Testicular Diffuse Large B-Cell Lymphoma—Clinical, Molecular, and Immunological Features
Abstract
:Simple Summary
Abstract
1. Introduction
2. Etiology and Pathogenesis
3. Lymphoma Classification
4. Clinical Presentation; Diagnosis, Staging, and Prognostic Factors
5. Treatment
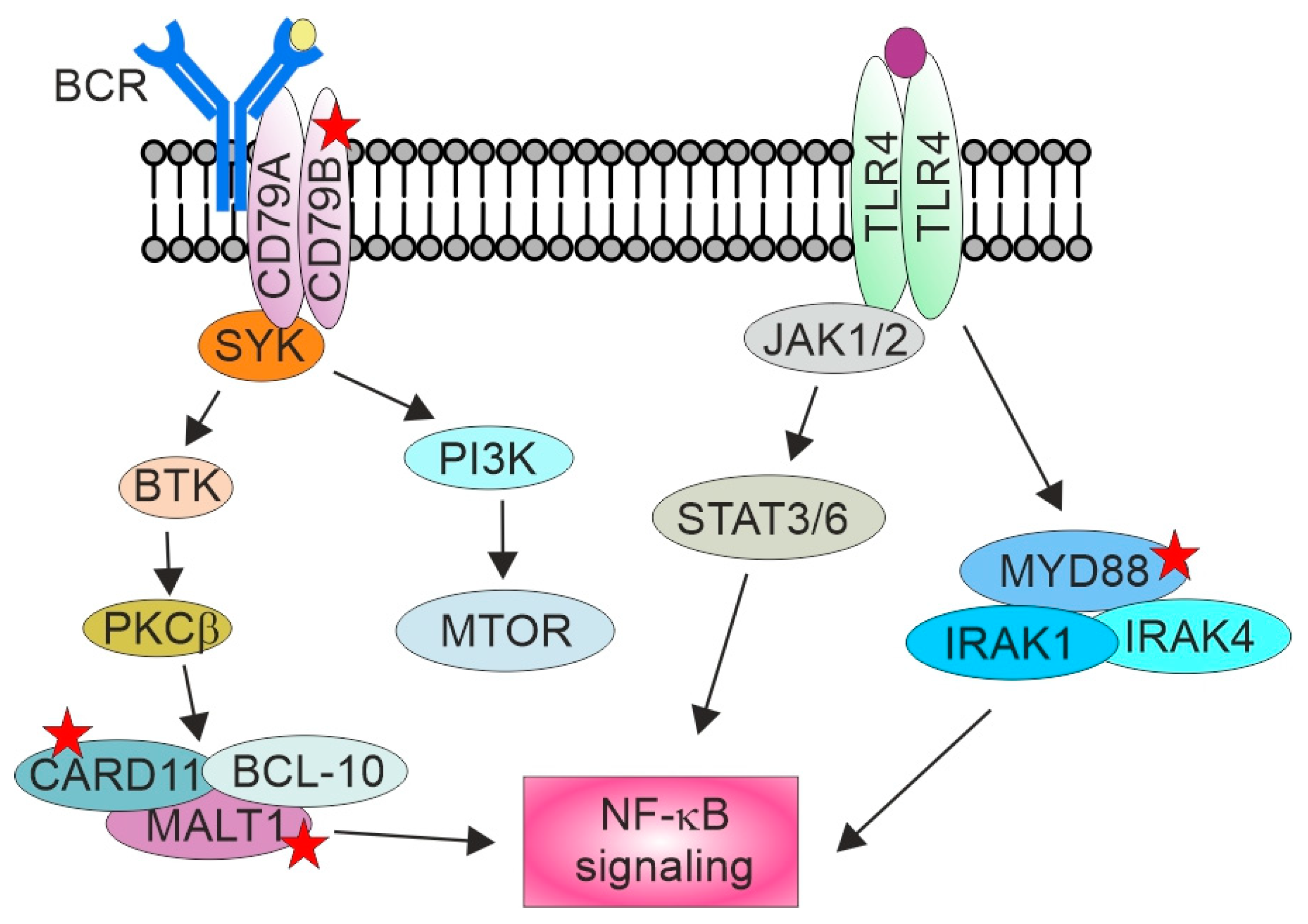
6. Genetic Landscape
7. The Tumor Microenvironment
7.1. Tumor-Infiltrating Lymphocytes
7.2. Macrophages
7.3. The Role of Host Immunity and Immune-Escape
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Mannisto, S.; Vahamurto, P.; Pollari, M.; Clausen, M.R.; Jyrkkio, S.; Kellokumpu-Lehtinen, P.L.; Kovanen, P.; Karjalainen-Lindsberg, M.L.; d’Amore, F.; Leppa, S. Intravenous but not intrathecal central nervous system-directed chemotherapy improves survival in patients with testicular diffuse large B-cell lymphoma. Eur. J. Cancer 2019, 115, 27–36. [Google Scholar] [CrossRef]
- Zucca, E.; Roggero, E.; Bertoni, F.; Cavalli, F. Primary extranodal non-Hodgkin’s lymphomas. Part 1: Gastrointestinal, cutaneous and genitourinary lymphomas. Ann. Oncol. 1997, 8, 727–737. [Google Scholar] [CrossRef]
- Xu, H.; Yao, F. Primary testicular lymphoma: A SEER analysis of 1,169 cases. Oncol. Lett. 2019, 17, 3113–3124. [Google Scholar] [CrossRef] [Green Version]
- Lagrange, J.L.; Ramaioli, A.; Theodore, C.H.; Terrier-Lacombe, M.J.; Beckendorf, V.; Biron, P.; Chevreau, C.H.; Chinet-Charrot, P.; Dumont, J.; Delobel-Deroide, A.; et al. Non-Hodgkin’s lymphoma of the testis: A retrospective study of 84 patients treated in the French anticancer centres. Ann. Oncol. 2001, 12, 1313–1319. [Google Scholar] [CrossRef]
- Gundrum, J.D.; Mathiason, M.A.; Moore, D.B.; Go, R.S. Primary testicular diffuse large B-cell lymphoma: A population-based study on the incidence, natural history, and survival comparison with primary nodal counterpart before and after the introduction of rituximab. J. Clin. Oncol. 2009, 27, 5227–5232. [Google Scholar] [CrossRef]
- Hasselblom, S.; Ridell, B.; Wedel, H.; Norrby, K.; Sender Baum, M.; Ekman, T. Testicular lymphoma—A retrospective, population-based, clinical and immunohistochemical study. Acta Oncol. 2004, 43, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Kemmerling, R.; Stintzing, S.; Muhlmann, J.; Dietze, O.; Neureiter, D. Primary testicular lymphoma: A strictly homogeneous hematological disease? Oncol. Rep. 2010, 23, 1261–1267. [Google Scholar] [PubMed] [Green Version]
- Oishi, N.; Kondo, T.; Nakazawa, T.; Mochizuki, K.; Tanioka, F.; Oyama, T.; Yamamoto, T.; Iizuka, J.; Tanabe, K.; Shibata, N.; et al. High prevalence of the MYD88 mutation in testicular lymphoma: Immunohistochemical and genetic analyses. Pathol. Int. 2015, 65, 528–535. [Google Scholar] [CrossRef]
- Deng, L.; Xu-Monette, Z.Y.; Loghavi, S.; Manyam, G.C.; Xia, Y.; Visco, C.; Huh, J.; Zhang, L.; Zhai, Q.; Wang, Y.; et al. Primary testicular diffuse large B-cell lymphoma displays distinct clinical and biological features for treatment failure in rituximab era: A report from the International PTL Consortium. Leukemia 2016, 30, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Al-Abbadi, M.A.; Hattab, E.M.; Tarawneh, M.S.; Amr, S.S.; Orazi, A.; Ulbright, T.M. Primary testicular diffuse large B-cell lymphoma belongs to the nongerminal center B-cell-like subgroup: A study of 18 cases. Mod. Pathol. 2006, 19, 1521–1527. [Google Scholar] [CrossRef]
- Li, D.; Xie, P.; Mi, C. Primary testicular diffuse large B-cell lymphoma shows an activated B-cell-like phenotype. Pathol. Res. Pract. 2010, 206, 611–615. [Google Scholar] [CrossRef]
- Menter, T.; Ernst, M.; Drachneris, J.; Dirnhofer, S.; Barghorn, A.; Went, P.; Tzankov, A. Phenotype profiling of primary testicular diffuse large B-cell lymphomas. Hematol. Oncol. 2014, 32, 72–81. [Google Scholar] [CrossRef]
- Bart, J.; Groen, H.J.; van der Graaf, W.T.; Hollema, H.; Hendrikse, N.H.; Vaalburg, W.; Sleijfer, D.T.; de Vries, E.G. An oncological view on the blood-testis barrier. Lancet Oncol. 2002, 3, 357–363. [Google Scholar] [CrossRef]
- Mital, P.; Hinton, B.T.; Dufour, J.M. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol. Reprod. 2011, 84, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Fijak, M.; Bhushan, S.; Meinhardt, A. Immunoprivileged sites: The testis. Methods Mol. Biol. 2011, 677, 459–470. [Google Scholar]
- Fonseca, R.; Habermann, T.M.; Colgan, J.P.; O’Neill, B.P.; White, W.L.; Witzig, T.E.; Egan, K.S.; Martenson, J.A.; Burgart, L.J.; Inwards, D.J. Testicular lymphoma is associated with a high incidence of extranodal recurrence. Cancer 2000, 88, 154–161. [Google Scholar] [CrossRef]
- Caumont, F.; Porter, C.; DeBerg, H.; Burns, J.; Frankel, J.; Flores, J.P. Combined chemotherapy and radiotherapy improves survival in 1897 testicular Lymphoma patients from a contemporary cohort. Urol. Oncol. 2020, 38, 641.e1–641.e8. [Google Scholar] [CrossRef]
- Vitolo, U.; Chiappella, A.; Ferreri, A.J.; Martelli, M.; Baldi, I.; Balzarotti, M.; Bottelli, C.; Conconi, A.; Gomez, H.; Lopez-Guillermo, A.; et al. First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: Final results of an international phase II trial. J. Clin. Oncol. 2011, 29, 2766–2772. [Google Scholar] [CrossRef]
- Aviles, A.; Nambo, M.J.; Cleto, S.; Neri, N.; Huerta-Guzman, J. Rituximab and dose-dense chemotherapy in primary testicular lymphoma. Clin. Lymphoma Myeloma 2009, 9, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, A.; Fowler, N.; Medeiros, L.J.; Iyengar, P.; Horace, P.; Dabaja, B.S. Outcome of patients with diffuse large B-cell lymphoma of the testis by era of treatment: The M. D. Anderson Cancer Center experience. Leuk. Lymphoma 2010, 51, 1217–1224. [Google Scholar] [CrossRef]
- Zucca, E.; Conconi, A.; Mughal, T.I.; Sarris, A.H.; Seymour, J.F.; Vitolo, U.; Klasa, R.; Ozsahin, M.; Mead, G.M.; Gianni, M.A.; et al. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J. Clin. Oncol. 2003, 21, 20–27. [Google Scholar] [CrossRef]
- Vitolo, U.; Seymour, J.F.; Martelli, M.; Illerhaus, G.; Illidge, T.; Zucca, E.; Campo, E.; Ladetto, M.; Committee, E.G. Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v91–v102. [Google Scholar] [CrossRef]
- Leivonen, S.K.; Pollari, M.; Bruck, O.; Pellinen, T.; Autio, M.; Karjalainen-Lindsberg, M.L.; Mannisto, S.; Kellokumpu-Lehtinen, P.L.; Kallioniemi, O.; Mustjoki, S.; et al. T-cell inflamed tumor microenvironment predicts favorable prognosis in primary testicular lymphoma. Haematologica 2019, 104, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Pollari, M.; Bruck, O.; Pellinen, T.; Vahamurto, P.; Karjalainen-Lindsberg, M.L.; Mannisto, S.; Kallioniemi, O.; Kellokumpu-Lehtinen, P.L.; Mustjoki, S.; Leivonen, S.K.; et al. PD-L1+ tumor-associated macrophages and PD-1+ tumor-infiltrating lymphocytes predict survival in primary testicular lymphoma. Haematologica 2018, 103, 1908–1914. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Xu, M.L. Microenvironment Cell Contribution to Lymphoma Immunity. Front. Oncol. 2018, 8, 288. [Google Scholar] [CrossRef] [Green Version]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Eyre, T.A.; Collins, G.P. Immune checkpoint inhibition in lymphoid disease. Br. J. Haematol. 2015, 170, 291–304. [Google Scholar] [CrossRef]
- Nayak, L.; Iwamoto, F.M.; LaCasce, A.; Mukundan, S.; Roemer, M.G.M.; Chapuy, B.; Armand, P.; Rodig, S.J.; Shipp, M.A. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood 2017, 129, 3071–3073. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef]
- Shaffer, A.L., 3rd; Young, R.M.; Staudt, L.M. Pathogenesis of human B cell lymphomas. Annu. Rev. Immunol. 2012, 30, 565–610. [Google Scholar] [CrossRef]
- Hans, C.P.; Weisenburger, D.D.; Greiner, T.C.; Gascoyne, R.D.; Delabie, J.; Ott, G.; Muller-Hermelink, H.K.; Campo, E.; Braziel, R.M.; Jaffe, E.S.; et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004, 103, 275–282. [Google Scholar] [CrossRef]
- Choi, W.W.; Weisenburger, D.D.; Greiner, T.C.; Piris, M.A.; Banham, A.H.; Delabie, J.; Braziel, R.M.; Geng, H.; Iqbal, J.; Lenz, G.; et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin. Cancer Res. 2009, 15, 5494–5502. [Google Scholar] [CrossRef] [Green Version]
- Meyer, P.N.; Fu, K.; Greiner, T.C.; Smith, L.M.; Delabie, J.; Gascoyne, R.D.; Ott, G.; Rosenwald, A.; Braziel, R.M.; Campo, E.; et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J. Clin. Oncol. 2011, 29, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Nyman, H.; Jerkeman, M.; Karjalainen-Lindsberg, M.L.; Banham, A.H.; Leppa, S. Prognostic impact of activated B-cell focused classification in diffuse large B-cell lymphoma patients treated with R-CHOP. Mod. Pathol. 2009, 22, 1094–1101. [Google Scholar] [CrossRef]
- Wright, G.; Tan, B.; Rosenwald, A.; Hurt, E.H.; Wiestner, A.; Staudt, L.M. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc. Natl. Acad. Sci. USA 2003, 100, 9991–9996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenwald, A.; Wright, G.; Chan, W.C.; Connors, J.M.; Campo, E.; Fisher, R.I.; Gascoyne, R.D.; Muller-Hermelink, H.K.; Smeland, E.B.; Giltnane, J.M.; et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002, 346, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Lenz, G.; Wright, G.; Dave, S.S.; Xiao, W.; Powell, J.; Zhao, H.; Xu, W.; Tan, B.; Goldschmidt, N.; Iqbal, J.; et al. Stromal gene signatures in large-B-cell lymphomas. N. Engl. J. Med. 2008, 359, 2313–2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staiger, A.M.; Ziepert, M.; Horn, H.; Scott, D.W.; Barth, T.F.E.; Bernd, H.W.; Feller, A.C.; Klapper, W.; Szczepanowski, M.; Hummel, M.; et al. Clinical Impact of the Cell-of-Origin Classification and the MYC/BCL2 Dual Expresser Status in Diffuse Large B-Cell Lymphoma Treated Within Prospective Clinical Trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group. J. Clin. Oncol. 2017, 35, 2515–2526. [Google Scholar] [CrossRef]
- Gutierrez-Garcia, G.; Cardesa-Salzmann, T.; Climent, F.; Gonzalez-Barca, E.; Mercadal, S.; Mate, J.L.; Sancho, J.M.; Arenillas, L.; Serrano, S.; Escoda, L.; et al. Gene-expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood 2011, 117, 4836–4843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.X.; Liu, Y.H.; Luo, D.L.; Zhang, F.; Cheng, Y.; Luo, X.L.; Xu, J.; Cheng, J.; Zhuang, H.G. MYC expression in concert with BCL2 and BCL6 expression predicts outcome in Chinese patients with diffuse large B-cell lymphoma, not otherwise specified. PLoS ONE 2014, 9, e104068. [Google Scholar] [CrossRef] [Green Version]
- Booman, M.; Douwes, J.; Glas, A.M.; de Jong, D.; Schuuring, E.; Kluin, P.M. Primary testicular diffuse large B-cell lymphomas have activated B-cell-like subtype characteristics. J. Pathol. 2006, 210, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Chaudhary, U.B.; Costa, L.J.; Gudena, V.; Lazarchick, J. Primary testicular lymphoma and AIDS. Ann. Clin. Lab. Sci. 2010, 40, 75–79. [Google Scholar]
- Cote, T.R.; Biggar, R.J.; Rosenberg, P.S.; Devesa, S.S.; Percy, C.; Yellin, F.J.; Lemp, G.; Hardy, C.; Geodert, J.J.; Blattner, W.A. Non-Hodgkin’s lymphoma among people with AIDS: Incidence, presentation and public health burden. AIDS/Cancer Study Group. Int. J. Cancer 1997, 73, 645–650. [Google Scholar] [CrossRef]
- Twa, D.D.W.; Mottok, A.; Savage, K.J.; Steidl, C. The pathobiology of primary testicular diffuse large B-cell lymphoma: Implications for novel therapies. Blood Rev. 2018, 32, 249–255. [Google Scholar] [CrossRef]
- Chapuy, B.; Roemer, M.G.; Stewart, C.; Tan, Y.; Abo, R.P.; Zhang, L.; Dunford, A.J.; Meredith, D.M.; Thorner, A.R.; Jordanova, E.S.; et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood 2016, 127, 869–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booman, M.; Douwes, J.; Glas, A.M.; Riemersma, S.A.; Jordanova, E.S.; Kok, K.; Rosenwald, A.; de Jong, D.; Schuuring, E.; Kluin, P.M. Mechanisms and effects of loss of human leukocyte antigen class II expression in immune-privileged site-associated B-cell lymphoma. Clin. Cancer Res. 2006, 12, 2698–2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemersma, S.A.; Jordanova, E.S.; Schop, R.F.; Philippo, K.; Looijenga, L.H.; Schuuring, E.; Kluin, P.M. Extensive genetic alterations of the HLA region, including homozygous deletions of HLA class II genes in B-cell lymphomas arising in immune-privileged sites. Blood 2000, 96, 3569–3577. [Google Scholar] [CrossRef]
- Swerdlow, S.; Campo, E.; Harris, N.; Jaffe, E.; Pileri, S.; Stein, H.; Thiele, J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; IARC: Lyon, France, 2017; Volume 2. [Google Scholar]
- Moller, M.B.; d’Amore, F.; Christensen, B.E. Testicular lymphoma: A population-based study of incidence, clinicopathological correlations and prognosis. The Danish Lymphoma Study Group, LYFO. Eur. J. Cancer 1994, 30A, 1760–1764. [Google Scholar] [CrossRef]
- NCCN. Clinical Practice Guidelines for B-Cell Lymphomas. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1480 (accessed on 22 June 2021).
- Carbone, P.P.; Kaplan, H.S.; Musshoff, K.; Smithers, D.W.; Tubiana, M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971, 31, 1860–1861. [Google Scholar]
- Sin, K.M.; Ho, S.K.; Wong, B.Y.; Gill, H.; Khong, P.L.; Lee, E.Y. Beyond the lymph nodes: FDG-PET/CT in primary extranodal lymphoma. Clin. Imaging 2017, 42, 25–33. [Google Scholar] [CrossRef] [PubMed]
- International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N. Engl. J. Med. 1993, 329, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Ferry, J.A.; Harris, N.L.; Young, R.H.; Coen, J.; Zietman, A.; Scully, R.E. Malignant lymphoma of the testis, epididymis, and spermatic cord. A clinicopathologic study of 69 cases with immunophenotypic analysis. Am. J. Surg. Pathol. 1994, 18, 376–390. [Google Scholar] [CrossRef]
- Seymour, J.F.; Solomon, B.; Wolf, M.M.; Janusczewicz, E.H.; Wirth, A.; Prince, H.M. Primary large-cell non-Hodgkin’s lymphoma of the testis: A retrospective analysis of patterns of failure and prognostic factors. Clin. Lymphoma 2001, 2, 109–115. [Google Scholar] [CrossRef]
- Crellin, A.M.; Hudson, B.V.; Bennett, M.H.; Harland, S.; Hudson, G.V. Non-Hodgkin’s lymphoma of the testis. Radiother. Oncol. 1993, 27, 99–106. [Google Scholar] [CrossRef]
- Tokiya, R.; Yoden, E.; Konishi, K.; Kamitani, N.; Hiratsuka, J.; Koresawa, R.; Hirose, T.; Sano, F.; Tokunaga, H.; Kondo, T.; et al. Efficacy of prophylactic irradiation to the contralateral testis for patients with advanced-stage primary testicular lymphoma: An analysis of outcomes at a single institution. Int. J. Hematol. 2017, 106, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Kridel, R.; Telio, D.; Villa, D.; Sehn, L.H.; Gerrie, A.S.; Shenkier, T.; Klasa, R.; Slack, G.W.; Tan, K.; Gascoyne, R.D.; et al. Diffuse large B-cell lymphoma with testicular involvement: Outcome and risk of CNS relapse in the rituximab era. Br. J. Haematol. 2017, 176, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Vahamurto, P.; Pollari, M.; Clausen, M.R.; d’Amore, F.; Leppa, S.; Mannisto, S. Low Absolute Lymphocyte Counts in the Peripheral Blood Predict Inferior Survival and Improve the International Prognostic Index in Testicular Diffuse Large B-Cell Lymphoma. Cancers 2020, 12, 1967. [Google Scholar] [CrossRef] [PubMed]
- Ziepert, M.; Hasenclever, D.; Kuhnt, E.; Glass, B.; Schmitz, N.; Pfreundschuh, M.; Loeffler, M. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J. Clin. Oncol. 2010, 28, 2373–2380. [Google Scholar] [CrossRef]
- Sehn, L.H.; Berry, B.; Chhanabhai, M.; Fitzgerald, C.; Gill, K.; Hoskins, P.; Klasa, R.; Savage, K.J.; Shenkier, T.; Sutherland, J.; et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007, 109, 1857–1861. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Sehn, L.H.; Rademaker, A.W.; Gordon, L.I.; Lacasce, A.S.; Crosby-Thompson, A.; Vanderplas, A.; Zelenetz, A.D.; Abel, G.A.; Rodriguez, M.A.; et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014, 123, 837–842. [Google Scholar] [CrossRef]
- Deng, L.; Song, Y.; Zhu, J.; Zheng, W.; Wang, X.; Xie, Y.; Lin, N.; Tu, M.; Ping, L.; Ying, Z.; et al. Secondary central nervous system involvement in 599 patients with diffuse large B-cell lymphoma: Are there any changes in the rituximab era? Int. J. Hematol. 2013, 98, 664–671. [Google Scholar] [CrossRef]
- Ho, J.C.; Dabaja, B.S.; Milgrom, S.A.; Smith, G.L.; Reddy, J.P.; Mazloom, A.; Young, K.H.; Deng, L.; Medeiros, L.J.; Dong, W.; et al. Radiation therapy improves survival in patients with testicular diffuse large B-cell lymphoma. Leuk. Lymphoma 2017, 58, 2833–2844. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.A.; Slack, G.W.; Savage, K.J.; Connors, J.M.; Ben-Neriah, S.; Rogic, S.; Scott, D.W.; Tan, K.L.; Steidl, C.; Sehn, L.H.; et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. 2012, 30, 3452–3459. [Google Scholar] [CrossRef]
- Hu, S.; Xu-Monette, Z.Y.; Tzankov, A.; Green, T.; Wu, L.; Balasubramanyam, A.; Liu, W.M.; Visco, C.; Li, Y.; Miranda, R.N.; et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: A report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 2013, 121, 4021–4031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, T.M.; Young, K.H.; Visco, C.; Xu-Monette, Z.Y.; Orazi, A.; Go, R.S.; Nielsen, O.; Gadeberg, O.V.; Mourits-Andersen, T.; Frederiksen, M.; et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. 2012, 30, 3460–3467. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.J.; Slack, G.W.; Mottok, A.; Sehn, L.H.; Villa, D.; Kansara, R.; Kridel, R.; Steidl, C.; Ennishi, D.; Tan, K.L.; et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood 2016, 127, 2182–2188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, A.M.; Alvarado-Bernal, Y.; Laurini, J.A.; Smith, L.M.; Slack, G.W.; Tan, K.L.; Sehn, L.H.; Fu, K.; Aoun, P.; Greiner, T.C.; et al. MYC and BCL2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with rituximab. Br. J. Haematol. 2014, 165, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Miura, K.; Nakagawa, M.; Sugitani, M.; Amano, Y.; Kurita, D.; Sakagami, M.; Ohtake, S.; Uchino, Y.; Kodaira, H.; et al. Negative impact of concurrent overexpression of MYC and BCL2 in patients with advanced diffuse large B-cell lymphoma treated with dose-intensified immunochemotherapy. Leuk. Lymphoma 2016, 57, 2784–2790. [Google Scholar] [CrossRef]
- Staiger, A.M.; Altenbuchinger, M.; Ziepert, M.; Kohler, C.; Horn, H.; Huttner, M.; Huttl, K.S.; Glehr, G.; Klapper, W.; Szczepanowski, M.; et al. A novel lymphoma-associated macrophage interaction signature (LAMIS) provides robust risk prognostication in diffuse large B-cell lymphoma clinical trial cohorts of the DSHNHL. Leukemia 2020, 34, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Horn, H.; Ziepert, M.; Becher, C.; Barth, T.F.; Bernd, H.W.; Feller, A.C.; Klapper, W.; Hummel, M.; Stein, H.; Hansmann, M.L.; et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood 2013, 121, 2253–2263. [Google Scholar] [CrossRef] [Green Version]
- Barrans, S.; Crouch, S.; Smith, A.; Turner, K.; Owen, R.; Patmore, R.; Roman, E.; Jack, A. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J. Clin. Oncol. 2010, 28, 3360–3365. [Google Scholar] [CrossRef] [PubMed]
- Akyurek, N.; Uner, A.; Benekli, M.; Barista, I. Prognostic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer 2012, 118, 4173–4183. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Xu-Monette, Z.Y.; Tzankov, A.; Deng, L.; Wang, X.; Manyam, G.C.; Visco, C.; Montes-Moreno, S.; Zhang, L.; Dybkaer, K.; et al. Prognostic impact of concurrent MYC and BCL6 rearrangements and expression in de novo diffuse large B-cell lymphoma. Oncotarget 2016, 7, 2401–2416. [Google Scholar] [CrossRef] [Green Version]
- Copie-Bergman, C.; Cuilliere-Dartigues, P.; Baia, M.; Briere, J.; Delarue, R.; Canioni, D.; Salles, G.; Parrens, M.; Belhadj, K.; Fabiani, B.; et al. MYC-IG rearrangements are negative predictors of survival in DLBCL patients treated with immunochemotherapy: A GELA/LYSA study. Blood 2015, 126, 2466–2474. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.A.; Savage, K.J.; Ludkovski, O.; Ben-Neriah, S.; Woods, R.; Steidl, C.; Dyer, M.J.; Siebert, R.; Kuruvilla, J.; Klasa, R.; et al. Lymphomas with concurrent BCL2 and MYC translocations: The critical factors associated with survival. Blood 2009, 114, 2273–2279. [Google Scholar] [CrossRef] [Green Version]
- Niitsu, N.; Okamoto, M.; Miura, I.; Hirano, M. Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia 2009, 23, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Tomita, N.; Tokunaka, M.; Nakamura, N.; Takeuchi, K.; Koike, J.; Motomura, S.; Miyamoto, K.; Kikuchi, A.; Hyo, R.; Yakushijin, Y.; et al. Clinicopathological features of lymphoma/leukemia patients carrying both BCL2 and MYC translocations. Haematologica 2009, 94, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.O.; Gang, A.O.; Poulsen, T.S.; Knudsen, H.; Lauritzen, A.F.; Nielsen, S.L.; Gang, U.O.; Nørgaard, P. Double-hit BCL2/MYC translocations in a consecutive cohort of patients with large B-cell lymphoma–a single centre’s experience. Eur. J. Haematol. 2012, 89, 63–71. [Google Scholar] [CrossRef]
- Oki, Y.; Noorani, M.; Lin, P.; Davis, R.E.; Neelapu, S.S.; Ma, L.; Ahmed, M.; Rodriguez, M.A.; Hagemeister, F.B.; Fowler, N.; et al. Double hit lymphoma: The MD Anderson Cancer Center clinical experience. Br. J. Haematol. 2014, 166, 891–901. [Google Scholar] [CrossRef]
- Snuderl, M.; Kolman, O.K.; Chen, Y.B.; Hsu, J.J.; Ackerman, A.M.; Dal Cin, P.; Ferry, J.A.; Harris, N.L.; Hasserjian, R.P.; Zukerberg, L.R.; et al. B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am. J. Surg. Pathol. 2010, 34, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Desai, P.; Lin, P.; Yin, C.C.; Tang, G.; Wang, X.J.; Konoplev, S.N.; Khoury, J.D.; Bueso-Ramos, C.E.; Medeiros, L.J. MYC/BCL6 double-hit lymphoma (DHL): A tumour associated with an aggressive clinical course and poor prognosis. Histopathology 2016, 68, 1090–1098. [Google Scholar] [CrossRef]
- Reddy, A.; Zhang, J.; Davis, N.S.; Moffitt, A.B.; Love, C.L.; Waldrop, A.; Leppa, S.; Pasanen, A.; Meriranta, L.; Karjalainen-Lindsberg, M.L.; et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell 2017, 171, 481–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.; Savage, K.J.; Kutok, J.L.; Feuerhake, F.; Kurtin, P.; Mihm, M.; Wu, B.; Pasqualucci, L.; Neuberg, D.; Aguiar, R.C.; et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood 2005, 105, 1851–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, R.L.; Goodlad, J.R.; Calaminici, M.; Dotlic, S.; Montes-Moreno, S.; Oschlies, I.; Ponzoni, M.; Traverse-Glehen, A.; Ott, G.; Ferry, J.A. Lymphomas arising in immune-privileged sites: Insights into biology, diagnosis, and pathogenesis. Virchows Arch. 2020, 476, 647–665. [Google Scholar] [CrossRef]
- Twa, D.D.; Mottok, A.; Chan, F.C.; Ben-Neriah, S.; Woolcock, B.W.; Tan, K.L.; Mungall, A.J.; McDonald, H.; Zhao, Y.; Lim, R.S.; et al. Recurrent genomic rearrangements in primary testicular lymphoma. J. Pathol. 2015, 236, 136–141. [Google Scholar] [CrossRef]
- Booman, M.; Szuhai, K.; Rosenwald, A.; Hartmann, E.; Kluin-Nelemans, H.; de Jong, D.; Schuuring, E.; Kluin, P. Genomic alterations and gene expression in primary diffuse large B-cell lymphomas of immune-privileged sites: The importance of apoptosis and immunomodulatory pathways. J. Pathol. 2008, 216, 209–217. [Google Scholar] [CrossRef]
- Kraan, W.; van Keimpema, M.; Horlings, H.M.; Schilder-Tol, E.J.; Oud, M.E.; Noorduyn, L.A.; Kluin, P.M.; Kersten, M.J.; Spaargaren, M.; Pals, S.T. High prevalence of oncogenic MYD88 and CD79B mutations in primary testicular diffuse large B-cell lymphoma. Leukemia 2014, 28, 719–720. [Google Scholar] [CrossRef]
- Chen, Y.P.; Ke, L.F.; Lu, J.P.; Wang, J.C.; Zhu, W.F.; Chen, F.F.; Lin, S.F.; Xu, C.W.; Wu, M.J.; Chen, G. Prevalence and Clinical Significance of Oncogenic CD79B and MYD88 Mutations in Primary Testicular Diffuse Large B-Cell Lymphoma: A Retrospective Study in China. OncoTargets Ther. 2019, 12, 10165–10175. [Google Scholar] [CrossRef] [Green Version]
- Elfrink, S.; de Winde, C.M.; van den Brand, M.; Berendsen, M.; Roemer, M.G.M.; Arnold, F.; Janssen, L.; van der Schaaf, A.; Jansen, E.; Groenen, P.; et al. High frequency of inactivating tetraspanin CD37 mutations in diffuse large B-cell lymphoma at immune-privileged sites. Blood 2019, 134, 946–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordanova, E.S.; Riemersma, S.A.; Philippo, K.; Giphart-Gassler, M.; Schuuring, E.; Kluin, P.M. Hemizygous deletions in the HLA region account for loss of heterozygosity in the majority of diffuse large B-cell lymphomas of the testis and the central nervous system. Genes Chromosomes Cancer 2002, 35, 38–48. [Google Scholar] [CrossRef]
- Jordanova, E.S.; Riemersma, S.A.; Philippo, K.; Schuuring, E.; Kluin, P.M. Beta2-microglobulin aberrations in diffuse large B-cell lymphoma of the testis and the central nervous system. Int. J. Cancer 2003, 103, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Twa, D.D.W.; Lee, D.G.; Tan, K.L.; Slack, G.W.; Ben-Neriah, S.; Villa, D.; Connors, J.M.; Sehn, L.H.; Mottok, A.; Gascoyne, R.D.; et al. Genomic predictors of central nervous system relapse in primary testicular diffuse large B-cell lymphoma (DLBCL). Blood 2021, 137, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, X.; Cai, W.; Bao, H.; Huang, H.; Liu, Y.; Yang, X.; Ruan, C.; Wu, D.; Shen, H.; et al. TBL1XR1 mutation predicts poor outcome in primary testicular diffuse large B-cell lymphoma patients. Biomark. Res. 2020, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Hatzl, S.; Posch, F.; Schulz, E.; Gornicec, M.; Deutsch, A.; Beham-Schmid, C.; Pichler, M.; Greinix, H.; Sill, H.; Zebisch, A.; et al. The Role of Immunohistochemical Overexpression of p53 as Adverse Prognostic Factor in Primary Testicular Diffuse Large B Cell Lymphoma. Pathol. Oncol. Res. 2020, 26, 2831–2833. [Google Scholar] [CrossRef] [PubMed]
- Riemersma, S.A.; Oudejans, J.J.; Vonk, M.J.; Dreef, E.J.; Prins, F.A.; Jansen, P.M.; Vermeer, M.H.; Blok, P.; Kibbelaar, R.E.; Muris, J.J.; et al. High numbers of tumour-infiltrating activated cytotoxic T lymphocytes, and frequent loss of HLA class I and II expression, are features of aggressive B cell lymphomas of the brain and testis. J. Pathol. 2005, 206, 328–336. [Google Scholar] [CrossRef]
- Rimsza, L.M.; Roberts, R.A.; Campo, E.; Grogan, T.M.; Bea, S.; Salaverria, I.; Zettl, A.; Rosenwald, A.; Ott, G.; Muller-Hermelink, H.K.; et al. Loss of major histocompatibility class II expression in non-immune-privileged site diffuse large B-cell lymphoma is highly coordinated and not due to chromosomal deletions. Blood 2006, 107, 1101–1107. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Medeiros, L.J.; Li, Y.; Li, J.; Young, K.H. Genetic alterations and their clinical implications in DLBCL. Nat. Rev. Clin. Oncol. 2019, 16, 634–652. [Google Scholar] [CrossRef]
- Wilson, W.H.; Young, R.M.; Schmitz, R.; Yang, Y.; Pittaluga, S.; Wright, G.; Lih, C.J.; Williams, P.M.; Shaffer, A.L.; Gerecitano, J.; et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 2015, 21, 922–926. [Google Scholar] [CrossRef]
- Godfrey, J.; Tumuluru, S.; Bao, R.; Leukam, M.; Venkataraman, G.; Phillip, J.; Fitzpatrick, C.; McElherne, J.; MacNabb, B.W.; Orlowski, R.; et al. PD-L1 gene alterations identify a subset of diffuse large B-cell lymphoma harboring a T-cell-inflamed phenotype. Blood 2019, 133, 2279–2290. [Google Scholar] [CrossRef]
- Shi, M.; Roemer, M.G.; Chapuy, B.; Liao, X.; Sun, H.; Pinkus, G.S.; Shipp, M.A.; Freeman, G.J.; Rodig, S.J. Expression of programmed cell death 1 ligand 2 (PD-L2) is a distinguishing feature of primary mediastinal (thymic) large B-cell lymphoma and associated with PDCD1LG2 copy gain. Am. J. Surg. Pathol. 2014, 38, 1715–1723. [Google Scholar] [CrossRef]
- Meriranta, L.; Pasanen, A.; Alkodsi, A.; Haukka, J.; Karjalainen-Lindsberg, M.L.; Leppa, S. Molecular background delineates outcome of double protein expressor diffuse large B-cell lymphoma. Blood Adv. 2020, 4, 3742–3753. [Google Scholar] [CrossRef]
- Pasqualucci, L.; Dalla-Favera, R. Genetics of diffuse large B-cell lymphoma. Blood 2018, 131, 2307–2319. [Google Scholar] [CrossRef]
- Tibiletti, M.G.; Martin, V.; Bernasconi, B.; Del Curto, B.; Pecciarini, L.; Uccella, S.; Pruneri, G.; Ponzoni, M.; Mazzucchelli, L.; Martinelli, G.; et al. BCL2, BCL6, MYC, MALT 1, and BCL10 rearrangements in nodal diffuse large B-cell lymphomas: A multicenter evaluation of a new set of fluorescent in situ hybridization probes and correlation with clinical outcome. Hum. Pathol. 2009, 40, 645–652. [Google Scholar] [CrossRef]
- Gascoyne, D.M.; Banham, A.H. The significance of FOXP1 in diffuse large B-cell lymphoma. Leuk. Lymphoma 2017, 58, 1037–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastian, E.; Alcoceba, M.; Balanzategui, A.; Marin, L.; Montes-Moreno, S.; Flores, T.; Gonzalez, D.; Sarasquete, M.E.; Chillon, M.C.; Puig, N.; et al. Molecular characterization of immunoglobulin gene rearrangements in diffuse large B-cell lymphoma: Antigen-driven origin and IGHV4-34 as a particular subgroup of the non-GCB subtype. Am. J. Pathol. 2012, 181, 1879–1888. [Google Scholar] [CrossRef]
- Davoodzadeh Gholami, M.; Kardar, G.A.; Saeedi, Y.; Heydari, S.; Garssen, J.; Falak, R. Exhaustion of T lymphocytes in the tumor microenvironment: Significance and effective mechanisms. Cell Immunol. 2017, 322, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Bronte, V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Investig. 2007, 117, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Huelsken, J.; Hanahan, D. A Subset of Cancer-Associated Fibroblasts Determines Therapy Resistance. Cell 2018, 172, 643–644. [Google Scholar] [CrossRef] [Green Version]
- Scott, D.W.; Gascoyne, R.D. The tumour microenvironment in B cell lymphomas. Nat. Rev. Cancer 2014, 14, 517–534. [Google Scholar] [CrossRef]
- Maj, T.; Wang, W.; Crespo, J.; Zhang, H.; Wang, W.; Wei, S.; Zhao, L.; Vatan, L.; Shao, I.; Szeliga, W.; et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat. Immunol. 2017, 18, 1332–1341. [Google Scholar] [CrossRef]
- Wang, D.; Quiros, J.; Mahuron, K.; Pai, C.C.; Ranzani, V.; Young, A.; Silveria, S.; Harwin, T.; Abnousian, A.; Pagani, M.; et al. Targeting EZH2 Reprograms Intratumoral Regulatory T Cells to Enhance Cancer Immunity. Cell Rep. 2018, 23, 3262–3274. [Google Scholar] [CrossRef]
- Pauken, K.E.; Wherry, E.J. Overcoming T cell exhaustion in infection and cancer. Trends. Immunol. 2015, 36, 265–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Noma, K.; Ohara, T.; Kashima, H.; Katsura, Y.; Sato, H.; Komoto, S.; Katsube, R.; Ninomiya, T.; Tazawa, H.; et al. Cancer-Associated Fibroblasts Affect Intratumoral CD8+ and FoxP3+ T Cells Via IL6 in the Tumor Microenvironment. Clin. Cancer Res. 2018, 24, 4820–4833. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borst, J.; Ahrends, T.; Babala, N.; Melief, C.J.M.; Kastenmuller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Pollari, M.; Pellinen, T.; Karjalainen-Lindsberg, M.L.; Kellokumpu-Lehtinen, P.L.; Leivonen, S.K.; Leppa, S. Adverse prognostic impact of regulatory T-cells in testicular diffuse large B-cell lymphoma. Eur. J. Haematol. 2020, 105, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.A.; Tucker-Heard, G.; Perdue, N.R.; Killebrew, J.R.; Urdahl, K.B.; Campbell, D.J. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 2009, 10, 595–602. [Google Scholar] [CrossRef]
- Ahrends, T.; Spanjaard, A.; Pilzecker, B.; Babala, N.; Bovens, A.; Xiao, Y.; Jacobs, H.; Borst, J. CD4+ T Cell Help Confers a Cytotoxic T Cell Effector Program Including Coinhibitory Receptor Downregulation and Increased Tissue Invasiveness. Immunity 2017, 47, 848–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottschalk, C.; Mettke, E.; Kurts, C. The Role of Invariant Natural Killer T Cells in Dendritic Cell Licensing, Cross-Priming, and Memory CD8+ T Cell Generation. Front. Immunol. 2015, 6, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef]
- Lazarevic, V.; Glimcher, L.H.; Lord, G.M. T-bet: A bridge between innate and adaptive immunity. Nat. Rev. Immunol. 2013, 13, 777–789. [Google Scholar] [CrossRef]
- Kachler, K.; Holzinger, C.; Trufa, D.I.; Sirbu, H.; Finotto, S. The role of Foxp3 and Tbet co-expressing Treg cells in lung carcinoma. Oncoimmunology 2018, 7, e1456612. [Google Scholar] [CrossRef] [Green Version]
- Jankovic, D.; Kullberg, M.C.; Feng, C.G.; Goldszmid, R.S.; Collazo, C.M.; Wilson, M.; Wynn, T.A.; Kamanaka, M.; Flavell, R.A.; Sher, A. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 2007, 204, 273–283. [Google Scholar] [CrossRef]
- Zheng, Y.; Chaudhry, A.; Kas, A.; deRoos, P.; Kim, J.M.; Chu, T.T.; Corcoran, L.; Treuting, P.; Klein, U.; Rudensky, A.Y. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control TH2 responses. Nature 2009, 458, 351–356. [Google Scholar] [CrossRef]
- Sakaguchi, S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005, 6, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, J.; Sun, Z.; Pagliano, O.; Guillaume, P.; Luescher, I.F.; Sander, C.; Kirkwood, J.M.; Olive, D.; Kuchroo, V.; Zarour, H.M. CD8+ T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012, 72, 887–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, L.S.; Sansom, D.M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 2011, 11, 852–863. [Google Scholar] [CrossRef]
- Wherry, E.J.; Blattman, J.N.; Murali-Krishna, K.; van der Most, R.; Ahmed, R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003, 77, 4911–4927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sica, A.; Larghi, P.; Mancino, A.; Rubino, L.; Porta, C.; Totaro, M.G.; Rimoldi, M.; Biswas, S.K.; Allavena, P.; Mantovani, A. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008, 18, 349–355. [Google Scholar] [CrossRef]
- Tan, B.; Shi, X.; Zhang, J.; Qin, J.; Zhang, N.; Ren, H.; Qian, M.; Siwko, S.; Carmon, K.; Liu, Q.; et al. Inhibition of Rspo-Lgr4 Facilitates Checkpoint Blockade Therapy by Switching Macrophage Polarization. Cancer Res. 2018, 78, 4929–4942. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.C.; Sun, X.; Ma, Q.; Fu, G.F.; Cong, L.L.; Zhang, H.; Fan, D.F.; Feng, J.; Lu, S.Y.; Liu, J.L.; et al. Metformin’s antitumour and anti-angiogenic activities are mediated by skewing macrophage polarization. J. Cell. Mol. Med. 2018, 22, 3825–3836. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Saccani, A.; Bottazzi, B.; Polentarutti, N.; Vecchi, A.; van Damme, J.; Mantovani, A. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J. Immunol. 2000, 164, 762–767. [Google Scholar] [CrossRef]
- Biswas, S.K.; Gangi, L.; Paul, S.; Schioppa, T.; Saccani, A.; Sironi, M.; Bottazzi, B.; Doni, A.; Vincenzo, B.; Pasqualini, F.; et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood 2006, 107, 2112–2122. [Google Scholar] [CrossRef] [Green Version]
- Ubil, E.; Caskey, L.; Holtzhausen, A.; Hunter, D.; Story, C.; Earp, H.S. Tumor-secreted Pros1 inhibits macrophage M1 polarization to reduce antitumor immune response. J. Clin. Investig. 2018, 128, 2356–2369. [Google Scholar] [CrossRef] [Green Version]
- Genard, G.; Wera, A.C.; Huart, C.; Le Calve, B.; Penninckx, S.; Fattaccioli, A.; Tabarrant, T.; Demazy, C.; Ninane, N.; Heuskin, A.C.; et al. Proton irradiation orchestrates macrophage reprogramming through NFkappaB signaling. Cell Death Dis. 2018, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef]
- Bally, A.P.; Lu, P.; Tang, Y.; Austin, J.W.; Scharer, C.D.; Ahmed, R.; Boss, J.M. NF-kappaB regulates PD-1 expression in macrophages. J. Immunol. 2015, 194, 4545–4554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, D.; Zhu, J.; Yu, W.; Hong, P.; Fan, Y.; Zhang, Z.; Li, J.; He, Q.; Han, W.; Shen, C.; et al. Expression of programmed cell death-ligand 1 in primary testicular diffuse large B cell lymphoma: A retrospective study. Oncol. Lett. 2019, 18, 2670–2676. [Google Scholar] [CrossRef] [Green Version]
- Riihijarvi, S.; Fiskvik, I.; Taskinen, M.; Vajavaara, H.; Tikkala, M.; Yri, O.; Karjalainen-Lindsberg, M.L.; Delabie, J.; Smeland, E.; Holte, H.; et al. Prognostic influence of macrophages in patients with diffuse large B-cell lymphoma: A correlative study from a Nordic phase II trial. Haematologica 2015, 100, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.J.; Go, H.; Paik, J.H.; Kim, T.M.; Heo, D.S.; Kim, C.W.; Jeon, Y.K. An increase of M2 macrophages predicts poor prognosis in patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk. Lymphoma 2014, 55, 2466–2476. [Google Scholar] [CrossRef]
- Lewis, C.E.; Pollard, J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Chong, L.C.; Twa, D.D.; Mottok, A.; Ben-Neriah, S.; Woolcock, B.W.; Zhao, Y.; Savage, K.J.; Marra, M.A.; Scott, D.W.; Gascoyne, R.D.; et al. Comprehensive characterization of programmed death ligand structural rearrangements in B-cell non-Hodgkin lymphomas. Blood 2016, 128, 1206–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abes, R.; Gelize, E.; Fridman, W.H.; Teillaud, J.L. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood 2010, 116, 926–934. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.P.; Lindorfer, M.A. Drug insight: The mechanism of action of rituximab in autoimmune disease—The immune complex decoy hypothesis. Nat. Clin. Pract. Rheumatol. 2007, 3, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Glennie, M.J.; French, R.R.; Cragg, M.S.; Taylor, R.P. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol. Immunol. 2007, 44, 3823–3837. [Google Scholar] [CrossRef]
- Di Gaetano, N.; Cittera, E.; Nota, R.; Vecchi, A.; Grieco, V.; Scanziani, E.; Botto, M.; Introna, M.; Golay, J. Complement activation determines the therapeutic activity of rituximab in vivo. J. Immunol. 2003, 171, 1581–1587. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Ilizaliturri, F.J.; Jupudy, V.; Ostberg, J.; Oflazoglu, E.; Huberman, A.; Repasky, E.; Czuczman, M.S. Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin’s lymphoma severe combined immunodeficiency mouse model. Clin. Cancer Res. 2003, 9, 5866–5873. [Google Scholar] [PubMed]
- Uchida, J.; Hamaguchi, Y.; Oliver, J.A.; Ravetch, J.V.; Poe, J.C.; Haas, K.M.; Tedder, T.F. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J. Exp. Med. 2004, 199, 1659–1669. [Google Scholar] [CrossRef]
- Leidi, M.; Gotti, E.; Bologna, L.; Miranda, E.; Rimoldi, M.; Sica, A.; Roncalli, M.; Palumbo, G.A.; Introna, M.; Golay, J. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. J. Immunol. 2009, 182, 4415–4422. [Google Scholar] [CrossRef] [Green Version]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Myklebust, J.H.; Varghese, B.; Gill, S.; Jan, M.; Cha, A.C.; Chan, C.K.; Tan, B.T.; et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 2010, 142, 699–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Hilchey, S.P.; Hyrien, O.; Mosmann, T.R.; Livingstone, A.M.; Friedberg, J.W.; Young, F.; Fisher, R.I.; Kelleher, R.J., Jr.; Bankert, R.B.; Bernstein, S.H. Rituximab immunotherapy results in the induction of a lymphoma idiotype-specific T-cell response in patients with follicular lymphoma: Support for a "vaccinal effect" of rituximab. Blood 2009, 113, 3809–3812. [Google Scholar] [CrossRef] [PubMed]
- Melssen, M.; Slingluff, C.L., Jr. Vaccines targeting helper T cells for cancer immunotherapy. Curr. Opin. Immunol. 2017, 47, 85–92. [Google Scholar] [CrossRef]
- Pardoll, D. Cancer and the Immune System: Basic Concepts and Targets for Intervention. Semin. Oncol. 2015, 42, 523–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strati, P.; Neelapu, S.S. Chimeric Antigen Receptor-Engineered T Cell Therapy in Lymphoma. Curr. Oncol. Rep. 2019, 21, 38. [Google Scholar] [CrossRef]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Park, J.J.; Omiya, R.; Matsumura, Y.; Sakoda, Y.; Kuramasu, A.; Augustine, M.M.; Yao, S.; Tsushima, F.; Narazaki, H.; Anand, S.; et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 2010, 116, 1291–1298. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, S.; Zhu, B.; Bedoret, D.; Bu, X.; Francisco, L.M.; Hua, P.; Duke-Cohan, J.S.; Umetsu, D.T.; Sharpe, A.H.; et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J. Exp. Med. 2014, 211, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Mittman, S.; Rodriguez, R.; Pacheco-Sanchez, P.; Moskalenko, M.; Yang, Y.; Elstrott, J.; Ritter, A.T.; Muller, S.; Nickles, D.; et al. Coexpression of Inhibitory Receptors Enriches for Activated and Functional CD8+ T Cells in Murine Syngeneic Tumor Models. Cancer Immunol. Res. 2019, 7, 963–976. [Google Scholar] [CrossRef]
- Kamada, T.; Togashi, Y.; Tay, C.; Ha, D.; Sasaki, A.; Nakamura, Y.; Sato, E.; Fukuoka, S.; Tada, Y.; Tanaka, A.; et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 9999–10008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Autio, M.; Leivonen, S.K.; Bruck, O.; Mustjoki, S.; Meszaros Jorgensen, J.; Karjalainen-Lindsberg, M.L.; Beiske, K.; Holte, H.; Pellinen, T.; Leppa, S. Immune cell constitution in the tumor microenvironment predicts the outcome in diffuse large B-cell lymphoma. Haematologica 2021, 106, 718–729. [Google Scholar] [CrossRef] [Green Version]
- Xu-Monette, Z.Y.; Xiao, M.; Au, Q.; Padmanabhan, R.; Xu, B.; Hoe, N.; Rodriguez-Perales, S.; Torres-Ruiz, R.; Manyam, G.C.; Visco, C.; et al. Immune Profiling and Quantitative Analysis Decipher the Clinical Role of Immune-Checkpoint Expression in the Tumor Immune Microenvironment of DLBCL. Cancer Immunol. Res. 2019, 7, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Minnema, M.C.; Johnson, P.; Timmerman, J.M.; Armand, P.; Shipp, M.A.; Rodig, S.J.; Ligon, A.H.; Roemer, M.G.M.; Reddy, N.; et al. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J. Clin. Oncol. 2019, 37, 481–489. [Google Scholar] [CrossRef] [PubMed]

| Stage | Description |
|---|---|
| I | Involvement of a single lymphatic region (I) or localized involvement of single extranodal organ or site (testis mono or bilateral) (IE) |
| II | Involvement of two or more lymphatic regions on the same side of the diaphragm (II) or localized involvement of a single extranodal organ or site (testis mono or bilateral) and of one or more lymphatic regions on the same side of the diaphragm (locoregional lymph nodes; iliac and/or lomboaortic) (IIE) |
| III | Involvement of lymphatic regions on both sides of the diaphragm |
| IV | Diffuse or disseminated involvement of one or more extranodal organs with or without lymphatic involvement |
| Risk Group | IPI Score | 3-Year OS, (95% CI), % |
|---|---|---|
| Low | 0 or 1 | 91 (89–94) |
| Intermediate low | 2 | 81 (76–86) |
| Intermediate high | 3 | 65 (58–73) |
| High | 4 or 5 | 59 (49–69) |
| Risk Group | aaIPI Score | 3-Year OS, (95% CI), % |
|---|---|---|
| Low | 0 | 98 (96–100) |
| Intermediate low | 1 | 92 (87–95) |
| Intermediate high | 2 | 75 (66–82) |
| High | 3 |
| Gene/Chromosome | Aberration | Occurrence in T-DLBCL | Occurrence in DLBCL | Occurrence in Non-GCB-DLBCL | Ref. |
|---|---|---|---|---|---|
| NFKBIZ | Copy number gain | 42% | 9% | 20% | [45] |
| MYD88 | Amplifications, mutations, and deletions | 60–82% | 18–27% | 29% | [8,45,91,92,101] |
| CD79b | Mutations and deletions | 19–34% | 14–15% | 23% | [91,92,101,102] |
| CDKN2A | Copy number alterations | 71% | 24% | 35% | [45] |
| 9p24.1 | Translocation and copy number alterations | 54% | <10% | <10% | [45] |
| CD274 | Rearrangements, copy number alterations, and increased protein expression | 35% | 27% | 45% | [45,89,103] |
| PDCD1LG2 | Rearrangements, copy number alterations, and increased protein expression | 47% | <5% | ND 1 | [45,89,104] |
| pSTAT1/pSTAT3 | Expression | 82% | ND | ND | [12] |
| BCL2/MYC | Rearrangements | 10–15% | 10–30% | ~35% | [44,101,105] |
| BCL6 | Rearrangements and deregulation | 16–48% | 35% | 19–60% | [12,45,86,91,106,107] |
| CIITA | Rearrangements | 10% | 3–6% | ND | [89,101] |
| FOXP1 | Rearrangements and increased protein expression | 7–78% | 8–10% | 15–30% | [9,89,106,108] |
| HLA region/genes | Mutations, deletions, and loss of expression | 61–77% | 4–22% | ND | [23,46,47,90,94,95,101] |
| IgH | V(D)J rearrangement and SHMs | 43% | <80% | ND | [9,109] |
| CD37 | Mutations | 26% | ND | ND | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollari, M.; Leivonen, S.-K.; Leppä, S. Testicular Diffuse Large B-Cell Lymphoma—Clinical, Molecular, and Immunological Features. Cancers 2021, 13, 4049. https://doi.org/10.3390/cancers13164049
Pollari M, Leivonen S-K, Leppä S. Testicular Diffuse Large B-Cell Lymphoma—Clinical, Molecular, and Immunological Features. Cancers. 2021; 13(16):4049. https://doi.org/10.3390/cancers13164049
Chicago/Turabian StylePollari, Marjukka, Suvi-Katri Leivonen, and Sirpa Leppä. 2021. "Testicular Diffuse Large B-Cell Lymphoma—Clinical, Molecular, and Immunological Features" Cancers 13, no. 16: 4049. https://doi.org/10.3390/cancers13164049
APA StylePollari, M., Leivonen, S.-K., & Leppä, S. (2021). Testicular Diffuse Large B-Cell Lymphoma—Clinical, Molecular, and Immunological Features. Cancers, 13(16), 4049. https://doi.org/10.3390/cancers13164049






