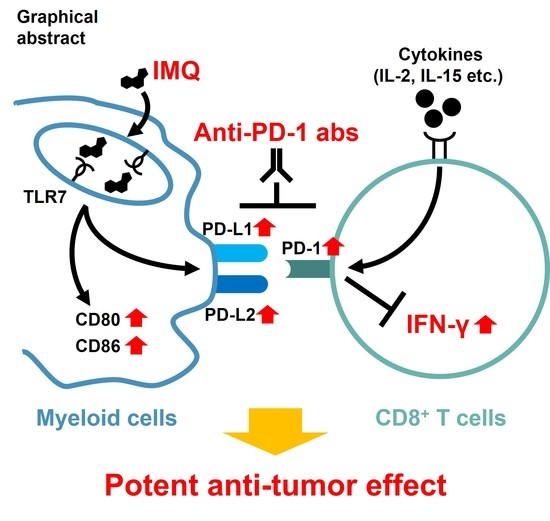
Combination Treatment of Topical Imiquimod Plus Anti-PD-1 Antibody Exerts Significantly Potent Antitumor Effect
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Cell Lines
2.3. Murine Tumor Model
2.4. Histopathologic Analyses
2.5. In Vitro Assay
2.6. Cell Isolation
2.7. Flow Cytometric Analysis
2.8. qRT-PCR
2.9. Measurement of Cytokines
2.10. Killing Assay
2.11. Statistical Analyses
3. Results
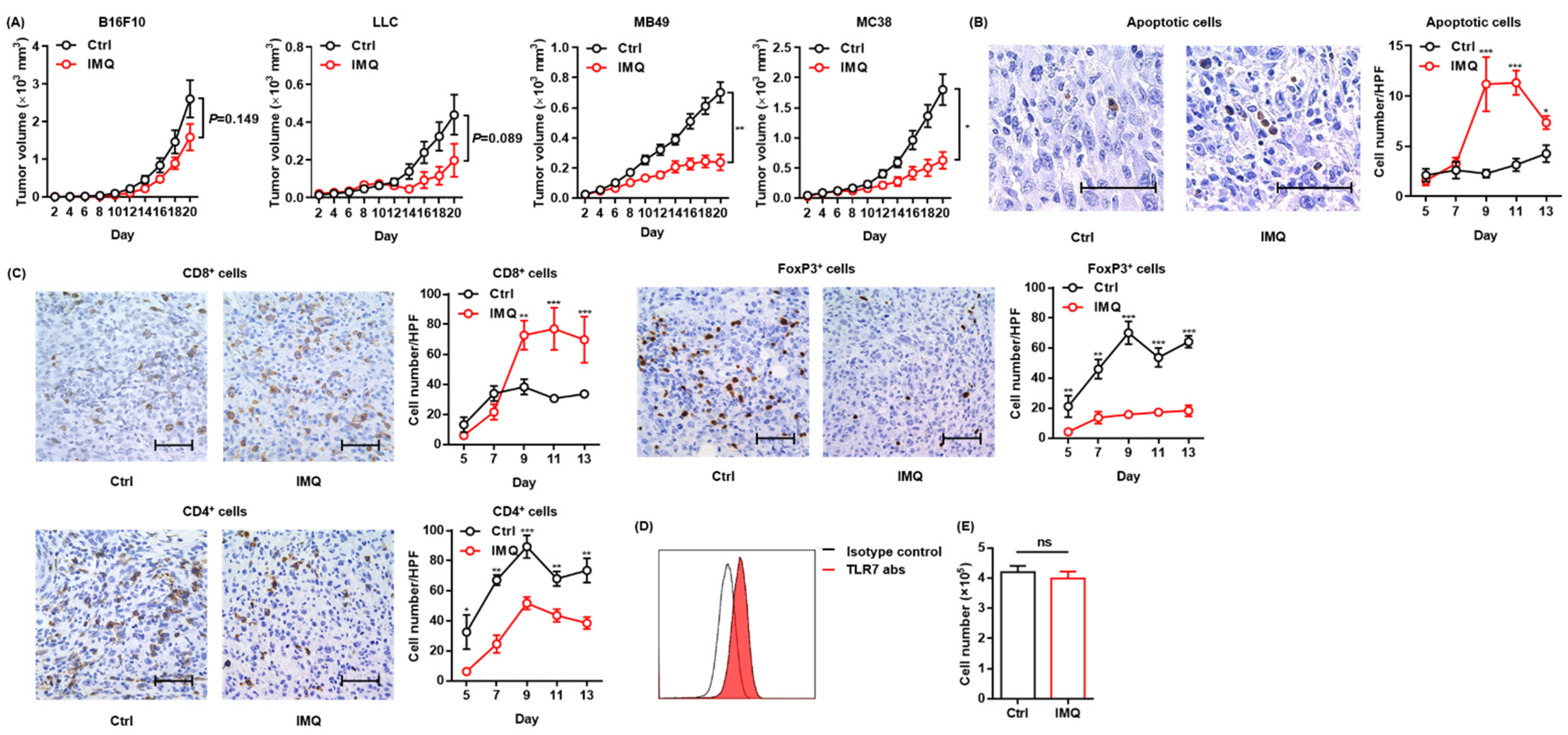
3.1. Topical IMQ Exerts an Antitumor Effect through Enhancement of the Immune Response to MC38 Colon Cancer
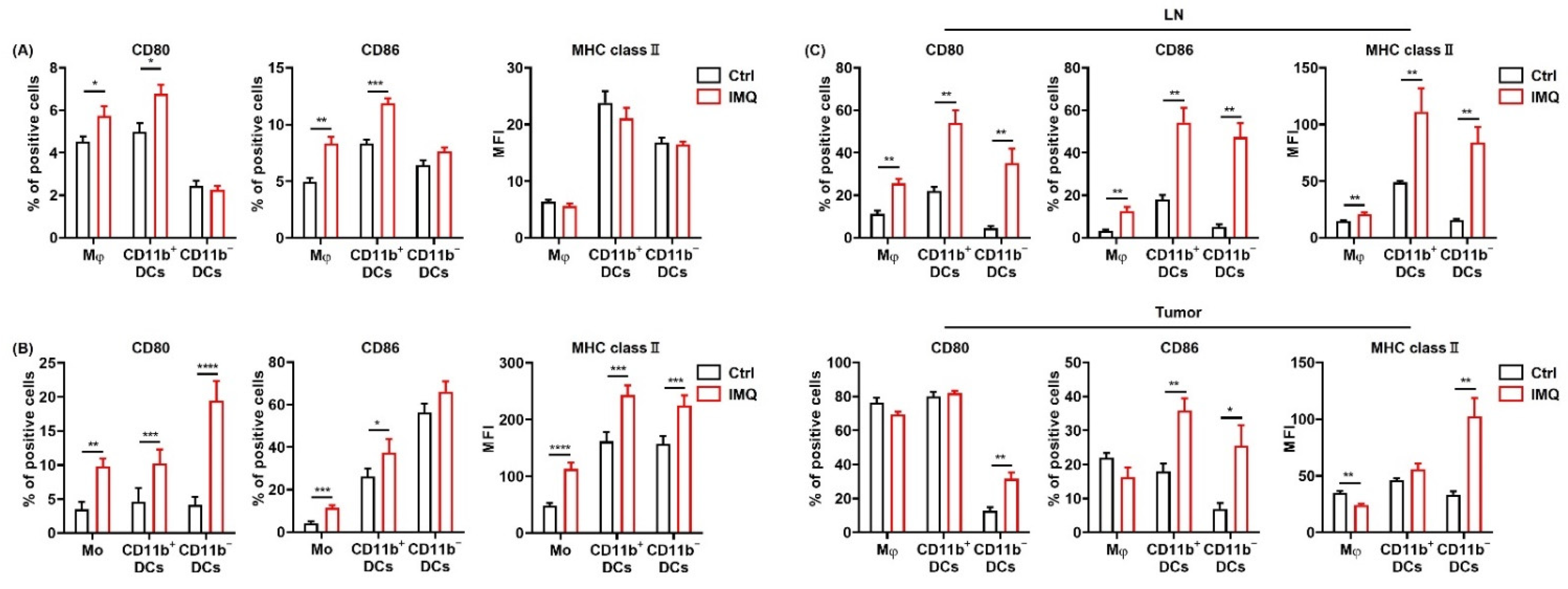
3.2. IMQ Activates Myeloid Cells, Leading to Upregulation of Costimulatory Molecules and MHC Class II
3.3. Antitumor Effect of IMQ Is Dependent on Adaptive Immunity
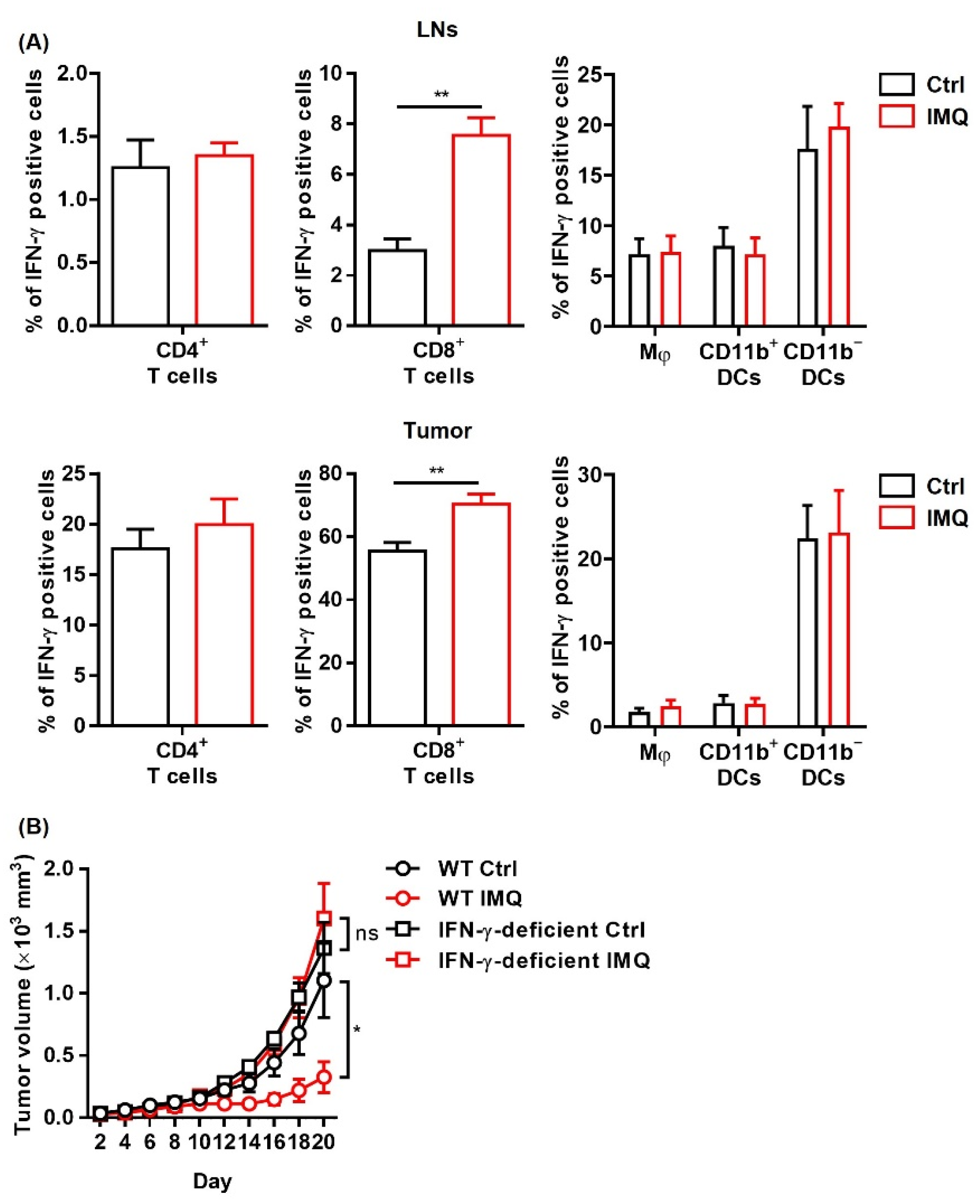
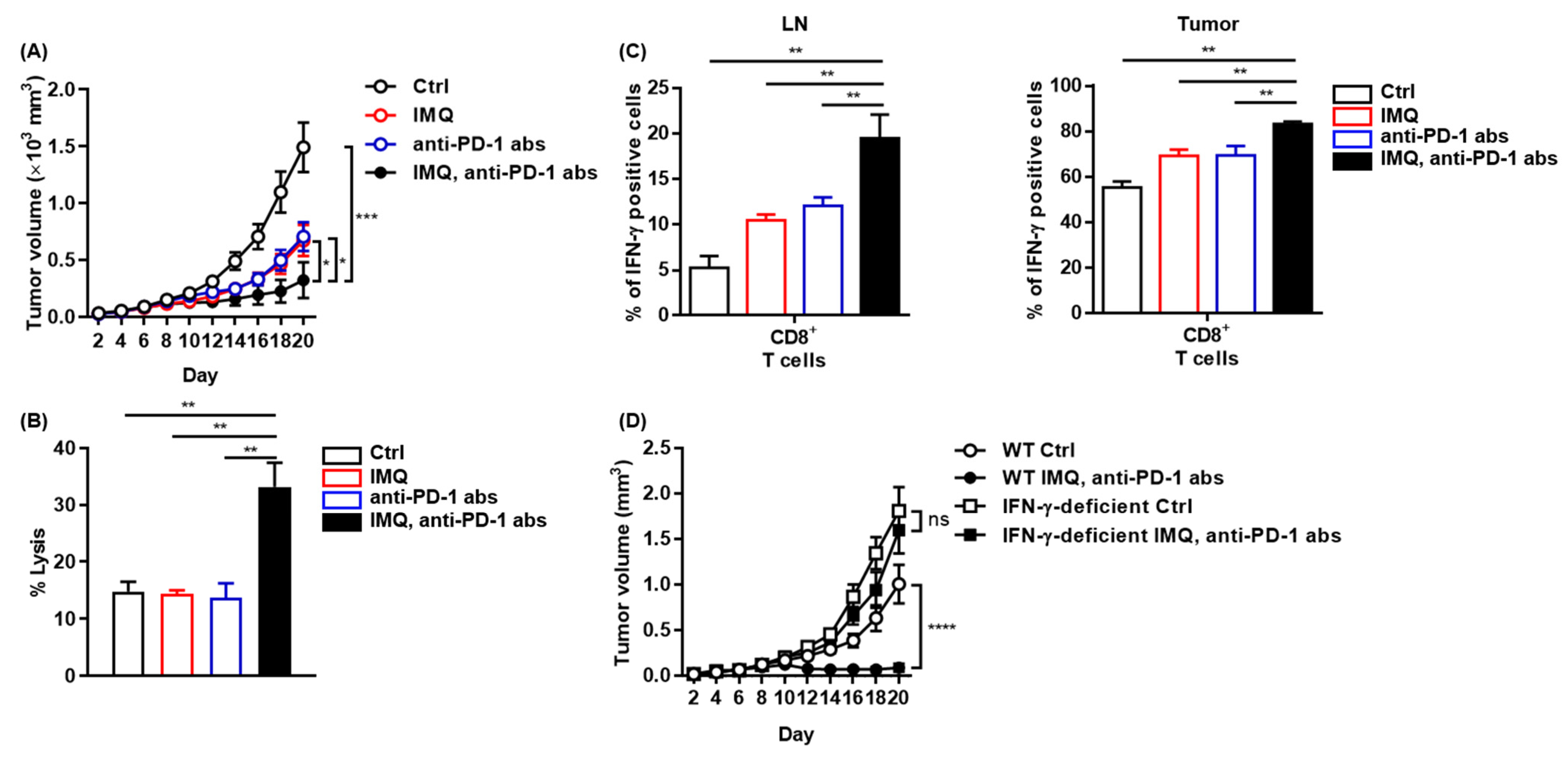
3.4. The IMQ-Induced Antitumor Effect Was Dependent on Increased IFN-γ Expression
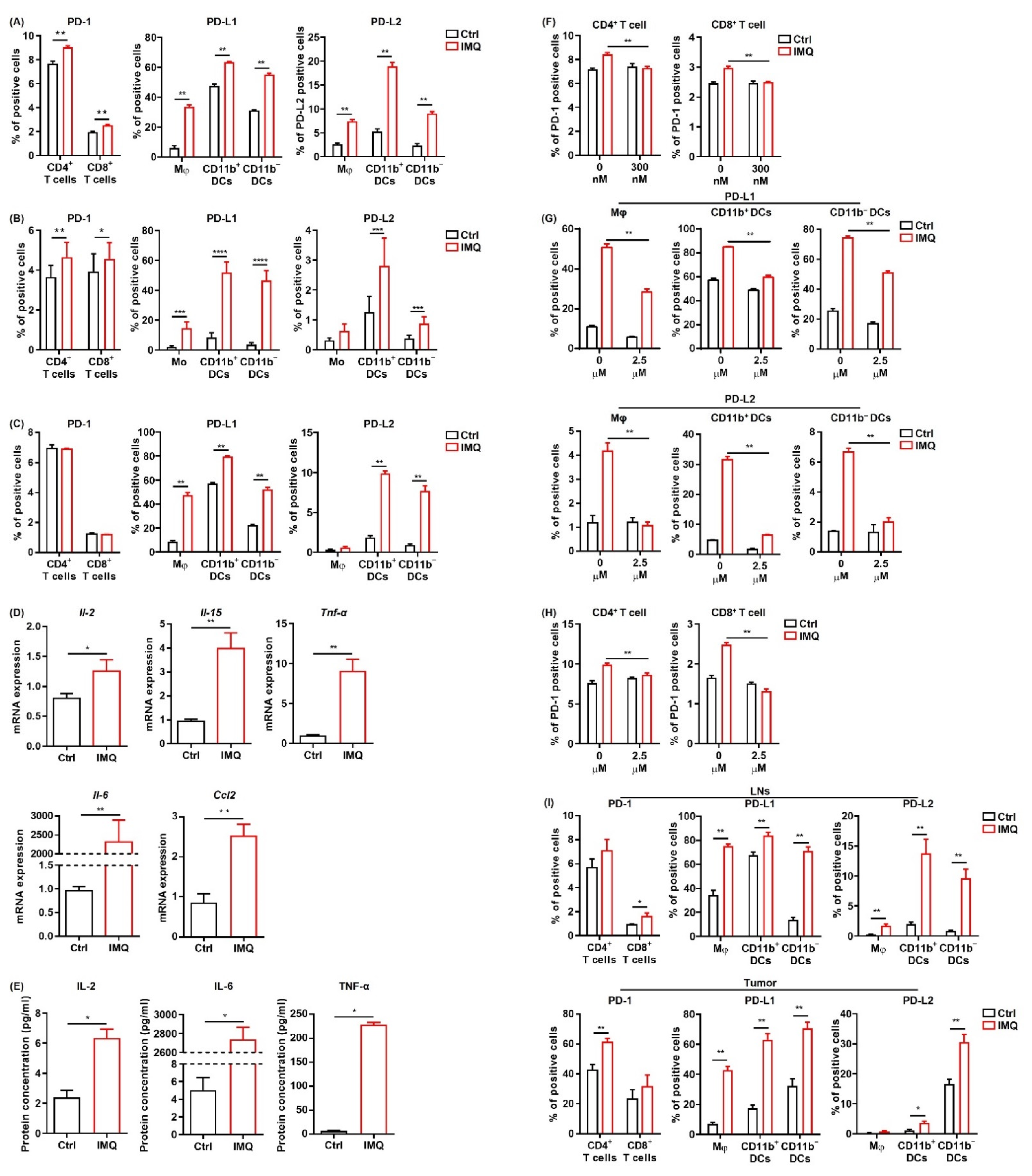
3.5. IMQ Upregulates Expression of PD-1/PD-L1/PD-L2 in Immune Cells
3.6. IFN-γ Is Not Involved in the IMQ-Induced Upregulation of PD-L1 Expression
3.7. Potent Antitumor Effect of Combination Therapy of Topical IMQ Plus Anti-PD-1 Antibody
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TLR | Toll-like receptor |
| IMQ | Imiquimod |
| IFN | Interferon |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| ITM | In-transit metastasis |
| LN | Lymph node |
| ORR | Overall response rate |
| BSA | Bovine serum albumin |
| qRT-PCR | Quantitative reverse transcription-polymerase chain reaction |
| PBMC | Peripheral blood mononuclear cell |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| DC | Dendritic cell |
| NF-κB | Nuclear factor-kappa B |
References
- Urban-Wojciuk, Z.; Khan, M.M.; Oyler, B.L.; Fåhraeus, R.; Marek-Trzonkowska, N.; Nita-Lazar, A.; Hupp, T.R.; Goodlett, D.R. The Role of TLRs in Anti-cancer Immunity and Tumor Rejection. Front. Immunol. 2019, 10, 2388. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, J.; Zhang, J.; Zhang, C. The TLR7 agonists imiquimod and gardiquimod improve DC-based immunotherapy for melanoma in mice. Cell Mol. Immunol. 2010, 7, 381–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, E.; Abadi, R.; Abbas, O. Imiquimod in dermatology: An overview. Int. J. Dermatol. 2016, 55, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chuang, T.H.; Redecke, V.; She, L.; Pitha, P.M.; Carson, D.A.; Raz, E.; Cottam, H.B. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: Activation of Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 2003, 100, 6646–6651. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Dietsch, G.N.; Matthews, M.A.; Yang, Y.; Ghanekar, S.; Inokuma, M.; Suni, M.; Maino, V.C.; Henderson, K.E.; Howbert, J.J.; et al. VTX-2337 is a novel TLR8 agonist that activates NK cells and augments ADCC. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 499–509. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Khong, H.; Dai, Z.; Huang, X.F.; Wargo, J.A.; Cooper, Z.A.; Vasilakos, J.P.; Hwu, P.; Overwijk, W.W. Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation. J. Immunol. 2014, 193, 4722–4731. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.J.; Hijnen, D.; Murphy, G.F.; Kupper, T.S.; Calarese, A.W.; Mollet, I.G.; Schanbacher, C.F.; Miller, D.M.; Schmults, C.D.; Clark, R.A. Imiquimod enhances IFN-gamma production and effector function of T cells infiltrating human squamous cell carcinomas of the skin. J. Investig. Dermatol. 2009, 129, 2676–2685. [Google Scholar] [CrossRef] [Green Version]
- Yokogawa, M.; Takaishi, M.; Nakajima, K.; Kamijima, R.; Digiovanni, J.; Sano, S. Imiquimod attenuates the growth of UVB-induced SCC in mice through Th1/Th17 cells. Mol. Carcinog. 2013, 52, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Aspord, C.; Tramcourt, L.; Leloup, C.; Molens, J.P.; Leccia, M.T.; Charles, J.; Plumas, J. Imiquimod inhibits melanoma development by promoting pDC cytotoxic functions and impeding tumor vascularization. J. Investig. Dermatol. 2014, 134, 2551–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zhou, Q.; Wang, X.; Wu, X.; Chen, X.; Li, J.; Zhu, Z.; Liu, B.; Su, L. The TLR7 agonist induces tumor regression both by promoting CD4+T cells proliferation and by reversing T regulatory cell-mediated suppression via dendritic cells. Oncotarget 2015, 6, 1779–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Li, S.; Yang, Y.; Zhu, S.; Zhang, M.; Qiao, Y.; Liu, Y.J.; Chen, J. TLR-activated plasmacytoid dendritic cells inhibit breast cancer cell growth in vitro and in vivo. Oncotarget 2017, 8, 11708–11718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stary, G.; Bangert, C.; Tauber, M.; Strohal, R.; Kopp, T.; Stingl, G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J. Exp. Med. 2007, 204, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Furudate, S.; Fujimura, T.; Kambayashi, Y.; Kakizaki, A.; Hidaka, T.; Aiba, S. Immunomodulatory Effect of Imiquimod Through CCL22 Produced by Tumor-associated Macrophages in B16F10 Melanomas. Anticancer Res. 2017, 37, 3461–3471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Read, T.; Lonne, M.; Sparks, D.S.; David, M.; Wagels, M.; Schaider, H.; Soyer, H.P.; Smithers, B.M. A systematic review and meta-analysis of locoregional treatments for in-transit melanoma. J. Surg. Oncol. 2019, 119, 887–896. [Google Scholar] [CrossRef]
- Kowalzick, L.; Eickenscheidt, L. Progress of multiple cutaneous and subcutaneous melanoma metastases of the face during imiquimod treatment. J. Dtsch. Dermatol. Ges. 2009, 7, 538–540. [Google Scholar] [CrossRef]
- Salazar, L.G.; Lu, H.; Reichow, J.L.; Childs, J.S.; Coveler, A.L.; Higgins, D.M.; Waisman, J.; Allison, K.H.; Dang, Y.; Disis, M.L. Topical Imiquimod Plus Nab-paclitaxel for Breast Cancer Cutaneous Metastases: A Phase 2 Clinical Trial. JAMA Oncol. 2017, 3, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Rozenblit, M.; Hendrickx, W.; Heguy, A.; Chiriboga, L.; Loomis, C.; Ray, K.; Darvishian, F.; Egeblad, M.; Demaria, S.; Marincola, F.M.; et al. Transcriptomic profiles conducive to immune-mediated tumor rejection in human breast cancer skin metastases treated with Imiquimod. Sci. Rep. 2019, 9, 8572. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, E.; Skitzki, J. The Role of Regional Therapies for in-Transit Melanoma in the Era of Improved Systemic Options. Cancers 2015, 7, 1154–1177. [Google Scholar] [CrossRef]
- Antonia, S.J.; Borghaei, H.; Ramalingam, S.S.; Horn, L.; De Castro Carpeño, J.; Pluzanski, A.; Burgio, M.A.; Garassino, M.; Chow, L.Q.M.; Gettinger, S.; et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: A pooled analysis. Lancet Oncol. 2019, 20, 1395–1408. [Google Scholar] [CrossRef]
- Planes-Laine, G.; Rochigneux, P.; Bertucci, F.; Chrétien, A.S.; Viens, P.; Sabatier, R.; Gonçalves, A. PD-1/PD-L1 Targeting in Breast Cancer: The First Clinical Evidences Are Emerging. A Literature Review. Cancers 2019, 11, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahlie, E.H.A.; Blankenstein, S.A.; van Houdt, W.J.; Wouters, M.; van Akkooi, A.C.J. A systematic review and meta-analysis of locoregional treatments for in-transit melanoma. J. Surg. Oncol. 2019, 120, 1056–1057. [Google Scholar] [CrossRef] [PubMed]
- Dika, E.; Ravaioli, G.M.; Melotti, B.; Patrizi, A.; Veronesi, G.; Lambertini, M.; Scarfì, F. In transit melanoma and imiquimod: A case of disease progression. Dermatol. Ther. 2020, 33, e13512. [Google Scholar] [CrossRef] [PubMed]
- Read, R.L.; Thompson, J.F. Managing in-transit melanoma metastases in the new era of effective systemic therapies for melanoma. Expert Rev. Clin. Pharmacol. 2019, 12, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Van den Boorn, J.G.; Konijnenberg, D.; Tjin, E.P.; Picavet, D.I.; Meeuwenoord, N.J.; Filippov, D.V.; van der Veen, J.P.; Bos, J.D.; Melief, C.J.; Luiten, R.M. Effective melanoma immunotherapy in mice by the skin-depigmenting agent monobenzone and the adjuvants imiquimod and CpG. PLoS ONE 2010, 5, e10626. [Google Scholar] [CrossRef]
- Zhong, W.; Myers, J.S.; Wang, F.; Wang, K.; Lucas, J.; Rosfjord, E.; Lucas, J.; Hooper, A.T.; Yang, S.; Lemon, L.A.; et al. Comparison of the molecular and cellular phenotypes of common mouse syngeneic models with human tumors. BMC Genom. 2020, 21, 2. [Google Scholar] [CrossRef] [Green Version]
- Mosely, S.I.; Prime, J.E.; Sainson, R.C.; Koopmann, J.O.; Wang, D.Y.; Greenawalt, D.M.; Ahdesmaki, M.J.; Leyland, R.; Mullins, S.; Pacelli, L.; et al. Rational Selection of Syngeneic Preclinical Tumor Models for Immunotherapeutic Drug Discovery. Cancer Immunol. Res. 2017, 5, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Coe, D.; Addey, C.; White, M.; Simpson, E.; Dyson, J.; Chai, J.G. The roles of antigen-specificity, responsiveness to transforming growth factor-β and antigen-presenting cell subsets in tumour-induced expansion of regulatory T cells. Immunology 2010, 131, 556–569. [Google Scholar] [CrossRef]
- Fukui, R.; Kanno, A.; Miyake, K. Type I IFN Contributes to the Phenotype of Unc93b1D34A/D34A Mice by Regulating TLR7 Expression in B Cells and Dendritic Cells. J. Immunol. 2016, 196, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Schon, M.P.; Schon, M. TLR7 and TLR8 as targets in cancer therapy. Oncogene 2008, 27, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Porciello, N.; Tuosto, L. CD28 costimulatory signals in T lymphocyte activation: Emerging functions beyond a qualitative and quantitative support to TCR signalling. Cytokine Growth Factor Rev. 2016, 28, 11–19. [Google Scholar] [CrossRef]
- Rickert, R.C.; Rajewsky, K.; Roes, J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature 1995, 376, 352–355. [Google Scholar] [CrossRef]
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. Pillars Article: IFNγ and Lymphocytes Prevent Primary Tumour Development and Shape Tumour Immunogenicity. J. Immunol. 2018, 201, 827–831. [Google Scholar] [PubMed]
- Hornung, V.; Rothenfusser, S.; Britsch, S.; Krug, A.; Jahrsdörfer, B.; Giese, T.; Endres, S.; Hartmann, G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 2002, 168, 4531–4537. [Google Scholar] [CrossRef] [Green Version]
- Zarember, K.A.; Godowski, P.J. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J. Immunol. 2002, 168, 554–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loke, P.; Allison, J.P. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc. Natl. Acad. Sci. USA 2003, 100, 5336–5341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greay, S.J.; Ireland, D.J.; Kissick, H.T.; Heenan, P.J.; Carson, C.F.; Riley, T.V.; Beilharz, M.W. Inhibition of established subcutaneous murine tumour growth with topical Melaleuca alternifolia (tea tree) oil. Cancer Chemother Pharmacol. 2010, 66, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Benonisson, H.; Sow, H.S.; Breukel, C.; Claassens, J.; Brouwers, C.; Linssen, M.M.; Fransen, M.F.; Sluijter, M.; Ossendorp, F.; van Hall, T.; et al. High FcγR Expression on Intratumoral Macrophages Enhances Tumor-Targeting Antibody Therapy. J. Immunol. 2018, 201, 3741–3749. [Google Scholar] [CrossRef] [Green Version]
- Nagato, T.; Lee, Y.R.; Harabuchi, Y.; Celis, E. Combinatorial immunotherapy of polyinosinic-polycytidylic acid and blockade of programmed death-ligand 1 induce effective CD8 T-cell responses against established tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 1223–1234. [Google Scholar] [CrossRef] [Green Version]
- Ordikhani, F.; Uehara, M.; Kasinath, V.; Dai, L.; Eskandari, S.K.; Bahmani, B.; Yonar, M.; Azzi, J.R.; Haik, Y.; Sage, P.T.; et al. Targeting antigen-presenting cells by anti-PD-1 nanoparticles augments antitumor immunity. JCI Insight 2018, 3, e122700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, D.; Kobayashi, A.; Jiang, P.; Ferrari de Andrade, L.; Tay, R.E.; Luoma, A.M.; Tsoucas, D.; Qiu, X.; Lim, K.; Rao, P.; et al. A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science 2018, 359, 770–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Vincent, K.M.; Postovit, L.M. Investigating the utility of human melanoma cell lines as tumour models. Oncotarget 2017, 8, 10498–10509. [Google Scholar] [CrossRef] [Green Version]
- Rothermel, L.D.; Sabesan, A.C.; Stephens, D.J.; Chandran, S.S.; Paria, B.C.; Srivastava, A.K.; Somerville, R.; Wunderlich, J.R.; Lee, C.C.; Xi, L.; et al. Identification of an Immunogenic Subset of Metastatic Uveal Melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 2237–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y. Biomarkers for Immune Checkpoint Inhibitor-Mediated Tumor Response and Adverse Events. Front. Med. 2019, 6, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F. Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int. Rev. Immunol. 2009, 28, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Tunger, A.; Sommer, U.; Wehner, R.; Kubasch, A.S.; Grimm, M.O.; Bachmann, M.P.; Platzbecker, U.; Bornhäuser, M.; Baretton, G.; Schmitz, M. The Evolving Landscape of Biomarkers for Anti-PD-1 or Anti-PD-L1 Therapy. J. Clin. Med. 2019, 8, 1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Lin, K.; Lin, C.; Wang, J.; Tang, Y. Prognostic and clinicopathological utility of PD-L2 expression in patients with digestive system cancers: A meta-analysis. Int. Immunopharmacol. 2020, 88, 106946. [Google Scholar] [CrossRef]
- Yasuoka, H.; Asai, A.; Ohama, H.; Tsuchimoto, Y.; Fukunishi, S.; Higuchi, K. Increased both PD-L1 and PD-L2 expressions on monocytes of patients with hepatocellular carcinoma was associated with a poor prognosis. Sci. Rep. 2020, 10, 10377. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, L.; Zhang, W.; Liu, Y.; Chen, B.; Zhao, S.; Li, W.; Wang, L.; Ye, L.; Jia, K.; et al. Expression of PD-1 and PD-L1 on Tumor-Infiltrating Lymphocytes Predicts Prognosis in Patients with Small-Cell Lung Cancer. Onco Targets Ther. 2020, 13, 6475–6483. [Google Scholar] [CrossRef]
- Curran, C.S.; Gupta, S.; Sanz, I.; Sharon, E. PD-1 immunobiology in systemic lupus erythematosus. J. Autoimmun. 2019, 97, 1–9. [Google Scholar] [CrossRef]
- Liang, S.C.; Latchman, Y.E.; Buhlmann, J.E.; Tomczak, M.F.; Horwitz, B.H.; Freeman, G.J.; Sharpe, A.H. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur. J. Immunol. 2003, 33, 2706–2716. [Google Scholar] [CrossRef]
- Meier, A.; Bagchi, A.; Sidhu, H.K.; Alter, G.; Suscovich, T.J.; Kavanagh, D.G.; Streeck, H.; Brockman, M.A.; LeGall, S.; Hellman, J.; et al. Upregulation of PD-L1 on monocytes and dendritic cells by HIV-1 derived TLR ligands. AIDS 2008, 22, 655–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, H.; Yan, L.; Liu, N.; Xu, M.; Cai, H. IFI16 promotes cervical cancer progression by upregulating PD-L1 in immunomicroenvironment through STING-TBK1-NF-kB pathway. Biomed. Pharmacother. 2020, 123, 109790. [Google Scholar] [CrossRef]
- Ju, X.; Zhang, H.; Zhou, Z.; Chen, M.; Wang, Q. Tumor-associated macrophages induce PD-L1 expression in gastric cancer cells through IL-6 and TNF-α signaling. Exp. Cell Res. 2020, 396, 112315. [Google Scholar] [CrossRef]
- Kinter, A.L.; Godbout, E.J.; McNally, J.P.; Sereti, I.; Roby, G.A.; O’Shea, M.A.; Fauci, A.S. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J. Immunol. 2008, 181, 6738–6746. [Google Scholar] [CrossRef]
- Bally, A.P.R.; Neeld, D.K.; Lu, P.; Majumder, P.; Tang, Y.; Barwick, B.G.; Wang, Q.; Boss, J.M. PD-1 Expression during Acute Infection Is Repressed through an LSD1-Blimp-1 Axis. J. Immunol. 2020, 204, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.; Gogoll, K.; Tenzer, S.; Schild, H.; Stevanovic, S.; Langguth, P.; Radsak, M.P. Efficacy of imiquimod-based transcutaneous immunization using a nano-dispersed emulsion gel formulation. PLoS ONE 2014, 9, e102664. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oya, K.; Nakamura, Y.; Zhenjie, Z.; Tanaka, R.; Okiyama, N.; Ichimura, Y.; Ishitsuka, Y.; Saito, A.; Kubota, N.; Watanabe, R.; et al. Combination Treatment of Topical Imiquimod Plus Anti-PD-1 Antibody Exerts Significantly Potent Antitumor Effect. Cancers 2021, 13, 3948. https://doi.org/10.3390/cancers13163948
Oya K, Nakamura Y, Zhenjie Z, Tanaka R, Okiyama N, Ichimura Y, Ishitsuka Y, Saito A, Kubota N, Watanabe R, et al. Combination Treatment of Topical Imiquimod Plus Anti-PD-1 Antibody Exerts Significantly Potent Antitumor Effect. Cancers. 2021; 13(16):3948. https://doi.org/10.3390/cancers13163948
Chicago/Turabian StyleOya, Kazumasa, Yoshiyuki Nakamura, Zhu Zhenjie, Ryota Tanaka, Naoko Okiyama, Yuki Ichimura, Yosuke Ishitsuka, Akimasa Saito, Noriko Kubota, Rei Watanabe, and et al. 2021. "Combination Treatment of Topical Imiquimod Plus Anti-PD-1 Antibody Exerts Significantly Potent Antitumor Effect" Cancers 13, no. 16: 3948. https://doi.org/10.3390/cancers13163948
APA StyleOya, K., Nakamura, Y., Zhenjie, Z., Tanaka, R., Okiyama, N., Ichimura, Y., Ishitsuka, Y., Saito, A., Kubota, N., Watanabe, R., Tahara, H., Fujimoto, M., & Fujisawa, Y. (2021). Combination Treatment of Topical Imiquimod Plus Anti-PD-1 Antibody Exerts Significantly Potent Antitumor Effect. Cancers, 13(16), 3948. https://doi.org/10.3390/cancers13163948