A HER2 Tri-Specific NK Cell Engager Mediates Efficient Targeting of Human Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of CAM1615HER2 TriKE
2.2. Purification of Protein from Inclusion Bodies
2.3. Cancer Cell Lines
2.4. Cell Products
2.5. Evaluation of NK Cell Activation and Tumor Cytotoxicity
2.6. NK Cell Expansion via IL-15 Stimulation
2.7. Spheroid Assays
2.8. In Vivo Mouse Study and Imaging
2.9. Statistical Analysis
3. Results
3.1. Generation of CAM1615HER2 TriKE
3.2. Evaluation of Proliferation Mediated by CAM1615HER2 TriKE
3.3. Determination of CAM1615HER2 TriKE Specificity and Function
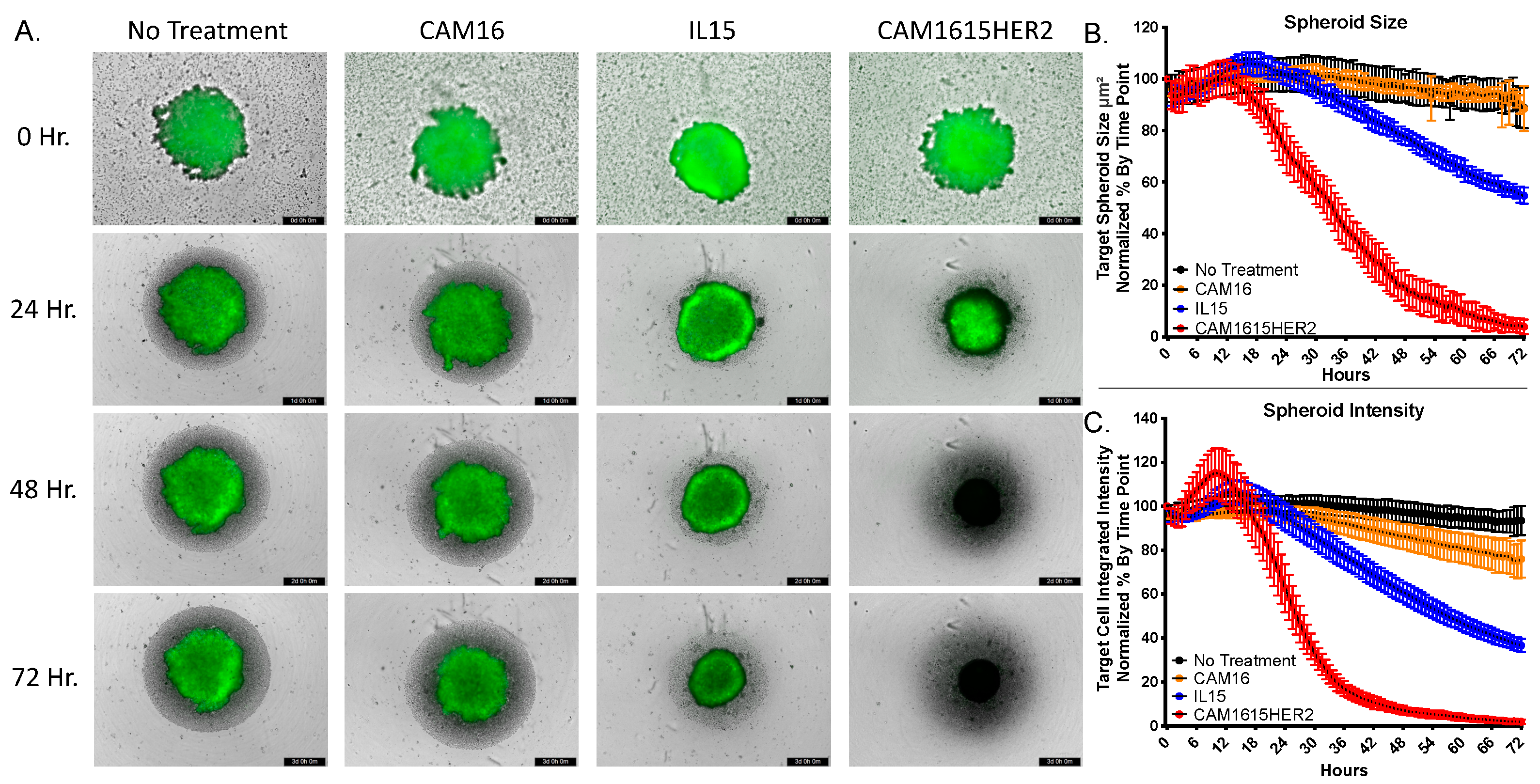
3.4. CAM1615HER2 TriKE Induces Dynamic Evaluation of Ovarian Cancer Spheroids
3.5. CAM1615HER2 TriKE Amplifies Function of NK Cells Derived from the Tumor Microenvironment
3.6. CAM1615HER2 TriKE Demonstrates Tumor Control in Xenogeneic Ovarian Cancer Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2021; American Cancer Society: Atlanta, CA, USA, 2021. [Google Scholar]
- Thibault, B.; Castells, M.; Delord, J.-P.; Couderc, B. Ovarian cancer microenvironment: Implications for cancer dissemination and chemoresistance acquisition. Cancer Metastasis Rev. 2013, 33, 17–39. [Google Scholar] [CrossRef]
- Timmermans, M.; Sonke, G.; Van de Vijver, K.; van der Aa, M.; Kruitwagen, R. No improvement in long-term survival for epithelial ovarian cancer patients: A population-based study between 1989 and 2014 in the Netherlands. Eur. J. Cancer 2018, 88, 31–37. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Luo, H.; Xu, X.; Ye, M.; Sheng, B.; Zhu, X. The prognostic value of HER2 in ovarian cancer: A meta-analysis of observational studies. PLoS ONE 2018, 13, e0191972. [Google Scholar] [CrossRef] [Green Version]
- Reibenwein, J.; Krainer, M. Targeting signaling pathways in ovarian cancer. Expert Opin. Ther. Targets 2008, 12, 353–365. [Google Scholar] [CrossRef]
- Rugo, H.S.; Barve, A.; Waller, C.F.; Hernandez-Bronchud, M.; Herson, J.; Yuan, J.; Sharma, R.; Baczkowski, M.; Kothekar, M.; Loganathan, S.; et al. Effect of a Proposed Trastuzumab Biosimilar Compared with trastuzumab on overall response rate in patients with erbb2 (her2)–positive metastatic breast cancer: A randomized clinical trial. JAMA 2017, 317, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Urruticoechea, A.; Rizwanullah, M.; Im, S.-A.; Ruiz, A.C.S.; Láng, I.; Tomasello, G.; Douthwaite, H.; Crnjevic, T.B.; Heeson, S.; Eng-Wong, J.; et al. Randomized Phase III Trial of trastuzumab plus capecitabine with or without pertuzumab in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer who experienced disease progression during or after trastuzumab-based therapy. J. Clin. Oncol. 2017, 35, 3030–3038. [Google Scholar] [CrossRef]
- Menderes, G.; Bonazzoli, E.; Bellone, S.; Altwerger, G.; Black, J.D.; Dugan, K.; Pettinella, F.; Masserdotti, A.; Riccio, F.; Bianchi, A.; et al. Superior in vitro and in vivo activity of trastuzumab-emtansine (T-DM1) in comparison to trastuzumab, pertuzumab and their combination in epithelial ovarian carcinoma with high HER2/neu expression. Gynecol. Oncol. 2017, 147, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Vallera, D.A.; Felices, M.; McElmurry, R.; McCullar, V.; Zhou, X.; Schmohl, J.U.; Zhang, B.; Lenvik, A.J.L.; Panoskaltsis-Mortari, A.; Verneris, M.R.; et al. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function. Clin. Cancer Res. 2016, 22, 3440–3450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felices, M.; Lenvik, T.R.; Kodal, B.; Lenvik, A.J.; Hinderlie, P.; Bendzick, L.E.; Schirm, D.K.; Kaminski, M.F.; McElmurry, R.T.; Geller, M.A.; et al. Potent Cytolytic Activity and Specific IL15 Delivery in a 2nd Generation Trispecific Killer Engager. Cancer Immunol. Res. 2020, 8, 1139–1149. [Google Scholar] [CrossRef]
- Vallera, D.A.; Ferrone, S.; Kodal, B.; Hinderlie, P.; Bendzick, L.; Ettestad, B.; Hallstrom, C.; Zorko, N.A.; Rao, A.; Fujioka, N.; et al. NK-Cell-Mediated Targeting of Various Solid Tumors Using a B7-H3 Tri-Specific Killer Engager In Vitro and In Vivo. Cancers 2020, 12, 2659. [Google Scholar] [CrossRef]
- Vincke, C.; Loris, R.; Saerens, D.; Martinez-Rodriguez, S.; Muyldermans, S.; Conrath, K. General Strategy to Humanize a Camelid Single-domain Antibody and Identification of a Universal Humanized Nanobody Scaffold. J. Biol. Chem. 2009, 284, 3273–3284. [Google Scholar] [CrossRef] [Green Version]
- Behar, G.; Siberil, S.; Groulet, A.; Chames, P.; Pugniere, M.; Boix, C.; Sautes-Fridman, C.; Teillaud, J.-L.; Baty, D. Isolation and characterization of anti-Fc RIII (CD16) llama single-domain antibodies that activate natural killer cells. Protein Eng. Des. Sel. 2007, 21, 1–10. [Google Scholar] [CrossRef]
- Batra, J.K.; Kasprzyk, P.G.; Bird, R.E.; Pastan, I.; King, C.R. Recombinant anti-erbB2 immunotoxins containing Pseudomonas exotoxin. Proc. Natl. Acad. Sci. USA 1992, 89, 5867–5871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallera, D.A.; Todhunter, D.; Kuroki, D.W.; Shu, Y.; Sicheneder, A.; Panoskaltsis-Mortari, A.; Vallera, V.D.; Chen, H. Molecular modification of a recombinant, bivalent anti-human CD3 immunotoxin (Bic3) results in reduced in vivo toxicity in mice. Leuk. Res. 2005, 29, 331–341. [Google Scholar] [CrossRef]
- Worsham, M.J.; Chen, K.M.; Meduri, V.; Nygren, A.O.H.; Errami, A.; Schouten, J.P.; Benninger, M.S. epigenetic events of disease progression in head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 668–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts, M.R.; Koup, R.A. Detection of T-Cell Degranulation: CD107a and b. Methods Cell Biol. 2004, 75, 497–512. [Google Scholar] [CrossRef]
- Alter, G.; Malenfant, J.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Felices, M.; Chu, S.; Kodal, B.; Bendzick, L.; Ryan, C.; Lenvik, A.; Boylan, K.; Wong, H.; Skubitz, A.; Miller, J.; et al. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol. Oncol. 2017, 145, 453–461. [Google Scholar] [CrossRef]
- Subramanian, I.V. Adeno-Associated Virus-Mediated Delivery of a Mutant Endostatin in Combination with Carboplatin Treatment Inhibits Orthotopic Growth of Ovarian Cancer and Improves Long-term Survival. Cancer Res. 2006, 66, 4319–4328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Mph, K.D.M.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Shang, A.-Q.; Wu, J.; Bi, F.; Zhang, Y.-J.; Xu, L.-R.; Li, L.-L.; Chen, F.-F.; Wang, W.-W.; Zhu, J.-J.; Liu, Y.-Y. Relationship between HER2 and JAK/STAT-SOCS3 signaling pathway and clinicopathological features and prognosis of ovarian cancer. Cancer Biol. Ther. 2017, 18, 314–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Wang, J.; Zhang, L.; Wu, D.; Yu, D.; Tian, X.; Liu, J.; Jiang, X.; Shen, Y.; Zhang, L.; et al. Expressions of fatty acid synthase and HER2 are correlated with poor prognosis of ovarian cancer. Med. Oncol. 2014, 32, 391. [Google Scholar] [CrossRef] [Green Version]
- Berchuck, A.; Kamel, A.; Whitaker, R.; Kerns, B.; Olt, G.; Kinney, R.; Soper, J.T.; Dodge, R.; Clarke-Pearson, D.L.; Marks, P. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990, 50, 4087–4091. [Google Scholar]
- McKeage, K.; Perry, C.M. Trastuzumab: A review of its use in the treatment of metastatic breast cancer overexpressing HER2. Drugs 2002, 62, 209–243. [Google Scholar] [CrossRef]
- Boku, N. HER2-positive gastric cancer. Gastric Cancer 2014, 17, 1–12. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. 19th WHO Model List of Essential Medicines. Available online: https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists (accessed on 8 December 2016).
- Hudis, C.A. Trastuzumab—Mechanism of Action and Use in Clinical Practice. New Engl. J. Med. 2007, 357, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, A.R.; Swain, S. Ongoing adjuvant trials with trastuzumab in breast cancer. Semin. Oncol. 2003, 30, 54–64. [Google Scholar] [CrossRef]
- Moja, L.; Tagliabue, L.; Balduzzi, S.; Parmelli, E.; Pistotti, V.; Guarneri, V.; D’Amico, R. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst. Rev. 2012, 2012, CD006243. [Google Scholar] [CrossRef]
- Teplinsky, E.; Muggia, F. EGFR and HER2: Is there a role in ovarian cancer? Transl. Cancer Res. 2015, 4, 107–117. [Google Scholar] [CrossRef]
- Evert, J.S.H.-V.; Maas, R.J.; Van Der Meer, J.; Cany, J.; Van Der Steen, S.; Jansen, J.H.; Miller, J.S.; Bekkers, R.; Hobo, W.; Massuger, L.; et al. Peritoneal NK cells are responsive to IL-15 and percentages are correlated with outcome in advanced ovarian cancer patients. Oncotarget 2018, 9, 34810–34820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romee, R.; Cooley, S.; Berrien-Elliott, M.M.; Westervelt, P.; Verneris, M.R.; Wagner, J.E.; Weisdorf, D.J.; Blazar, B.R.; Ustun, C.; DeFor, T.E.; et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 2018, 131, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Cooley, S.; He, F.; Bachanova, V.; Vercellotti, G.M.; DeFor, T.E.; Curtsinger, J.M.; Robertson, P.; Grzywacz, B.; Conlon, K.C.; Waldmann, T.A.; et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 2019, 3, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Morishima, C.; McNeel, D.G.; Patel, M.R.; Kohrt, H.E.; Thompson, J.A.; Sondel, P.M.; Wakelee, H.A.; Disis, M.L.; Kaiser, J.C.; et al. A First-in-Human Phase I Study of Subcutaneous Outpatient Recombinant Human IL15 (rhIL15) in Adults with Advanced Solid Tumors. Clin. Cancer Res. 2017, 24, 1525–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conlon, K.C.; Lugli, E.; Welles, H.; Rosenberg, S.A.; Fojo, A.T.; Morris, J.C.; Fleisher, T.A.; Dubois, S.P.; Perera, L.P.; Stewart, D.M.; et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 2015, 33, 74–82. [Google Scholar] [CrossRef]
- Conlon, K.C.; Potter, E.L.; Pittaluga, S.; Lee, C.-C.; Miljković, M.; Fleisher, T.A.; Dubois, S.; Bryant, B.R.; Petrus, M.N.; Perera, L.P.; et al. IL15 by Continuous Intravenous Infusion to Adult Patients with Solid Tumors in a Phase I Trial Induced Dramatic NK-Cell Subset Expansion. Clin. Cancer Res. 2019, 25, 4945–4954. [Google Scholar] [CrossRef]
- Yigit, R.; Massuger, L.F.; Figdor, C.; Torensma, R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol. Oncol. 2010, 117, 366–372. [Google Scholar] [CrossRef]
- Belisle, J.A.; Gubbels, J.A.A.; Raphael, C.A.; Migneault, M.; Rancourt, C.; Connor, J.P.; Patankar, M.S. Peritoneal natural killer cells from epithelial ovarian cancer patients show an altered phenotype and bind to the tumour marker MUC16 (CA125). Immunology 2007, 122, 418–429. [Google Scholar] [CrossRef]
- Liu, R.B.; Engels, B.; Arina, A.; Schreiber, K.; Hyjek, E.; Schietinger, A.; Binder, D.C.; Butz, E.; Krausz, T.; Rowley, D.A.; et al. Densely Granulated Murine NK Cells Eradicate Large Solid Tumors. Cancer Res. 2012, 72, 1964–1974. [Google Scholar] [CrossRef] [Green Version]
- Wagner, J.A.; Rosario, M.; Romee, R.; Berrien-Elliott, M.; Schneider, S.E.; Leong, J.W.; Sullivan, R.P.; Jewell, B.A.; Becker-Hapak, M.; Schappe, T.; et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J. Clin. Investig. 2017, 127, 4042–4058. [Google Scholar] [CrossRef] [Green Version]
- Steel, J.; Waldmann, T.A.; Morris, J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 2012, 33, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, K.; Liu, Y.; Guo, Y.; Qiu, J.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Han, W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell 2018, 9, 838–847. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor–Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Gerken, C.; Liu, E.; Dakhova, O.; Ashoori, A.; Corder, A.; et al. Human Epidermal Growth Factor Receptor 2 (HER2)—Specific Chimeric Antigen Receptor–Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015, 33, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Lanitis, E.; Dangaj, D.; Hagemann, I.; Song, D.-G.; Best, A.; Sandaltzopoulos, R.; Coukos, G.; Powell, D.J., Jr. Primary Human Ovarian Epithelial Cancer Cells Broadly Express HER2 at Immunologically-Detectable Levels. PLoS ONE 2012, 7, e49829. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallera, D.A.; Oh, F.; Kodal, B.; Hinderlie, P.; Geller, M.A.; Miller, J.S.; Felices, M. A HER2 Tri-Specific NK Cell Engager Mediates Efficient Targeting of Human Ovarian Cancer. Cancers 2021, 13, 3994. https://doi.org/10.3390/cancers13163994
Vallera DA, Oh F, Kodal B, Hinderlie P, Geller MA, Miller JS, Felices M. A HER2 Tri-Specific NK Cell Engager Mediates Efficient Targeting of Human Ovarian Cancer. Cancers. 2021; 13(16):3994. https://doi.org/10.3390/cancers13163994
Chicago/Turabian StyleVallera, Daniel A., Felix Oh, Behiye Kodal, Peter Hinderlie, Melissa A. Geller, Jeffrey S. Miller, and Martin Felices. 2021. "A HER2 Tri-Specific NK Cell Engager Mediates Efficient Targeting of Human Ovarian Cancer" Cancers 13, no. 16: 3994. https://doi.org/10.3390/cancers13163994
APA StyleVallera, D. A., Oh, F., Kodal, B., Hinderlie, P., Geller, M. A., Miller, J. S., & Felices, M. (2021). A HER2 Tri-Specific NK Cell Engager Mediates Efficient Targeting of Human Ovarian Cancer. Cancers, 13(16), 3994. https://doi.org/10.3390/cancers13163994






