, D.; Mahadevan, D. Genetic and cytokine changes associated with symptomatic stages of CLL. Leuk. Res. 2014, 38, 1097–1101. [Google Scholar] [CrossRef]
- Vitale, C.; Griggio, V.; Riganti, C.; Todaro, M.; Kopecka, J.; Jones, R.; Salvetti, C.; Boccellato, E.; Perutelli, F.; Voena, C.; et al. Targeting HIF-1α Regulatory Pathways as a Strategy to Hamper Tumor-Microenvironment Interactions in CLL. Cancers 2021, 13, 2883. [Google Scholar] [CrossRef]
- Rigoni, M.; Riganti, C.; Vitale, C.; Griggio, V.; Campia, I.; Robino, M.; Foglietta, M.; Castella, B.; Sciancalepore, P.; Buondonno, I.; et al. Simvastatin and downstream inhibitors circumvent constitutive and stromal cell-induced resistance to doxorubicin in IGHV unmutated CLL cells. Oncotarget 2015, 6, 29833–29846. [Google Scholar] [CrossRef] [Green Version]
- Griggio, V.; Vitale, C.; Todaro, M.; Riganti, C.; Kopecka, J.; Salvetti, C.; Bomben, R.; Bo, M.D.; Magliulo, D.; Rossi, D.; et al. HIF-1alpha is over-expressed in leukemic cells from TP53-disrupted patients and is a promising therapeutic target in chronic lymphocytic leukemia. Haematologica 2020, 105, 1042–1054. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, M.K.; Amaya-Chanaga, C.I.; Kumar, D.; Simmons, B.; Huser, N.; Gu, Y.; Hallin, M.; Lindquist, K.; Yafawi, R.; Choi, M.Y.; et al. Targeting the CXCR4 pathway using a novel anti-CXCR4 IgG1 antibody (PF-06747143) in chronic lymphocytic leukemia. J. Hematol. Oncol. 2017, 10, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashyap, M.K.; Kumar, D.; Jones, H.; Amaya-Chanaga, C.I.; Choi, M.Y.; Melo-Cardenas, J.; Ale-Ali, A.; Kuhne, M.R.; Sabbatini, P.; Cohen, L.J.; et al. Ulocuplumab (BMS-936564 / MDX1338): A fully human anti-CXCR4 antibody induces cell death in chronic lymphocytic leukemia mediated through a reactive oxygen species-dependent pathway. Oncotarget 2016, 7, 2809–2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, M.; Hartmann, T.; Krome, M.; Rawluk, J.; Tamamura, H.; Fujii, N.; Kipps, T.J.; Burger, J.A. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood 2005, 106, 1824–1830. [Google Scholar] [CrossRef] [Green Version]
- Musolino, C.; Di Cesare, E.; Alonci, A.; Allegra, A.; Orlando, A.; Grosso, P.; Squadrito, G. Serum levels of CD8 antigen and soluble interleukin 2 receptors in patients with B cell chronic lymphocytic leukemia. Acta Haematol. 1991, 85, 57–61. [Google Scholar] [CrossRef]
- Kara, I.O.; Sahin, B.; Gunesacar, R. Expression of soluble CD27 and interleukins-8 and -10 in B-cell chronic lymphocytic leukemia: Correlation with disease stage and prognosis. Adv. Ther. 2007, 24, 29–40. [Google Scholar] [CrossRef]
- Wang, H.Q.; Jia, L.; Li, Y.T.; Farren, T.; Agrawal, S.G.; Liu, F.T. Increased autocrine interleukin-6 production is significantly associated with worse clinical outcome in patients with chronic lymphocytic leukemia. J. Cell. Physiol. 2019, 234, 13994–14006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.Y.; Lafarge, S.; Dawe, D.; Lakhi, S.; Kumar, R.; Morales, C.; Marshall, A.; Gibson, S.B.; Johnston, J.B. Association of interleukin-6 and interleukin-8 with poor prognosis in elderly patients with chronic lymphocytic leukemia. Leuk. Lymphoma 2012, 53, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Molica, S.; Vitelli, G.; Levato, D.; Levato, L.; Dattilo, A.; Gandolfo, G.M. Clinico-biological implications of increased serum levels of interleukin-8 in B-cell chronic lymphocytic leukemia. Haematologica 1999, 84, 208–211. [Google Scholar]
- Wierda, W.G.; Johnson, M.M.; Do, K.A.; Manshouri, T.; Dey, A.; O’Brien, S.; Giles, F.J.; Kantarjian, H.; Thomas, D.; Faderl, S.; et al. Plasma interleukin 8 level predicts for survival in chronic lymphocytic leukaemia. Br. J. Haematol. 2003, 120, 452–456. [Google Scholar] [CrossRef]
- Parfieńczyk, A.; Kiersnowska-Rogowska, B.; Rogowski, F. Cytokine and adhesion molecule concentrations in blood of patients with B-cell chronic lymphocytic leukaemia with regard to disease progression. Rocz. Akad. Med. Bialymst. 2003, 48, 90–94. [Google Scholar]
- Chen, N.; Lv, X.; Li, P.; Lu, K.; Wang, X. Role of high expression of IL-9 in prognosis of CLL. Int. J. Clin. Exp. Pathol. 2014, 7, 716–721. [Google Scholar] [PubMed]
- Abbassy, H.A.; Aboelwafa, R.A.; Ghallab, O.M. Evaluation of Interleukin-9 Expression as a Potential Therapeutic Target in Chronic Lymphocytic Leukemia in a Cohort of Egyptian Patients. Indian J. Hematol. Blood Transfus. 2017, 33, 477–482. [Google Scholar] [CrossRef]
- Sjöberg, J.; Aguilar-Santelises, M.; Sjögren, A.M.; Pisa, E.K.; Ljungdahl, A.; Björkholm, M.; Jondal, M.; Mellstedt, H.; Pisa, P. Interleukin-10 mRNA expression in B-cell chronic lymphocytic leukaemia inversely correlates with progression of disease. Br. J. Haematol. 1996, 92, 393–400. [Google Scholar] [CrossRef]
- Lech-Maranda, E.; Grzybowska-Izydorczyk, O.; Wyka, K.; Mlynarski, W.; Borowiec, M.; Antosik, K.; Cebula-Obrzut, B.; Makuch-Lasica, H.; Nowak, G.; Klimkiewicz-Wojciechowska, G.; et al. Serum tumor necrosis factor-α and interleukin-10 levels as markers to predict outcome of patients with chronic lymphocytic leukemia in different risk groups defined by the IGHV mutation status. Arch. Immunol. Ther. Exp. (Warsz) 2012, 60, 477–486. [Google Scholar] [CrossRef]
- Cutrona, G.; Tripodo, C.; Matis, S.; Recchia, A.G.; Massucco, C.; Fabbi, M.; Colombo, M.; Emionite, L.; Sangaletti, S.; Gulino, A.; et al. Microenvironmental regulation of the IL-23R/IL-23 axis overrides chronic lymphocytic leukemia indolence. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Ferrajoli, A.; Keating, M.J.; Manshouri, T.; Giles, F.J.; Dey, A.; Estrov, Z.; Koller, C.A.; Kurzrock, R.; Thomas, D.A.; Faderl, S.; et al. The clinical significance of tumor necrosis factor-alpha plasma level in patients having chronic lymphocytic leukemia. Blood 2002, 100, 1215–1219. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Bone, N.D.; Stenson, M.J.; Novak, A.; Hedin, K.E.; Kay, N.E.; Ansell, S.M. Expression of the chemokine receptors CXCR4 and CCR7 and disease progression in B-cell chronic lymphocytic leukemia/ small lymphocytic lymphoma. Mayo Clin. Proc. 2004, 79, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, E.; Delgado-Pérez, L.; Campos-Caro, A.; Muñóz, J.; Paz, A.; Franco, R.; Brieva, J.A. The prognostic role of CXCR3 expression by chronic lymphocytic leukemia B cells. Haematologica 2007, 92, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Morabito, F.; Merendino, R.A.; Penna, G.; Cuzzola, M.; Stelitano, C.; Callea, V.; Di Pasquale, G.; Minciullo, P.L.; Gangemi, S. The CX3C chemokine fractalkine (CX3CL1) is detectable in serum of B cell chronic lymphocytic leukemia patients with lymph node involvement. Acta Haematol. 2005, 113, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, A.; Ollila, J.; Tobin, G.; Nagy, B.; Thunberg, U.; Aalto, Y.; Vihinen, M.; Vilpo, J.; Rosenquist, R.; Knuutila, S. Different gene expression in immunoglobulin-mutated and immunoglobulin-unmutated forms of chronic lymphocytic leukemia. Cancer Genet. Cytogenet. 2004, 153, 69–72. [Google Scholar] [CrossRef]
- Sivina, M.; Hartmann, E.; Kipps, T.J.; Rassenti, L.; Krupnik, D.; Lerner, S.; LaPushin, R.; Xiao, L.; Huang, X.; Werner, L.; et al. CCL3 (MIP-1α) plasma levels and the risk for disease progression in chronic lymphocytic leukemia. Blood 2011, 117, 1662–1669. [Google Scholar] [CrossRef] [Green Version]
- Forconi, F.; Moss, P. Perturbation of the normal immune system in patients with CLL. Blood 2015, 126, 573–581. [Google Scholar] [CrossRef] [Green Version]
- Morrison, V.A. Infectious complications of chronic lymphocytic leukaemia: Pathogenesis, spectrum of infection, preventive approaches. Best Pract. Res. Clin. Haematol. 2010, 23, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Hilal, T.; Gea-Banacloche, J.C.; Leis, J.F. Chronic lymphocytic leukemia and infection risk in the era of targeted therapies: Linking mechanisms with infections. Blood Rev. 2018, 32, 387–399. [Google Scholar] [CrossRef]
- Vitale, C.; Montalbano, M.C.; Salvetti, C.; Boccellato, E.; Griggio, V.; Boccadoro, M.; Coscia, M. Autoimmune Complications in Chronic Lymphocytic Leukemia in the Era of Targeted Drugs. Cancers 2020, 12, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasanu, C.A.; Alexandrescu, D.T. Risk for second nonlymphoid neoplasms in chronic lymphocytic leukemia. MedGenMed Medscape Gen. Med. 2007, 9, 35. [Google Scholar]
- Falchi, L.; Vitale, C.; Keating, M.J.; Lerner, S.; Wang, X.; Elhor Gbito, K.Y.; Strom, S.; Wierda, W.G.; Ferrajoli, A. Incidence and prognostic impact of other cancers in a population of long-term survivors of chronic lymphocytic leukemia. Ann. Oncol. 2016, 27, 1100–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcellini, W.; Capalbo, S.; Agostinelli, R.M.; Mauro, F.R.; Ambrosetti, A.; Calori, R.; Cortelezzi, A.; Laurenti, L.; Pogliani, E.M.; Pedotti, P.; et al. Relationship between autoimmune phenomena and disease stage and therapy in B-cell chronic lymphocytic leukemia. Haematologica 2006, 91, 1689–1692. [Google Scholar]
- Demir, C.; Ekinci, O. Clinical and serological autoimmune complications in chronic lymphocytic leukemia. Wien. Klin. Wochenschr. 2017, 129, 552–557. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef] [Green Version]
- Kyasa, M.J.; Parrish, R.S.; Schichman, S.A.; Zent, C.S. Autoimmune cytopenia does not predict poor prognosis in chronic lymphocytic leukemia/small lymphocytic lymphoma. Am. J. Hematol. 2003, 74, 1–8. [Google Scholar] [CrossRef]
- Zent, C.S.; Ding, W.; Schwager, S.M.; Reinalda, M.S.; Hoyer, J.D.; Jelinek, D.F.; Tschumper, R.C.; Bowen, D.A.; Call, T.G.; Shanafelt, T.D.; et al. The prognostic significance of cytopenia in chronic lymphocytic leukaemia/small lymphocytic lymphoma. Br. J. Haematol. 2008, 141, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Moreno, C.; Hodgson, K.; Ferrer, G.; Elena, M.; Filella, X.; Pereira, A.; Baumann, T.; Montserrat, E. Autoimmune cytopenia in chronic lymphocytic leukemia: Prevalence, clinical associations, and prognostic significance. Blood 2010, 116, 4771–4776. [Google Scholar] [CrossRef] [PubMed]
- Mauro, F.R.; Foa, R.; Cerretti, R.; Giannarelli, D.; Coluzzi, S.; Mandelli, F.; Girelli, G. Autoimmune hemolytic anemia in chronic lymphocytic leukemia: Clinical, therapeutic, and prognostic features. Blood 2000, 95, 2786–2792. [Google Scholar] [CrossRef] [PubMed]
- Duek, A.; Shvidel, L.; Braester, A.; Berrebi, A. Clinical and immunologic aspects of B chronic lymphocytic leukemia associated with autoimmune disorders. Isr. Med. Assoc. J. IMAJ 2006, 8, 828–831. [Google Scholar] [PubMed]
- Visco, C.; Ruggeri, M.; Laura Evangelista, M.; Stasi, R.; Zanotti, R.; Giaretta, I.; Ambrosetti, A.; Madeo, D.; Pizzolo, G.; Rodeghiero, F. Impact of immune thrombocytopenia on the clinical course of chronic lymphocytic leukemia. Blood 2008, 111, 1110–1116. [Google Scholar] [CrossRef] [Green Version]
- Zent, C.S.; Ding, W.; Reinalda, M.S.; Schwager, S.M.; Hoyer, J.D.; Bowen, D.A.; Jelinek, D.F.; Tschumper, R.C.; Call, T.G.; Shanafelt, T.D.; et al. Autoimmune cytopenia in chronic lymphocytic leukemia/small lymphocytic lymphoma: Changes in clinical presentation and prognosis. Leuk. Lymphoma 2009, 50, 1261–1268. [Google Scholar] [CrossRef]
- Maura, F.; Visco, C.; Falisi, E.; Reda, G.; Fabris, S.; Agnelli, L.; Tuana, G.; Lionetti, M.; Guercini, N.; Novella, E.; et al. B-cell receptor configuration and adverse cytogenetics are associated with autoimmune hemolytic anemia in chronic lymphocytic leukemia. Am. J. Hematol. 2013, 88, 32–36. [Google Scholar] [CrossRef]
- Visentin, A.; Imbergamo, S.; Gurrieri, C.; Frezzato, F.; Trimarco, V.; Martini, V.; Severin, F.; Raggi, F.; Scomazzon, E.; Facco, M.; et al. Major infections, secondary cancers and autoimmune diseases occur in different clinical subsets of chronic lymphocytic leukaemia patients. Eur. J. Cancer 2017, 72, 103–111. [Google Scholar] [CrossRef]
- Hampel, P.J.; Larson, M.C.; Kabat, B.; Call, T.G.; Ding, W.; Kenderian, S.S.; Bowen, D.; Boysen, J.; Schwager, S.M.; Leis, J.F.; et al. Autoimmune cytopenias in patients with chronic lymphocytic leukaemia treated with ibrutinib in routine clinical practice at an academic medical centre. Br. J. Haematol. 2018, 183, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Atef, B.; Azmy, E.; Aladle, D.; Mabed, M. The prevalence and prognostic significance of autoimmune cytopenias in a cohort of Egyptian patients with chronic lymphocytic leukemia. Hematol. Oncol. Stem Cell Ther. 2019, 12, 97–104. [Google Scholar] [CrossRef]
- Vitale, C.; Salvetti, C.; Griggio, V.; Porrazzo, M.; Schiattone, L.; Zamprogna, G.; Visentin, A.; Vassallo, F.; Cassin, R.; Rigolin, G.M.; et al. Pre-existing and treatment-emergent autoimmune cytopenias in patients with CLL treated with targeted drugs. Blood 2021, 137, 3507–3517. [Google Scholar] [CrossRef] [PubMed]
- Visco, C.; Novella, E.; Peotta, E.; Paolini, R.; Giaretta, I.; Rodeghiero, F. Autoimmune hemolytic anemia in patients with chronic lymphocytic leukemia is associated with IgVH status. Haematologica 2010, 95, 1230–1232. [Google Scholar] [CrossRef] [PubMed]
- Dearden, C.; Wade, R.; Else, M.; Richards, S.; Milligan, D.; Hamblin, T.; Catovsky, D. The prognostic significance of a positive direct antiglobulin test in chronic lymphocytic leukemia: A beneficial effect of the combination of fludarabine and cyclophosphamide on the incidence of hemolytic anemia. Blood 2008, 111, 1820–1826. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Li, J.Y.; Miao, K.R.; Cao, X.; Liu, Q.; Fan, L.; Qiao, C.; Wu, Y.J. The negative prognostic significance of positive direct antiglobulin test in Chinese patients with chronic lymphocytic leukemia. Leuk. Lymphoma 2009, 50, 1482–1487. [Google Scholar] [CrossRef]
- Ricci, F.; Tedeschi, A.; Vismara, E.; Colombo, C.; Veronese, S.; Nichelatti, M.; Cairoli, R.; Morra, E.; Montillo, M. Should a positive direct antiglobulin test be considered a prognostic predictor in chronic lymphocytic leukemia? Clin. Lymphoma Myeloma Leuk. 2013, 13, 441–446. [Google Scholar] [CrossRef]
- Quinquenel, A.; Al Nawakil, C.; Baran-Marszak, F.; Eclache, V.; Letestu, R.; Khalloufi, M.; Boubaya, M.; Le Roy, C.; Varin-Blank, N.; Delmer, A.; et al. Old DAT and new data: Positive direct antiglobulin test identifies a subgroup with poor outcome among chronic lymphocytic leukemia stage A patients. Am. J. Hematol. 2015, 90, E5–E8. [Google Scholar] [CrossRef]
- Shvidel, L.; Tadmor, T.; Braester, A.; Bairey, O.; Rahimi-Levene, N.; Herishanu, Y.; Klepfish, A.; Shtalrid, M.; Berrebi, A.; Polliack, A. Pathogenesis, prevalence, and prognostic significance of cytopenias in chronic lymphocytic leukemia (CLL): A retrospective comparative study of 213 patients from a national CLL database of 1518 cases. Ann. Hematol. 2013, 92, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Niemann, C.U.; Kater, A.P.; Gregor, M.; Cymbalista, F.; Buske, C.; Hillmen, P.; et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Abdallah, G.E.M.; Aly, M.M.; Abdelsalam, E.M.N.; Mohammed Saleh, M.F. Revisiting Autoimmunity in Chronic Lymphocytic Leukemia: Prognostic Value of Positive Direct Antiglobulin Test in a Retrospective Study and Literature Review. J. Blood Med. 2021, 12, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; O’Brien, S. Evolution of CLL treatment—From chemoimmunotherapy to targeted and individualized therapy. Nat. Rev. Clin. Oncol. 2018, 15, 510–527. [Google Scholar] [CrossRef] [PubMed]
- Strati, P.; Parikh, S.A.; Chaffee, K.G.; Kay, N.E.; Call, T.G.; Achenbach, S.J.; Cerhan, J.R.; Slager, S.L.; Shanafelt, T.D. Relationship between co-morbidities at diagnosis, survival and ultimate cause of death in patients with chronic lymphocytic leukaemia (CLL): A prospective cohort study. Br. J. Haematol. 2017, 178, 394–402. [Google Scholar] [CrossRef]
- Rotbain, E.C.; Niemann, C.U.; Rostgaard, K.; da Cunha-Bang, C.; Hjalgrim, H.; Frederiksen, H. Mapping comorbidity in chronic lymphocytic leukemia: Impact of individual comorbidities on treatment, mortality, and causes of death. Leukemia 2021. [Google Scholar] [CrossRef]
- Moreira, J.; Rabe, K.G.; Cerhan, J.R.; Kay, N.E.; Wilson, J.W.; Call, T.G.; Leis, J.F.; Jelinek, D.F.; Schwager, S.M.; Bowen, D.A.; et al. Infectious complications among individuals with clinical monoclonal B-cell lymphocytosis (MBL): A cohort study of newly diagnosed cases compared to controls. Leukemia 2013, 27, 136–141. [Google Scholar] [CrossRef]
- Agius, R.; Brieghel, C.; Andersen, M.A.; Pearson, A.T.; Ledergerber, B.; Cozzi-Lepri, A.; Louzoun, Y.; Andersen, C.L.; Bergstedt, J.; von Stemann, J.H.; et al. Machine learning can identify newly diagnosed patients with CLL at high risk of infection. Nat. Commun. 2020, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.A.; Niemann, C.U. Immune failure, infection and survival in chronic lymphocytic leukemia in Denmark. Haematologica 2018, 103, e330. [Google Scholar] [CrossRef] [Green Version]
- Crassini, K.R.; Best, O.G.; Mulligan, S.P. Immune failure, infection and survival in chronic lymphocytic leukemia. Haematologica 2018, 103, e329. [Google Scholar] [CrossRef] [PubMed]
- Mauro, F.R.; Giannarelli, D.; Visentin, A.; Reda, G.; Sportoletti, P.; Frustaci, A.M.; Chiarenza, A.; Ciolli, S.; Vitale, C.; Laurenti, L.; et al. Prognostic Impact and Risk Factors of Infections in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib. Cancers 2021, 13, 3240. [Google Scholar] [CrossRef]
- Scarfò, L.; Chatzikonstantinou, T.; Rigolin, G.M.; Quaresmini, G.; Motta, M.; Vitale, C.; Garcia-Marco, J.A.; Hernández-Rivas, J.; Mirás, F.; Baile, M.; et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: A joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia 2020, 34, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Langerbeins, P.; Eichhorst, B. Immune Dysfunction in Patients with Chronic Lymphocytic Leukemia and Challenges during COVID-19 Pandemic. Acta Haematol. 2021, 1–11. [Google Scholar] [CrossRef]
- SEER Data. Available online: https://seer.cancer.gov/statfacts/html/clyl.html (accessed on 30 May 2021).
- Travis, L.B.; Curtis, R.E.; Hankey, B.F.; Fraumeni, J.F., Jr. Second cancers in patients with chronic lymphocytic leukemia. J. Natl. Cancer Inst. 1992, 84, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Ailawadhi, S.; Bojanini, L.; Mehta, A.; Biswas, S.; Sher, T.; Roy, V.; Vishnu, P.; Marin-Acevedo, J.; Alegria, V.R.; et al. Trends in the risk of second primary malignancies among survivors of chronic lymphocytic leukemia. Blood Cancer J. 2019, 9, 75. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Wen, S.; McLaughlin, P.; O’Brien, S.; Wierda, W.G.; Lerner, S.; Strom, S.; Freireich, E.J.; Medeiros, L.J.; Kantarjian, H.M.; et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 904–910. [Google Scholar] [CrossRef] [Green Version]
- Maddocks-Christianson, K.; Slager, S.L.; Zent, C.S.; Reinalda, M.; Call, T.G.; Habermann, T.M.; Bowen, D.A.; Hoyer, J.D.; Schwager, S.; Jelinek, D.F.; et al. Risk factors for development of a second lymphoid malignancy in patients with chronic lymphocytic leukaemia. Br. J. Haematol. 2007, 139, 398–404. [Google Scholar] [CrossRef]
- Bond, D.A.; Huang, Y.; Fisher, J.L.; Ruppert, A.S.; Owen, D.H.; Bertino, E.M.; Rogers, K.A.; Bhat, S.A.; Grever, M.R.; Jaglowski, S.M.; et al. Second cancer incidence in CLL patients receiving BTK inhibitors. Leukemia 2020, 34, 3197–3205. [Google Scholar] [CrossRef] [PubMed]
- Toro, J.R.; Blake, P.W.; Björkholm, M.; Kristinsson, S.Y.; Wang, Z.; Landgren, O. Prior history of non-melanoma skin cancer is associated with increased mortality in patients with chronic lymphocytic leukemia. Haematologica 2009, 94, 1460–1464. [Google Scholar] [CrossRef]
- Royle, J.A.; Baade, P.D.; Joske, D.; Girschik, J.; Fritschi, L. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: A population-based study. Br. J. Cancer 2011, 105, 1076–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mhibik, M.; Wiestner, A.; Sun, C. Harnessing the Effects of BTKi on T Cells for Effective Immunotherapy against CLL. Int. J. Mol. Sci. 2019, 21, 68. [Google Scholar] [CrossRef] [Green Version]
- Pleyer, C.; Wiestner, A.; Sun, C. Immunological changes with kinase inhibitor therapy for chronic lymphocytic leukemia. Leuk. Lymphoma 2018, 59, 2792–2800. [Google Scholar] [CrossRef] [PubMed]
| NK-Cell Aberrations | Consequence | Impact on Disease | Ref. |
|---|---|---|---|
| Reduction of NKp30 and NKp46 activating receptors | Exhaustion state on NK cells | Immune escape | [63] |
| Increased expression of Tim-3 immune checkpoint | Exhaustion state on NK cells | Immune escape | [63] |
| Abundance of immature CD56bright NK cells | Reduced NKG2D activating receptor and cytokine (IL-10 and IL-13) secretion | CLL cells survival and proliferation | [69,70] |
| Irregular NKG2AR activity and reduced killer Ig-like receptors (KIRs) | Hampering of NK-cell cytotoxicity and viability | Compromised immune system | [1] |
| Lower natural cytokine receptors (NCRs) expression | Immune escape | [69] | |
| NKG2D downregulation | Hampering of NK cytotoxicity | [65] |
| Cytokines | Alteration Compared to Healthy Controls | Correlation with Biological Characteristics and/or Prognosis | Ref. |
|---|---|---|---|
| sCD8 | Increased | Active disease, advanced Rai stage. | [120] |
| sCD27 | Increased | Advanced Rai stage, elevated β2-microglobulin. | [121] |
| sIL-2R | Increased | Active disease, advanced Rai stage, high lymphocyte count. | [120] |
| IL-6 | Increased | Advanced Rai stage, previous treatment, elevated β2-microglobulin, elevated LDH, worse OS *. | [104] |
| Increased | Advanced Binet stage, previous treatment, non-CR status, presence of del(17p)/del(11q), shorter absolute LDT, worse TTFT, worse PFS. | [122] | |
| Increased | Elevated β2-microglobulin. IL-6, IL-8 and TNFα levels correlated with each other. In patients ≥ 70 years, IL-6 is a better prognostic marker than IGHV mutational status. | [123] | |
| IL-8 | Increased | Active disease (progression from Binet stage A to B/C). | [124] |
| Increased | Elevated β2-microglobulin. IL-8, IL-6 and TNFα levels correlated with each other. | [123] | |
| Increased | Advanced Rai stage, elevated β2-microglobulin. | [121,125,126] | |
| IL-9 | Increased | Advanced stage, elevated β2-microglobulin, higher ZAP70 expression. | [127] |
| Increased | Advanced Rai stage, higher ZAP70 and CD38 expression. | [128] | |
| IL-10 | Increased | Advanced Rai stage, previous treatment, elevated β2-microglobulin, elevated LDH, worse OS *. | [104] |
| Decreased | Active disease. | [129] | |
| Increased | High-risk and active disease. Worse TFS (in high-risk group, regardless of IGHV mutational status), worse OS §. | [130] | |
| Increased | Advanced Rai stage, elevated β2-microglobulin. | [121] | |
| IL-23R | Decreased | Worse prognosis in early stage CLL, worse TTFT. | [131] |
| TNFα | Increased | Advanced stage, elevated β2-microglobulin, higher CD38 expression, presence of del(11q), tris(12), chromosome 17 aberrations, worse OS. | [132] |
| Increased | High-risk and active disease. Worse TFS (in high-risk group, regardless of IGHV mutational status), worse OS §. | [130] | |
| Increased | Elevated β2-microglobulin. IL-6, IL-8 and TNFα levels correlated with each other. In patients ≥ 70 years, IL-6 is a better prognostic marker than IGHV mutational status. | [123] | |
| SDF-1 and uPAR | Increased | Advanced stage. | [113] |
| SDF-1 and CXCR4 | Increased | Advanced Rai stage. | [133] |
| IGFBP-2, BMP-4, MCP-4 | Decreased | Advanced stage. | [113] |
| CCR7 | Increased | Advanced Rai stage. | [133] |
| CXCR3 | Decreased | Advanced stage, higher CD38 expression, unmutated IGHV status, worse OS. | [134] |
| CX3CL1 | Increased | Lymph node involvement, worse TTT, high risk of progression (especially in earlier stages of disease). | [135] |
| Increased | Higher ZAP70 expression. | [136] | |
| CCL3/MIP-1α | Increased | Advanced stage, higher CD38 and ZAP70 expression, unmutated IGHV status. | [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, C.; Boccellato, E.; Comba, L.; Jones, R.; Perutelli, F.; Griggio, V.; Coscia, M. Impact of Immune Parameters and Immune Dysfunctions on the Prognosis of Patients with Chronic Lymphocytic Leukemia. Cancers 2021, 13, 3856. https://doi.org/10.3390/cancers13153856
Vitale C, Boccellato E, Comba L, Jones R, Perutelli F, Griggio V, Coscia M. Impact of Immune Parameters and Immune Dysfunctions on the Prognosis of Patients with Chronic Lymphocytic Leukemia. Cancers. 2021; 13(15):3856. https://doi.org/10.3390/cancers13153856
Chicago/Turabian StyleVitale, Candida, Elia Boccellato, Lorenzo Comba, Rebecca Jones, Francesca Perutelli, Valentina Griggio, and Marta Coscia. 2021. "Impact of Immune Parameters and Immune Dysfunctions on the Prognosis of Patients with Chronic Lymphocytic Leukemia" Cancers 13, no. 15: 3856. https://doi.org/10.3390/cancers13153856







