Genetic Profiling of a Cohort of Italian Patients with ACTH-Secreting Pituitary Tumors and Characterization of a Novel USP8 Gene Variant
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction and Sanger Sequencing
2.3. Plasmids and Mutagenesis
2.4. ACTH-Secreting Pituitary Cell Culture
2.5. Cell Transfection
2.6. Western Blot Analysis
2.7. Hormone Levels Detection
2.8. Immunofluorescence Analysis and In Situ Proximity Ligation Assay
2.9. Cell Proliferation Assay
2.10. Statistical Analysis
3. Results
3.1. Clinical Characteristics and Prevalence of USP8 and USP48 Mutations in Patients
3.2. Identification of a New USP8 Variant
3.3. Overexpression of USP8 G664R Variant in AtT-20 Cells Increased ACTH Secretion and Cell Proliferation
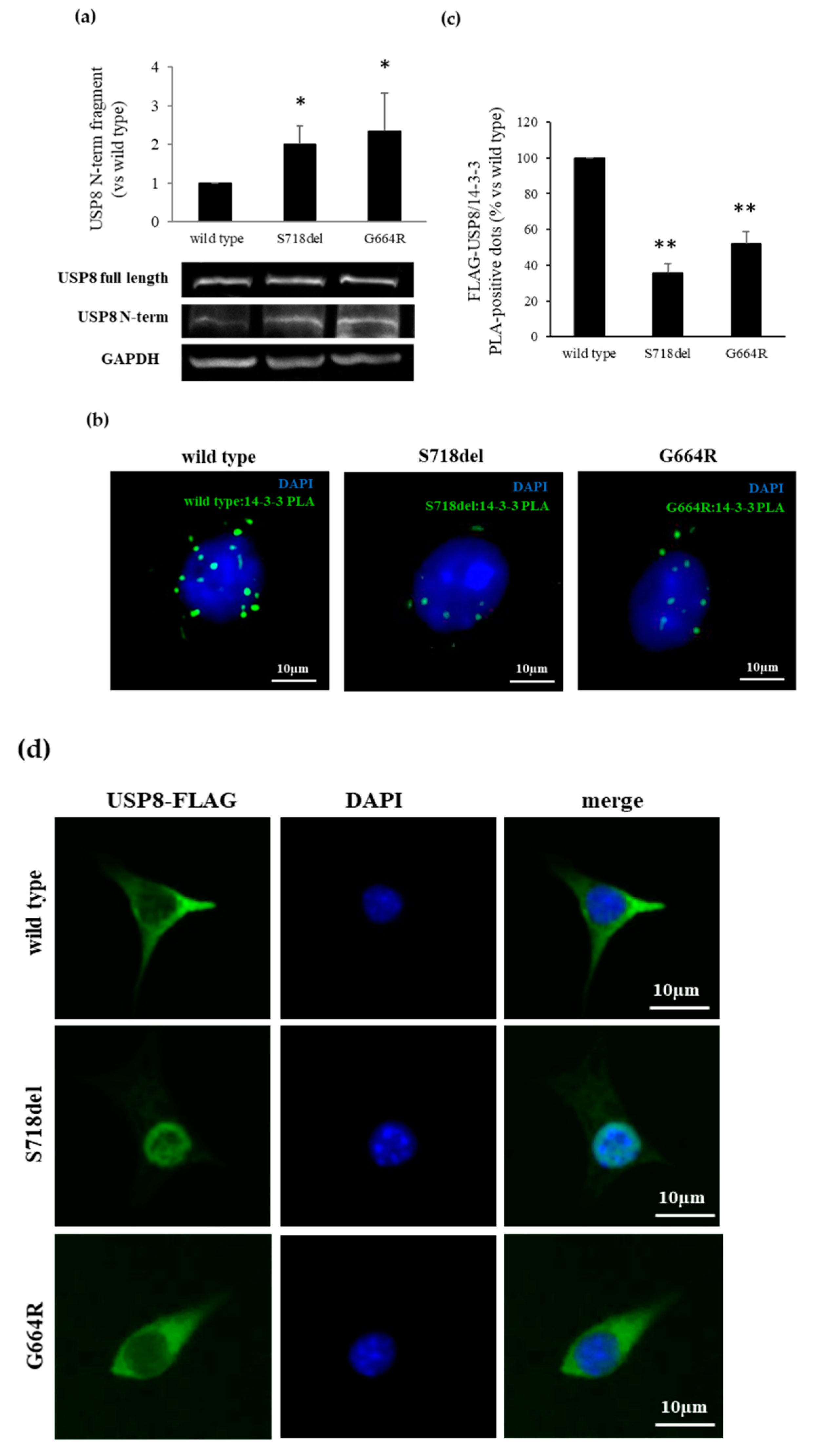
3.4. USP8 G664R Variant Leads to USP8 Proteolytic Cleavage by Affecting USP8/14-3-3 Proteins Binding
3.5. Clinical Significance of Novel USP8 Variant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pivonello, R.; Ferrigno, R.; De Martino, M.C.; Simeoli, C.; Di Paola, N.; Pivonello, C.; Barba, L.; Negri, M.; De Angelis, C.; Colao, A. Medical treatment of Cushing’s disease: An overview of the current and recent clinical trials. Front. Endocrinol. 2020, 11, 648. [Google Scholar] [CrossRef] [PubMed]
- Reincke, M.; Sbiera, S.; Hayakawa, A.; Theodoropoulou, M.; Osswald, A.; Beuschlein, F.; Meitinger, T.; Mizuno-Yamasaki, E.; Kawaguchi, K.; Saeki, Y.; et al. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat. Genet. 2015, 47, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Y.; Song, Z.J.; Chen, J.H.; Wang, Y.F.; Li, S.Q.; Zhou, L.F.; Mao, Y.; Li, Y.M.; Hu, R.G.; Zhang, Z.Y.; et al. Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res. 2015, 25, 306–317. [Google Scholar] [CrossRef]
- Mevissen, T.E.T.; Komander, D. Mechanisms of deubiquitinase dpecificity and regulation. Annu. Rev. Biochem. 2017, 86, 159–192. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, E.; Iura, T.; Mukai, A.; Yoshimori, T.; Kitamura, N.; Komada, M. Regulation of epidermal growth factor receptor down-regulation by UBPY-mediated deubiquitination at endosomes. Mol. Biol. Cell 2005, 11, 5163–5174. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, E.; Kitamura, N.; Komada, M. 14-3-3-Dependent inhibition of the deubiquitinating activity of UBPY and its cancellation in the M phase. Exp. Cell Res. 2007, 313, 3624–3634. [Google Scholar] [CrossRef]
- Centorrino, F.; Ballone, A.; Wolter, M.; Ottmann, C. Biophysical and structural insight into the USP8/14-3-3 interaction. FEBS Lett. 2018, 592, 1211–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufner, A.; Knobeloch, K.P. Ubiquitin-specific protease 8 (USP8/UBPy): A prototypic multidomain deubiquitinating enzyme with pleiotropic functions. Biochem. Soc. Trans. 2019, 47, 1867–1879. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jian, X.; Deng, S.; Ma, Z.; Shou, X.; Shen, Y.; Zhang, Q.; Song, Z.; Li, Z.; Peng, H.; et al. Identification of recurrent USP48 and BRAF mutations in Cushing’s disease. Nat. Commun. 2018, 9, 3171. [Google Scholar] [CrossRef] [Green Version]
- Sbiera, S.; Kunz, M.; Weigand, I.; Deutschbein, T.; Dandekar, T.; Fassnacht, M. The new genetic landscape of Cushing’s disease: Deubiquitinases in the spotlight. Cancers 2019, 11, 1761. [Google Scholar] [CrossRef] [Green Version]
- Perez-Rivas, L.G.; Theodoropoulou, M.; Ferraù, F.; Nusser, C.; Kawaguchi, K.; Stratakis, C.A.; Faucz, F.R.; Wildemberg, L.E.; Assié, G.; Beschorner, R.; et al. The gene of the ubiquitin-specific protease 8 is frequently mutated in adenomas causing Cushing’s disease. J. Clin. Endocrinol. Metab. 2015, 100, E997–E1004. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Inoshita, N.; Kawaguchi, K.; Ibrahim Ardisasmita, A.; Suzuki, H.; Fukuhara, N.; Okada, M.; Nishioka, H.; Takeuchi, Y.; Komada, M.; et al. The USP8 mutational status may predict drug susceptibility in corticotroph adenomas of Cushing’s disease. Eur. J. Endocrinol. 2016, 174, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Faucz, F.R.; Tirosh, A.; Tatsi, C.; Berthon, A.; Hernández-Ramírez, L.C.; Settas, N.; Angelousi, A.; Correa, R.; Papadakis, G.Z.; Chittiboina, P.; et al. Somatic USP8 gene mutations are a common cause of pediatric Cushing disease. J. Clin. Endocrinol. Metab. 2017, 102, 2836–2843. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, L.J.; Lerario, A.M.; de Castro, M.; Martins, C.S.; Bronstein, M.D.; Machado, M.C.; Trarbach, E.B.; Villares Fragoso, M.C. Transcriptome analysis showed a differential signature between invasive and non-invasive corticotrophinomas. Front. Endocrinol. 2017, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Rivas, L.G.; Theodoropoulou, M.; Puar, T.H.; Fazel, J.; Stieg, M.R.; Ferraù, F.; Assié, G.; Gadelha, M.R.; Deutschbein, T.; Fragoso, M.C.; et al. Somatic USP8 mutations are frequent events in corticotroph tumor progression causing Nelson’s tumor. Eur. J. Endocrinol. 2018, 178, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Ballmann, C.; Thiel, A.; Korah, H.E.; Reis, A.C.; Saeger, W.; Stepanow, S.; Köhrer, K.; Reifenberger, G.; Knobbe-Thomsen, C.B.; Knappe, U.J.; et al. USP8 mutations in pituitary Cushing adenomas-targeted analysis by next-generation sequencing. J. Endocr. Soc. 2018, 2, 266–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sesta, A.; Cassarino, M.F.; Terreni, M.; Ambrogio, A.G.; Libera, L.; Bardelli, D.; Lasio, G.; Losa, M.; Pecori Giraldi, F. Ubiquitin-specific protease 8 mutant corticotrope adenomas present unique secretory and molecular features and shed light on the role of ubiquitylation on ACTH processing. Neuroendocrinology 2020, 110, 119–129. [Google Scholar] [CrossRef]
- Wanichi, I.Q.; de Paula Mariani, B.M.; Frassetto, F.P.; Siqueira, S.A.C.; de Castro Musolino, N.R.; Cunha-Neto, M.B.C.; Ochman, G.; Cescato, V.A.S.; Machado, M.C.; Trarbach, E.B.; et al. Cushing’s disease due to somatic USP8 mutations: A systematic review and meta-analysis. Pituitary 2019, 22, 435–442. [Google Scholar] [CrossRef]
- Weigand, I.; Knobloch, L.; Flitsch, J.; Saeger, W.; Monoranu, C.M.; Höfner, K.; Herterich, S.; Rotermund, R.; Ronchi, C.L.; Buchfelder, M.; et al. Impact of USP8 gene mutations on protein deregulation in Cushing disease. J. Clin. Endocrinol. Metab. 2019, 104, 2535–2546. [Google Scholar] [CrossRef]
- Bujko, M.; Kober, P.; Boresowicz, J.; Rusetska, N.; Paziewska, A.; Dąbrowska, M.; Piaścik, A.; Pękul, M.; Zieliński, G.; Kunicki, J.; et al. USP8 mutations in corticotroph adenomas determine a distinct gene expression profile irrespective of functional tumour status. Eur. J. Endocrinol. 2019, 181, 615–627. [Google Scholar] [CrossRef]
- Martins, C.S.; Camargo, R.C.; Coeli-Lacchini, F.B.; Saggioro, F.P.; Moreira, A.C.; de Castro, M. USP8 Mutations and Cell Cycle Regulation in Corticotroph Adenomas. Horm. Metab. Res. 2020, 52, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellnou, S.; Vasiljevic, A.; Lapras, V.; Raverot, V.; Alix, E.; Borson-Chazot, F.; Jouanneau, E.; Raverot, G.; Lasolle, H. SST5 expression and USP8 mutation in functioning and silent corticotroph pituitary tumors. Endocr. Connect. 2020, 9, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Ji, C.; Jian, X.; Zhou, J.; Zhang, Q.; Qiao, N.; Zhang, Y.; Shou, X.; Zhou, X.; Ma, Z. Regulation of the EGFR Pathway by HSP90 Is Involved in the Pathogenesis of Cushing’s Disease. Front. Endocrinol. 2021, 11, 601984. [Google Scholar] [CrossRef]
- Sbiera, S.; Perez-Rivas, L.G.; Taranets, L.; Weigand, I.; Flitsch, J.; Graf, E.; Monoranu, C.M.; Saeger, W.; Hagel, C.; Honegger, J.; et al. Driver mutations in USP8 wild-type Cushing’s disease. Neuro-Oncology 2019, 21, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L.; Mamon, H.; Kulke, M.H.; Berbeco, R.; Makrigiorgos, G.M. Replacing PCR with COLD-PCR enriches variant DNA sequences and redefines the sensitivity of genetic testing. Nat. Med. 2008, 14, 579–584. [Google Scholar] [CrossRef]
- Mangili, F.; Treppiedi, D.; Catalano, R.; Marra, G.; Di Muro, G.; Spada, A.; Arosio, M.; Peverelli, E.; Mantovani, G. A novel mechanism regulating dopamine receptor type 2 signal transduction in pituitary tumoral cells: The role of cAMP/PKA-induced filamin A phosphorylation. Front. Endocrinol. 2021, 11, 611752. [Google Scholar] [CrossRef]
- Treppiedi, D.; Di Muro, G.; Mangili, F.; Catalano, R.; Giardino, E.; Barbieri, A.M.; Locatelli, M.; Arosio, M.; Spada, A.; Peverelli, E.; et al. Filamin A is required for somatostatin receptor type 5 expression and pasireotide-mediated signaling in pituitary corticotroph tumor cells. Mol. Cell. Endocrinol. 2021, 524, 111159. [Google Scholar] [CrossRef]
- Serban, A.L.; Del Sindaco, G.; Sala, E.; Carosi, G.; Indirli, R.; Rodari, G.; Giavoli, C.; Locatelli, M.; Carrabba, G.; Bertani, G. Determinants of outcome of transsphenoidal surgery for Cushing disease in a single-centre series. J. Endocrinol. Investig. 2020, 43, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Boutillier, A.L.; Gaiddon, C.; Lorang, D.; Roberts, J.L.; Loeffler, J.P. Transcriptional activation of the proopiomelanocortin gene by cyclic AMP-responsive element binding protein. Pituitary 1998, 1, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, K.; Ikeda, H.; Nigawara, T.; Sakihara, S.; Suda, T. Expression of adrenocorticotropic hormone, prolactin and transcriptional factors in clinically nonfunctioning pituitary adenoma. Endocr. J. 2007, 54, 961–968. [Google Scholar] [CrossRef] [Green Version]
- Bamberger, C.M.; Fehn, M.; Bamberger, A.M.; Ludecke, D.K.; Beil, F.U.; Saeger, W.; Schulte, H.M. Reduced expression levels of the cell-cycle inhibitor p27Kip1 in human pituitary adenomas. Eur. J. Endocrinol. 1999, 140, 250–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lidhar, K.; Korbonits, M.; Jordan, S.; Khalimova, Z.; Kaltsas, G.; Lu, X.; Clayton, R.N.; Jenkins, P.J.; Monson, J.P.; Besser, G.M.; et al. Low expression of the cell cycle inhibitor p27Kip1 in normal corticotroph cells, corticotroph tumors, and malignant pituitary tumors. J. Clin. Endocrinol. Metab. 1999, 84, 3823–3830. [Google Scholar] [CrossRef]
- D’Angelo, D.; De Martino, M.; Arra, C.; Fusco, A. Emerging Role of USP8, HMGA, and Non-Coding RNAs in Pituitary Tumorigenesis. Cancers 2019, 11, 1302. [Google Scholar] [CrossRef] [Green Version]
- Losa, M.; Mortini, P.; Pagnano, A.; Detomas, M.; Cassarino, M.F.; Pecori Giraldi, F. Clinical characteristics and surgical outcome in USP8-mutated human adrenocorticotropic hormone-secreting pituitary adenomas. Endocrine 2019, 63, 240–246. [Google Scholar] [CrossRef]
- Bujko, M.; Kober, P.; Boresowicz, J.; Rusetska, N.; Zeber-Lubecka, N.; Paziewska, A.; Pekul, M.; Zielinski, G.; Styk, A.; Kunicki, J. Differential microRNA Expression in USP8-Mutated and Wild-Type Corticotroph Pituitary Tumors Reflect the Difference in Protein Ubiquitination Processes. J. Clin. Med. 2021, 10, 375. [Google Scholar] [CrossRef]
- Stroud, A.; Dhaliwal, P.; Alvarado, R.; Winder, M.J.; Jonker, B.P.; Grayson, J.W.; Hamizan, A.; Harvey, R.J.; McCormack, A. Outcomes of pituitary surgery for Cushing’s disease: A systematic review and meta-analysis. Pituitary 2020, 23, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Cassarino, M.F.; Ambrogio, A.G.; Cassarino, A.; Terreni, M.R.; Gentilini, D.; Sesta, A.; Cavagnini, F.; Losa, M.; Pecori Giraldi, F. Gene expression profiling in human corticotroph tumours reveals distinct, neuroendocrine profiles. J. Neuroendocrinol. 2018, 30, e12628. [Google Scholar] [CrossRef] [PubMed]
- Brar, B.K.; Chen, A.; Perrin, M.H.; Vale, W. Specificity and regulation of extracellularly regulated kinase1/2 phosphorylation through corticotropin-releasing factor (CRF) receptors 1 and 2beta by the CRF/urocortin family of peptides. Endocrinology 2004, 145, 1718–1729. [Google Scholar] [CrossRef] [Green Version]
- Hubina, E.; Nanzer, A.M.; Hanson, M.R.; Ciccarelli, E.; Losa, M.; Gaia, D.; Papotti, M.; Terreni, M.R.; Khalaf, S.; Jordan, S.; et al. Somatostatin analogues stimulate p27 expression and inhibit the MAP kinase pathway in pituitary tumours. Eur. J. Endocrinol. 2006, 155, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Treppiedi, D.; Giardino, E.; Catalano, R.; Mangili, F.; Vercesi, P.; Sala, E.; Locatelli, M.; Arosio, M.; Spada, A.; Mantovani, G.; et al. Somatostatin analogs regulate tumor corticotrophs growth by reducing ERK1/2 activity. Mol. Cell. Endocrinol. 2019, 483, 31–38. [Google Scholar] [CrossRef]
- Fukuoka, H.; Cooper, O.; Ben-Shlomo, A.; Mamelak, A.; Ren, S.G.; Bruyette, D.; Melmed, S. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J. Clin. Investig. 2011, 121, 4712–4721. [Google Scholar] [CrossRef] [Green Version]
- Asari, Y.; Kageyama, K.; Sugiyama, A.; Kogawa, H.; Niioka, K.; Daimon, M. Lapatinib decreases the ACTH production and proliferation of corticotroph tumor cells. Endocr. J. 2019, 66, 515–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnár, J.; Szakács, G.; Tusnády, G.E. Characterization of disease-associated mutations in human transmembrane proteins. PLoS ONE 2016, 11, e0151760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiang, R.; Thompson, L.M.; Zhu, Y.Z.; Church, D.M.; Fielder, T.J.; Bocian, M.; Winokur, S.T.; Wasmuth, J.J. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell 1994, 78, 335–342. [Google Scholar] [CrossRef]
- Su, M.A.; Trenor, C.C.; Fleming, J.C.; Fleming, M.D.; Andrews, N.C. The G185R mutation disrupts function of the iron transporter Nramp2. Blood 1998, 92, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, A.; De Falco, L.; Borgese, F.; Esposito, M.R.; Avvisati, R.A.; Izzo, P.; Piscopo, C.; Guizouarn, H.; Biondani, A.; Pantaleo, A.; et al. A novel erythroid anion exchange variant (Gly796Arg) of hereditary stomatocytosis associated with dyserythropoiesis. Haematologica 2009, 94, 1049–1059. [Google Scholar] [CrossRef]


| All (n = 60) | WT (n = 50) | USP8 (n = 7) * | p§ | |
|---|---|---|---|---|
| Age (years, mean ± SD) | 45 ± 15.1 | 46 ± 15.1 | 46.8 ± 14.8 | 0.88 |
| Females [n/tot (%)] | 45/60 (75%) | 37/50 (74%) | 6/7 (85%) | 0.67 |
| ACTH pg/mL (median, IQR) | 44.3 (33.1–77.6) | 43.6 (32–76) | 57 (38–110) | 0.308 |
| Relative UFC (median, IQR) | 2.3 (1.2–3.7) | 2.3 (1.2–3.3) | 2.1 (0.6–7.1) | 0.832 |
| Microadenoma [n/tot (%)] | 39/60 (65) | 35/50 (70) | 2/7 (28) | 0.041 |
| Macroadenoma [n/tot (%)] | 9/60 (15%) | 4/50 (8) | 4/7 (57.1) | 0.005 |
| Negative imaging [n/tot (%)] | 12/60 (20) | 11/50 (22) | 1/7 (14.2) | 1 |
| Remission [n/tot (%)] | 46/60 (76.6) | 38/50 (76) | 6/7 (86.5) | 1 |
| Recurrence [n/tot (%)] | 9/46 (19.5%) | 5/38 (13.1) | 3/6 (50) | 0.009 |
| Follow-up [years, median IQR)] | 5 (2–8) | 5 (2–8) | 4 (3–6) | 0.614 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treppiedi, D.; Barbieri, A.M.; Di Muro, G.; Marra, G.; Mangili, F.; Catalano, R.; Esposito, E.; Ferrante, E.; Serban, A.L.; Locatelli, M.; et al. Genetic Profiling of a Cohort of Italian Patients with ACTH-Secreting Pituitary Tumors and Characterization of a Novel USP8 Gene Variant. Cancers 2021, 13, 4022. https://doi.org/10.3390/cancers13164022
Treppiedi D, Barbieri AM, Di Muro G, Marra G, Mangili F, Catalano R, Esposito E, Ferrante E, Serban AL, Locatelli M, et al. Genetic Profiling of a Cohort of Italian Patients with ACTH-Secreting Pituitary Tumors and Characterization of a Novel USP8 Gene Variant. Cancers. 2021; 13(16):4022. https://doi.org/10.3390/cancers13164022
Chicago/Turabian StyleTreppiedi, Donatella, Anna Maria Barbieri, Genesio Di Muro, Giusy Marra, Federica Mangili, Rosa Catalano, Emanuela Esposito, Emanuele Ferrante, Andreea Liliana Serban, Marco Locatelli, and et al. 2021. "Genetic Profiling of a Cohort of Italian Patients with ACTH-Secreting Pituitary Tumors and Characterization of a Novel USP8 Gene Variant" Cancers 13, no. 16: 4022. https://doi.org/10.3390/cancers13164022
APA StyleTreppiedi, D., Barbieri, A. M., Di Muro, G., Marra, G., Mangili, F., Catalano, R., Esposito, E., Ferrante, E., Serban, A. L., Locatelli, M., Lania, A. G., Spada, A., Arosio, M., Peverelli, E., & Mantovani, G. (2021). Genetic Profiling of a Cohort of Italian Patients with ACTH-Secreting Pituitary Tumors and Characterization of a Novel USP8 Gene Variant. Cancers, 13(16), 4022. https://doi.org/10.3390/cancers13164022







