Intravesical Instillation of Azacitidine Suppresses Tumor Formation through TNF-R1 and TRAIL-R2 Signaling in Genotoxic Carcinogen-Induced Bladder Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. MTT Assays
2.3. Clonogenic Assay
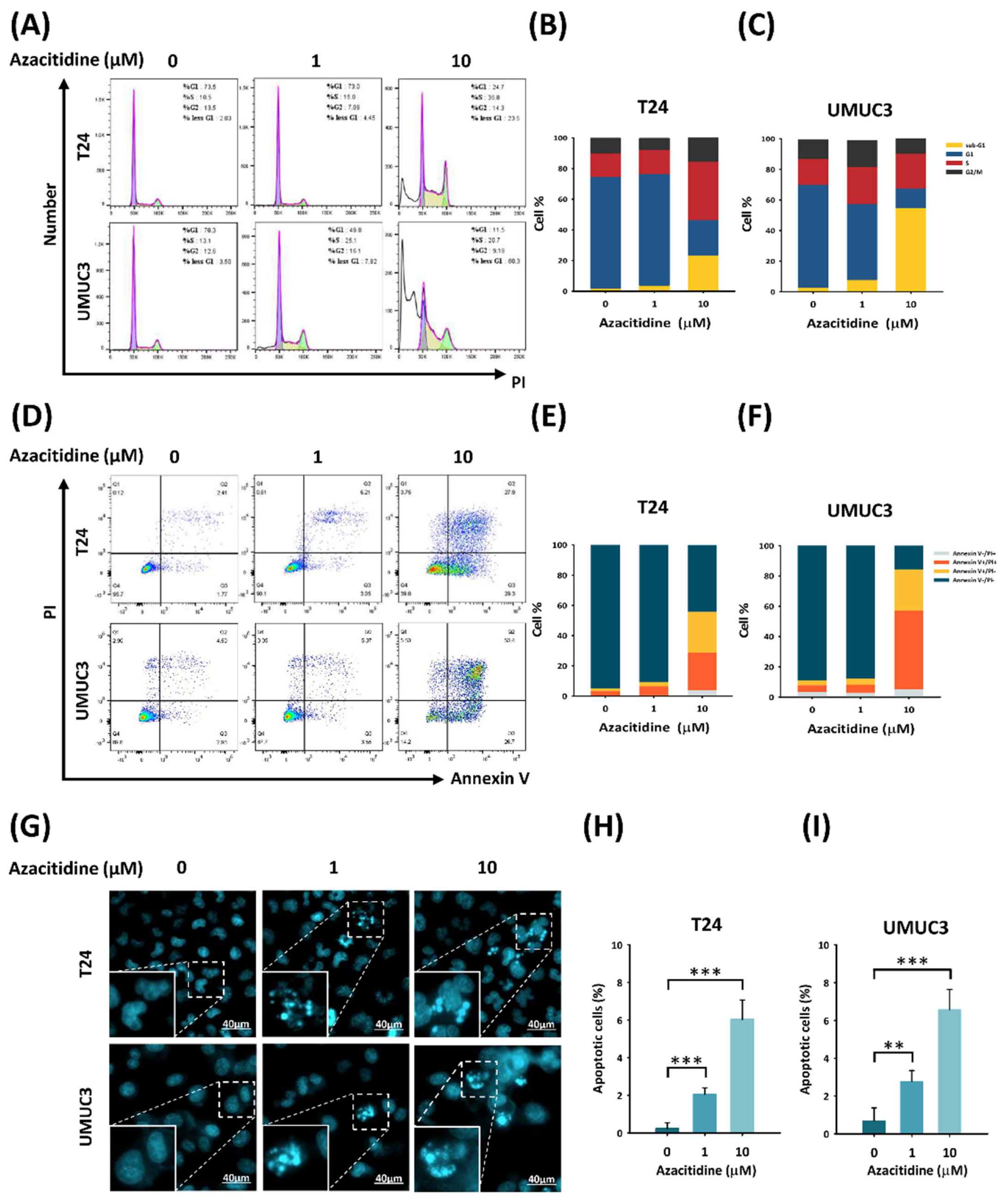
2.4. Flow Cytometry Analysis
2.5. Hoechst Staining Assay
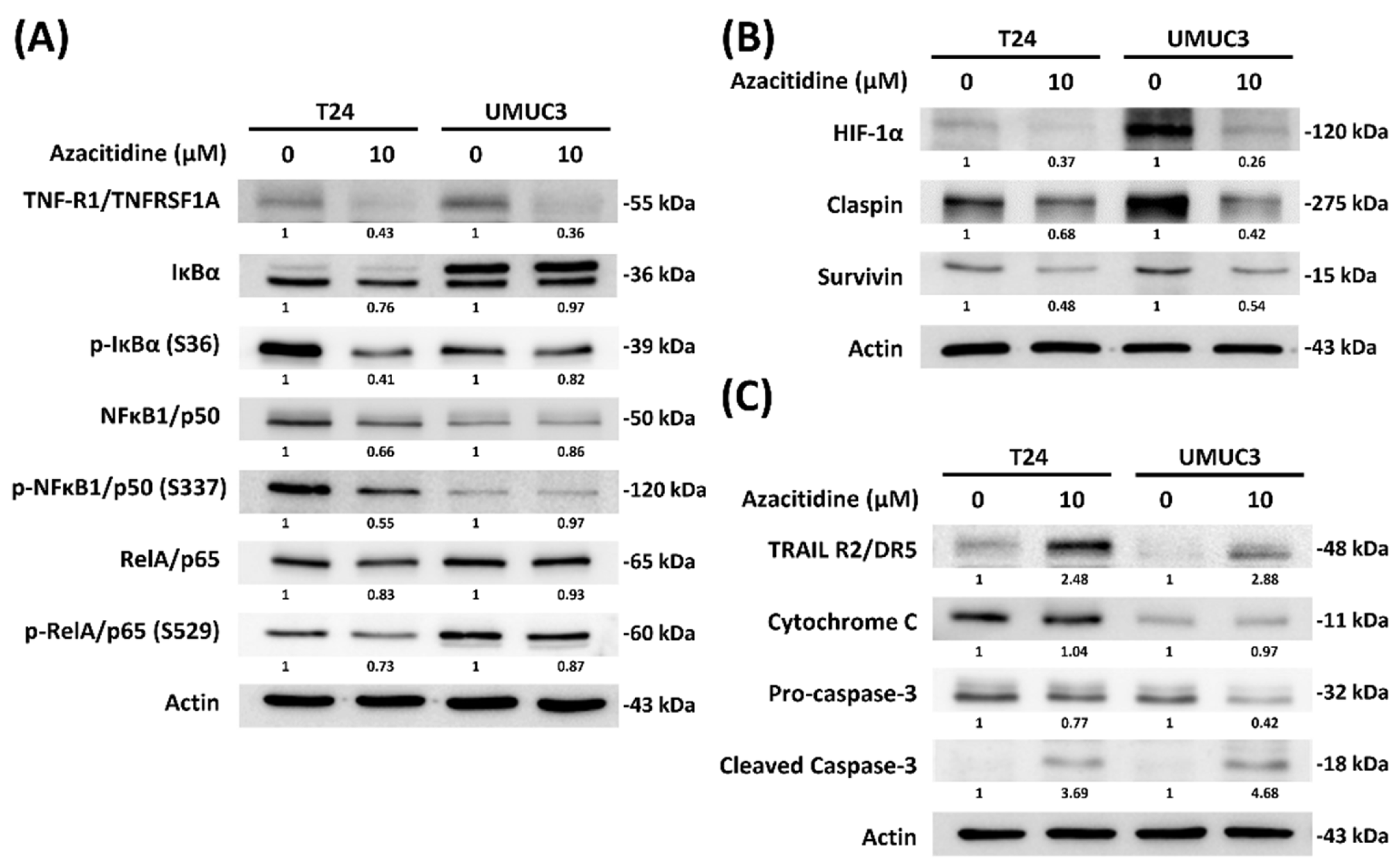
2.6. Immunoblotting Assays
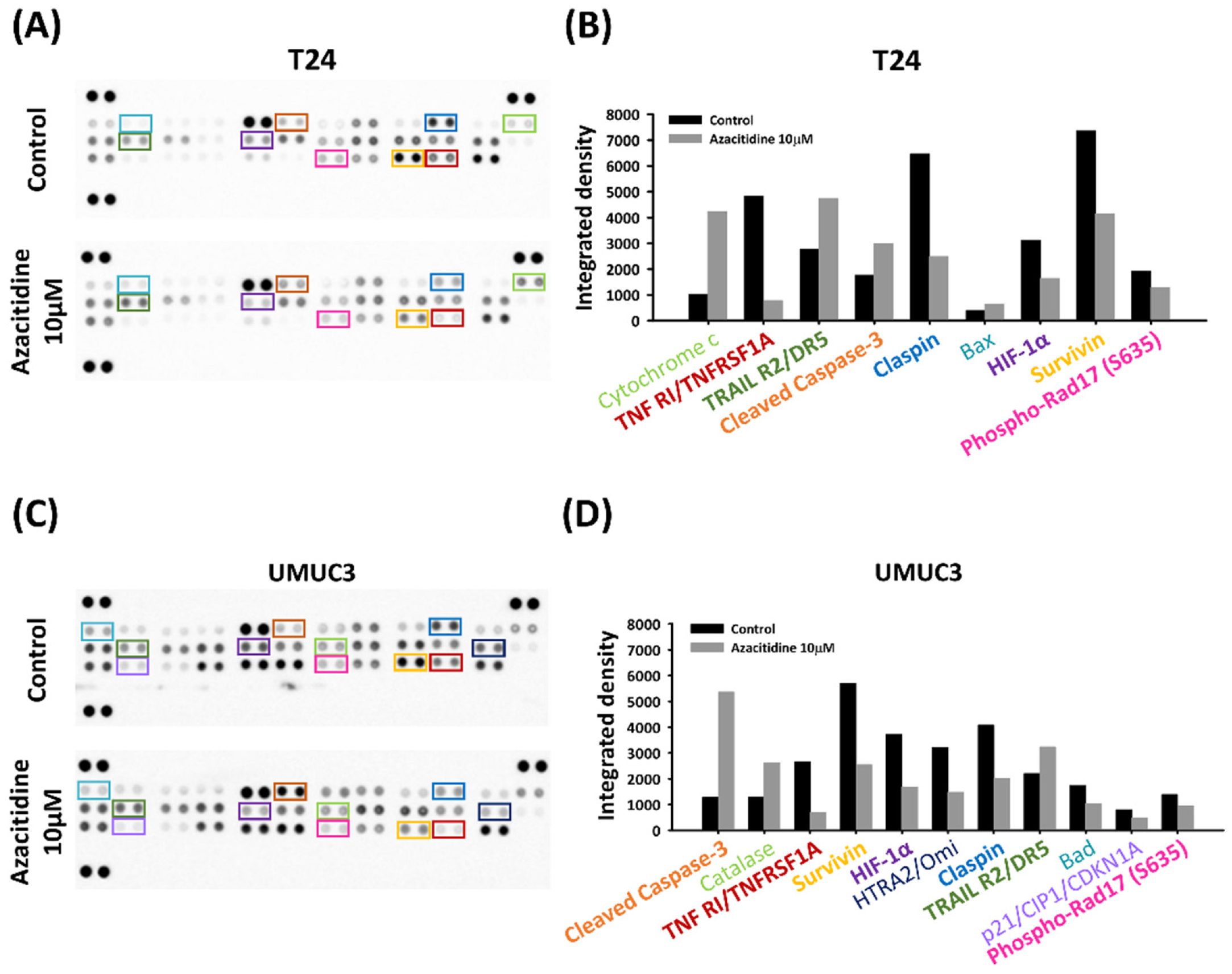
2.7. Human Apoptosis Array for Proteome Profiling
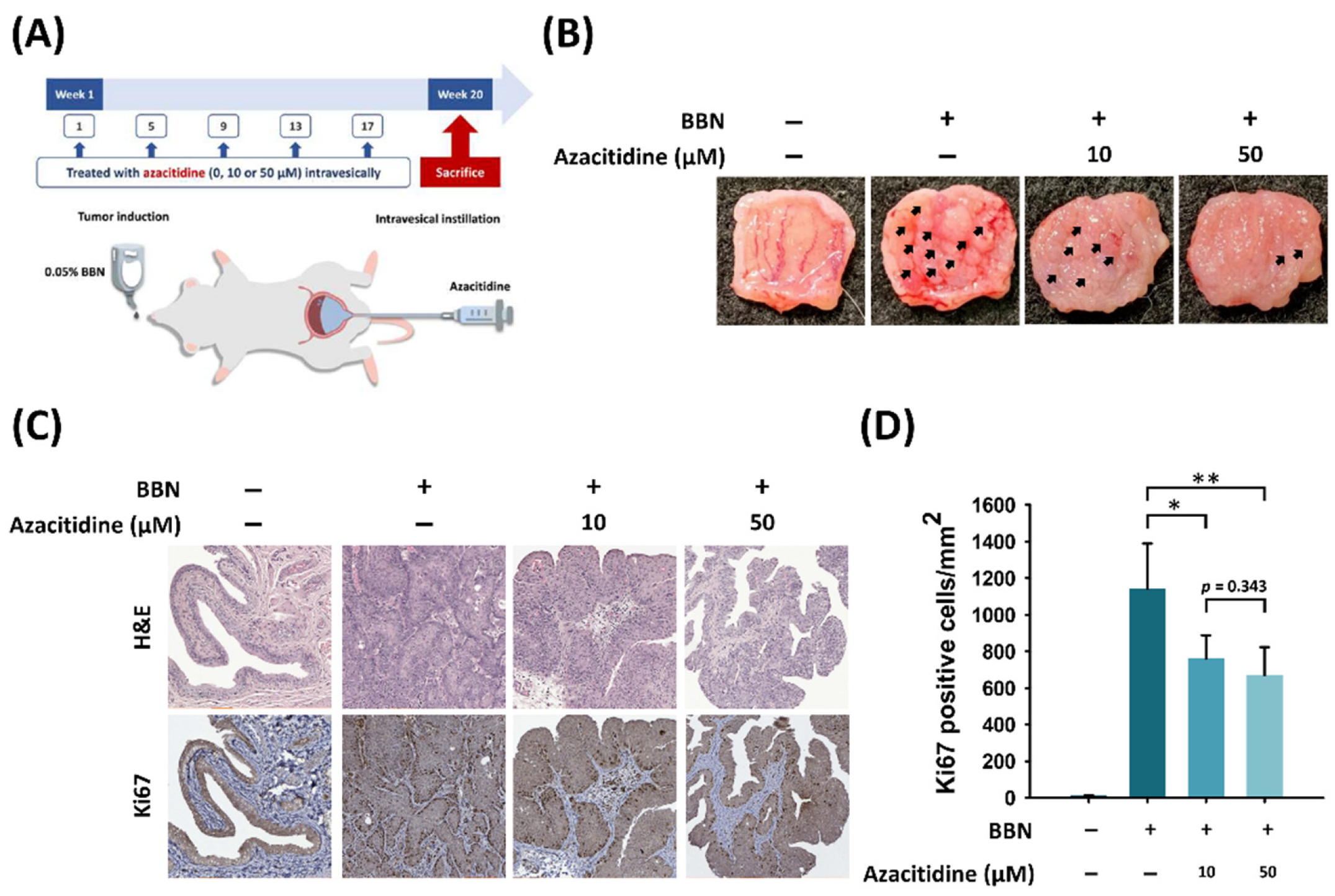
2.8. BBN-Induced Bladder Cancer Animal Model (Azacitidine Treatment)
2.9. Hematoxylin and Eosin Staining
2.10. Immunohistochemistry
2.11. Statistical Analysis
3. Results
3.1. Azacitidine Treatment of the UBUC Cell Lines Suppresses DNMT Expression and Cell Growth
3.2. Azacitidine Induces Apoptosis of UBUC Cell Lines through Claspin and Survivin
3.3. Intravesical Instillation of Azacitidine Suppresses Bladder Cancer Growth in a BBN Rat Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; MSHS, M. Bladder cancer: A review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Piñeiro, J.A.; Martínez-Piñeiro, L. BCG update: Intravesical therapy. Eur. Urol. 1997, 31, 31. [Google Scholar] [CrossRef] [PubMed]
- Bandari, J.; Maganty, A.; MacLeod, L.C.; Davies, B.J. Manufacturing and the market: Rationalizing the shortage of Bacillus Calmette-Guérin. Eur. Urol. Focus 2018, 4, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.E.; Smelser, W.W.; Chang, S.S. Side effects of intravesical BCG and chemotherapy for bladder cancer: What they are and how to manage them. Urology 2021, 149, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Gasión, J.P.B.; Cruz, J.F.J. Improving efficacy of intravesical chemotherapy. Eur. Urol. 2006, 50, 225–234. [Google Scholar] [CrossRef]
- Michalak, E.; Burr, M.; Bannister, A.J.; Dawson, M.A. The roles of DNA, RNA and histone methylation in ageing and cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 573–589. [Google Scholar] [CrossRef]
- Rhee, I.; Bachman, K.E.; Park, B.H.; Jair, K.-W.; Yen, R.-W.C.; Schuebel, K.E.; Cui, H.; Feinberg, A.; Lengauer, C.; Kinzler, K.W.; et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nat. Cell Biol. 2002, 416, 552–556. [Google Scholar] [CrossRef]
- Jones, P.A.; Issa, J.-P.; Baylin, P.A.J.S. Targeting the cancer epigenome for therapy. Nat. Rev. Genet. 2016, 17, 630–641. [Google Scholar] [CrossRef]
- Faleiro, I.; Leão, R.; Binnie, A.; De Mello, R.A.; Maia, A.-T.; Castelo-Branco, P. Epigenetic therapy in urologic cancers: An update on clinical trials. Oncotarget 2016, 8, 12484–12500. [Google Scholar] [CrossRef]
- Issa, J.P.; Kantarjian, H.M.; Kirkpatrick, P. Azacitidine. Nat. Rev. Drug Discov. 2005, 4, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Christman, J.K. 5-azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy. Oncogene 2002, 21, 5483–5495. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M. Myelodysplastic syndromes. Haematologica 2020, 383, 1358–1374. [Google Scholar]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs. conventional care regimens in older patients with newly diagnosed AML with > 30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef]
- Cowan, L.A.; Talwar, S.; Yang, A.S. Will DNA methylation inhibitors work in solid tumors? A review of the clinical experience with azacitidine and decitabine in solid tumors. Epigenomics 2010, 2, 71–86. [Google Scholar] [CrossRef]
- O’Rourke, C.J.; Knabben, V.; Bolton, E.; Moran, D.; Lynch, T.; Hollywood, D.; Perry, A.S. Manipulating the epigenome for the treatment of urological malignancies. Pharmacol. Ther. 2013, 138, 185–196. [Google Scholar] [CrossRef]
- Wang, X.; Chen, E.; Yang, X.; Wang, Y.; Quan, Z.; Wu, X.; Luo, C. 5-azacytidine inhibits the proliferation of bladder cancer cells via reversal of the aberrant hypermethylation of the hepaCAM gene. Oncol. Rep. 2015, 35, 1375–1384. [Google Scholar] [CrossRef][Green Version]
- Ramachandran, K.; Gordian, E.; Singal, R. 5-azacytidine reverses drug resistance in bladder cancer cells. Anticancer Res. 2011, 31, 31. [Google Scholar]
- Sung, W.-W.; Wang, Y.-C.; Lin, P.-L.; Cheng, Y.-W.; Chen, C.-Y.; Wu, T.-C.; Lee, H. IL-10 promotes tumor aggressiveness via upregulation of CIP2A transcription in lung adenocarcinoma. Clin. Cancer Res. 2013, 19, 4092–4103. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.W.; Wang, Y.C.; Cheng, Y.W.; Lee, M.C.; Yeh, K.T.; Wang, L.; Wang, J.; Chen, C.Y.; Lee, H. A polymorphic -844T/C in FasL promoter predicts survival and relapse in non-small cell lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 5991–5999. [Google Scholar] [CrossRef]
- John, B.A.; Said, N. Insights from animal models of bladder cancer: Recent advances, challenges, and opportunities. Oncotarget 2017, 8, 57766–57781. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Huang, Y.-H.; Wei, T.-Y.; Huang, K.-M.; Ho, S.-H.; Bih, L.-I. Motor and bladder dysfunctions in patients with vertebral fractures at the thoracolumbar junction. Eur. Spine J. 2011, 21, 844–849. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kratzsch, T.; Kuhn, S.A.; Joedicke, A.; Hanisch, U.K.; Vajkoczy, P.; Hoffmann, J.; Fichtner, I. Treatment with 5-azacitidine delay growth of glioblastoma xenografts: A potential new treatment approach for glioblastomas. J. Cancer Res. Clin. Oncol. 2018, 144, 809–819. [Google Scholar] [CrossRef]
- Oing, C.; Verem, I.; Mansour, W.Y.; Bokemeyer, C.; Dyshlovoy, S.; Honecker, F. 5-azacitidine exerts prolonged pro-apoptotic effects and overcomes cisplatin-resistance in non-seminomatous germ cell tumor cells. Int. J. Mol. Sci. 2018, 20, 21. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Li, Z.; Zhu, M.; Zhang, Z.; Wang, Y.; Jing, H. Promoter hypermethylation of PTPL1, PTPN6, DAPK, p16 and 5-azacitidine inhibits growth in DLBCL. Oncol. Rep. 2015, 35, 139–146. [Google Scholar] [CrossRef]
- Agrawal, K.; Das, V.; Vyas, P.; Hajdúch, M. Nucleosidic DNA demethylating epigenetic drugs—A comprehensive review from discovery to clinic. Pharmacol. Ther. 2018, 188, 45–79. [Google Scholar] [CrossRef]
- Li, H.; Chiappinelli, K.B.; Guzzetta, A.A.; Easwaran, H.; Yen, R.-W.C.; Vatapalli, R.; Topper, M.J.; Luo, J.; Connolly, R.M.; Azad, N.S.; et al. Immune regulation by low doses of the DNA methyltransferase inhibitor 5-azacitidine in common human epithelial cancers. Oncotarget 2014, 5, 587–598. [Google Scholar] [CrossRef]
- Buchi, F.; Masala, E.; Rossi, A.; Valencia, A.; Spinelli, E.; Sanna, A.; Gozzini, A.; Santini, V. Redistribution of H3K27me3 and acetylated histone H4 upon exposure to azacitidine and decitabine results in de-repression of the AML1/ETO target geneIL3. Epigenetics 2013, 9, 387–395. [Google Scholar] [CrossRef]
- Wang, X.M.; Wang, X.; Li, J.; Evers, B.M. Effects of 5-azacytidine and butyrate on differentiation and apoptosis of hepatic cancer cell lines. Ann. Surg. 1998, 227, 922–931. [Google Scholar] [CrossRef] [PubMed]
- von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef]
- Lund, P.; Kotova, I.; Kedinger, V.; Khanwalkar, H.; Voltz, E.; Hahn, W.C.; Gronemeyer, H. Transformation-dependent silencing of tumor-selective apoptosis-inducing TRAIL by DNA hypermethylation is antagonized by decitabine. Mol. Cancer Ther. 2011, 10, 1611–1623. [Google Scholar] [CrossRef] [PubMed]
- Soncini, M.; Santoro, F.; Gutierrez, A.; Frigè, G.; Romanenghi, M.; Botrugno, O.A.; Pallavicini, I.; Pelicci, P.; Di Croce, L.; Minucci, S. The DNA demethylating agent decitabine activates the TRAIL pathway and induces apoptosis in acute myeloid leukemia. Biochim. Biophys. Acta 2013, 1832, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Shen, D.; Kong, C.; Zhang, Z.; Zeng, Y.; Lin, X.; Liu, X. NF-κB suppresses apoptosis and promotes bladder cancer cell proliferation by upregulating survivin expression in vitro and in vivo. Sci. Rep. 2017, 7, 40723. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, D.; Ramos-Vara, J.A.; Hahn, N.M.; Waddell, J.; Olbricht, G.R.; Zheng, R.; Stewart, J.C.; Knapp, D.W. DNMT1: An emerging target in the treatment of invasive urinary bladder cancer. Urol. Oncol. 2013, 31, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.I.; Smits, V.A.; Fernaud, J.R.H.; Mamely, I.; Freire, R. Cleavage and degradation of Claspin during apoptosis by caspases and the proteasome. Cell Death Differ. 2007, 14, 1433–1442. [Google Scholar] [CrossRef][Green Version]
- Kenneth, N.S.; Mudie, S.; Rocha, S. IKK and NF-kappaB-mediated regulation of Claspin impacts on ATR checkpoint function. EMBO J. 2010, 29, 2966–2978. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Giallongo, C.; La Cava, P.; Parrinello, N.L.; Chiechi, A.; Vetro, C.; Tibullo, D.; Di Raimondo, F.; Liotta, L.A.; Espina, V.; et al. Proteomic analysis reveals autophagy as pro-survival pathway elicited by long-term exposure with 5-azacitidine in high-risk myelodysplasia. Front. Pharmacol. 2017, 8, 204. [Google Scholar] [CrossRef]
- Owen, K.L.; Brockwell, N.K.; Parker, B.S. JAK-STAT signaling: A double-edged sword of immune regulation and cancer progression. Cancers 2019, 11, 2002. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, S.; Ibrahim, A.N.; Wang, J.; Deng, Z.; Wang, M. RCC2 promotes proliferation and radio-resistance in glioblastoma via activating transcription of DNMT1. Biochem. Biophys. Res. Commun. 2019, 516, 999–1006. [Google Scholar] [CrossRef]
- Robert, G.; Auberger, P. Azacitidine resistance caused by LAMP2 deficiency: A therapeutic window for the use of autophagy inhibitors in MDS/AML patients? Autophagy 2019, 15, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, S.T.; Han, Y.; Visconte, V.; Przychodzen, B.; Espitia, C.M.; Phillips, J.; Anwer, F.; Advani, A.; Carraway, H.E.; Kelly, K.R.; et al. The novel autophagy inhibitor ROC-325 augments the antileukemic activity of azacitidine. Leukemia 2019, 33, 2971–2974. [Google Scholar] [CrossRef] [PubMed]
- Schnekenburger, M.; Grandjenette, C.; Ghelfi, J.; Karius, T.; Foliguet, B.; Dicato, M.; Diederich, M. Sustained exposure to the DNA demethylating agent, 2′-deoxy-5-azacytidine, leads to apoptotic cell death in chronic myeloid leukemia by promoting differentiation, senescence, and autophagy. Biochem. Pharmacol. 2011, 81, 364–378. [Google Scholar] [CrossRef]
- Zhang, H.H.; Huang, B.; Cao, Y.H.; Li, Q.; Xu, H.F. Role of 5-aza-CdR in mitomycin-C chemosensitivity of T24 bladder cancer cells. Oncol. Lett. 2017, 14, 5652–5656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hahn, N.M.; Bonney, P.L.; Dhawan, D.; Jones, D.R.; Balch, C.; Guo, Z.; Hartman-Frey, C.; Fang, F.; Parker, H.G.; Kwon, E.M.; et al. Subcutaneous 5-azacitidine treatment of naturally occurring canine urothelial carcinoma: A novel epigenetic approach to human urothelial carcinoma drug development. J. Urol. 2012, 187, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Min, M.; Yang, C.; Tian, C.; Gookin, S.; Carter, D.; Spencer, S.L. Ki67 is a graded rather than a binary marker of proliferation versus quiescence. Cell Rep. 2018, 24, 1105–1112.e5. [Google Scholar] [CrossRef]
- Fantini, D.; Glaser, A.; Rimar, K.J.; Wang, Y.; Schipma, M.; Varghese, N.; Rademaker, A.; Behdad, A.; Yellapa, A.; Yu, Y.; et al. A carcinogen-induced mouse model recapitulates the molecular alterations of human muscle invasive bladder cancer. Oncogene 2018, 37, 1911–1925. [Google Scholar] [CrossRef]
- Wei, A.H.; Döhner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.-H.; Porkka, K.; et al. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N. Engl. J. Med. 2020, 383, 2526–2537. [Google Scholar] [CrossRef]
- Peterson, B.A.; Collins, A.J.; Vogelzang, N.J.; Bloomfield, C.D. 5-azacytidine and renal tubular dysfunction. Blood 1981, 57, 182–185. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-C.; Chang, Y.-C.; Wu, M.-Y.; Yu, C.-Y.; Chen, S.-L.; Sung, W.-W. Intravesical Instillation of Azacitidine Suppresses Tumor Formation through TNF-R1 and TRAIL-R2 Signaling in Genotoxic Carcinogen-Induced Bladder Cancer. Cancers 2021, 13, 3933. https://doi.org/10.3390/cancers13163933
Wang S-C, Chang Y-C, Wu M-Y, Yu C-Y, Chen S-L, Sung W-W. Intravesical Instillation of Azacitidine Suppresses Tumor Formation through TNF-R1 and TRAIL-R2 Signaling in Genotoxic Carcinogen-Induced Bladder Cancer. Cancers. 2021; 13(16):3933. https://doi.org/10.3390/cancers13163933
Chicago/Turabian StyleWang, Shao-Chuan, Ya-Chuan Chang, Min-You Wu, Chia-Ying Yu, Sung-Lang Chen, and Wen-Wei Sung. 2021. "Intravesical Instillation of Azacitidine Suppresses Tumor Formation through TNF-R1 and TRAIL-R2 Signaling in Genotoxic Carcinogen-Induced Bladder Cancer" Cancers 13, no. 16: 3933. https://doi.org/10.3390/cancers13163933
APA StyleWang, S.-C., Chang, Y.-C., Wu, M.-Y., Yu, C.-Y., Chen, S.-L., & Sung, W.-W. (2021). Intravesical Instillation of Azacitidine Suppresses Tumor Formation through TNF-R1 and TRAIL-R2 Signaling in Genotoxic Carcinogen-Induced Bladder Cancer. Cancers, 13(16), 3933. https://doi.org/10.3390/cancers13163933









