Clinical Impact of FDG-PET/CT Compared with CE-CT in Response Monitoring of Metastatic Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
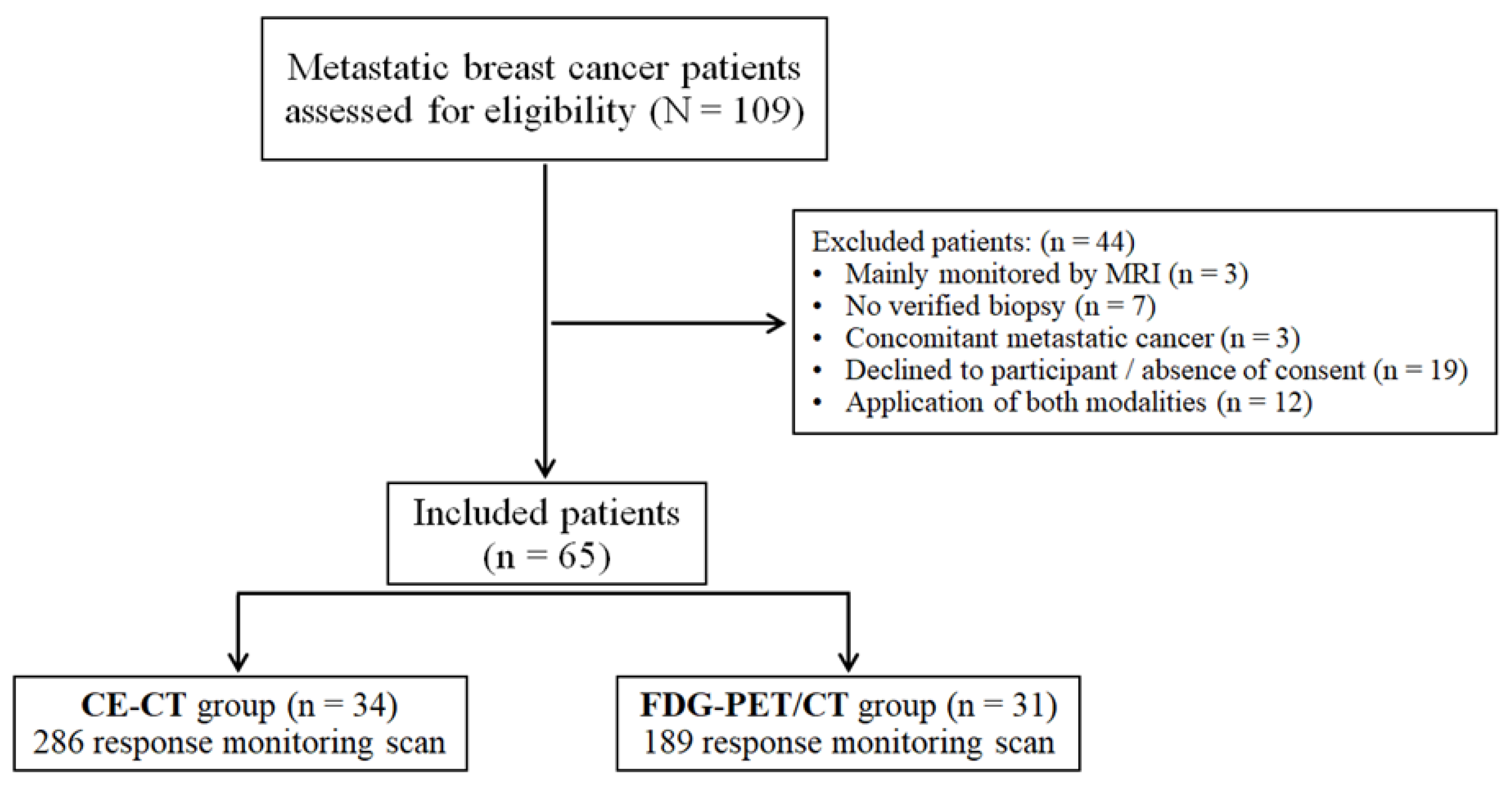
2.1. Study Design and Subjects
2.2. Imaging Techniques
2.2.1. FDG-PET/CT
2.2.2. CE-CT
2.3. Data Collection and Variables
2.4. Outcome Measures and Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Treatment Protocol and Metastatic Site
3.3. Response Monitoring Scans
3.4. Response Categories, Clinical Impact, and Positive Predictive Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Carioli, G.; Malvezzi, M.; Rodriguez, T.; Bertuccio, P.; Negri, E.; La Vecchia, C. Trends and predictions to 2020 in breast cancer mortality in Europe. Breast 2017, 36, 89–95. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Cochet, A.; David, S.; Moodie, K.; Drummond, E.; Dutu, G.; MacManus, M.; Chua, B.; Hicks, R.J. The utility of 18 F-FDG PET/CT for suspected recurrent breast cancer: Impact and prognostic stratification. Cancer Imaging 2014, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, M.G.; Lauridsen, J.F.; Vogsen, M.; Holm, J.; Vilstrup, M.H.; Braad, P.-E.; Gerke, O.; Thomassen, M.; Ewertz, M.; Høilund-Carlsen, P.F.; et al. FDG-PET/CT for Response monitoring in metastatic breast cancer: Today, tomorrow, and beyond. Cancers 2019, 11, 1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Semiglazov, V. RECIST for Response (Clinical and Imaging) in neoadjuvant clinical trials in operable breast cancer. J. Natl. Cancer Inst. Monogr. 2015, 2015, 21–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; André, F.; Harbeck, N.; Lopez, B.A.; Barrios, C.; Bergh, J.; et al. 4th ESO–ESMO International consensus guidelines for advanced breast cancer (ABC 4). Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; Van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Van Uden, D.J.P.; van Maaren, M.; Strobbe, L.J.A.; Bult, P.; Van Der Hoeven, J.J.; Siesling, S.; De Wilt, J.H.W.; Blanken-Peeters, C.F.J.M. Metastatic behavior and overall survival according to breast cancer subtypes in stage IV inflammatory breast cancer. Breast Cancer Res. 2019, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, R.; Zeng, F.; Zhao, J.; Peng, S.; Ma, Y.; Chen, S.; Ding, S.; Zhong, L.; Guo, W.; et al. Impact of molecular subtypes on metastatic behavior and overall survival in patients with metastatic breast cancer: A single-center study combined with a large cohort study based on the Surveillance, Epidemiology and End Results database. Oncol. Lett. 2020, 20, 1. [Google Scholar] [CrossRef]
- Yang, H.-L.; Liu, T.; Wang, X.-M.; Xu, Y.; Deng, S.-M. Diagnosis of bone metastases: A meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur. Radiol. 2011, 21, 2604–2617. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.G.; Gerke, O.; Baun, C.; Falch, K.; Hansen, J.A.; Farahani, Z.A.; Petersen, H.; Larsen, L.B.; Duvnjak, S.; Buskevica, I.; et al. [18F] fluorodeoxyglucose (FDG)-positron emission tomography (PET)/Computed Tomography (CT) in Suspected Recurrent Breast Cancer: A prospective comparative study of dual-time-point FDG-PET/CT, contrast-enhanced CT, and bone scintigraphy. J. Clin. Oncol. 2016, 34, 1889–1897. [Google Scholar] [CrossRef]
- Bretschi, M.; Fränzle, A.; Merz, M.; Hillengass, J.; Semmler, W.; Bendl, R.; Bäuerle, T. Assessing treatment response of osteolytic lesions by manual volumetry, automatic segmentation, and recist in experimental bone metastases. Acad. Radiol. 2014, 21, 1177–1184. [Google Scholar] [CrossRef]
- Sun, Z.; Yi, Y.L.; Liu, Y.; Xiong, J.P.; He, C.Z. Comparison of whole-body PET/PET-CT and conventional imaging procedures for distant metastasis staging in patients with breast cancer: A meta-analysis. Eur. J. Gynaecol. Oncol. 2015, 36, 672–676. [Google Scholar]
- Riedl, C.C.; Pinker-Domenig, K.; Ulaner, G.A.; Ong, L.T.; Baltzer, P.; Jochelson, M.S.; McArthur, H.L.; Gönen, M.; Dickler, M.; Weber, W.A. Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1428–1437. [Google Scholar] [CrossRef]
- Joo Hyun, O.; Lodge, M.A.; Wahl, R.L. Practical PERCIST: A simplified guide to pet response criteria in solid tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef] [Green Version]
- Paydary, K.; Seraj, S.M.; Zadeh, M.Z.; Emamzadehfard, S.; Shamchi, S.P.; Gholami, S.; Werner, T.J.; Alavi, A. The Evolving Role of FDG-PET/CT in the diagnosis, staging, and treatment of breast cancer. Mol. Imaging Biol. 2019, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.J.; Shupe, M.P.; Schneble, E.J.; Flynt, F.L.; Clemenshaw, M.N.; Kirkpatrick, A.D.; Gallagher, C.; Nissan, A.; Henry, L.; Stojadinovic, A.; et al. Current approaches and challenges in monitoring treatment responses in breast cancer. J. Cancer 2014, 5, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Sok, M.; Zavrl, M.; Greif, B.; Srpčič, M. Objective assessment of WHO/ECOG performance status. Support. Care Cancer 2019, 27, 3793–3798. [Google Scholar] [CrossRef] [PubMed]
- Groheux, D.; Hindie, E. Breast cancer: Initial workup and staging with FDG PET/CT. Clin. Transl. Imaging 2021, 9, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi-Jehanno, N.; Giraudet, A.-L.; Champion, L.; Lerebours, F.; Le Stanc, E.; Edeline, V.; Madar, O.; Bellet, D.; Pecking, A.P.; Alberini, J.-L. Assessment of response to endocrine therapy using FDG PET/CT in metastatic breast cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 450–460. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, H.; Liu, J.; Xu, Y.; Chen, B.; Yang, Y.; Yan, N.; Song, S.; Lin, Y.; Xu, Y. 18F-FDG PET/CT for the early prediction of the response rate and survival of patients with recurrent or metastatic breast cancer. Oncol. Lett. 2018, 16, 4151–4158. [Google Scholar] [CrossRef] [Green Version]
- Helland, F.; Henriksen, M.H.; Gerke, O.; Vogsen, M.; Høilund-Carlsen, P.F.; Hildebrandt, M.G. FDG-PET/CT Versus contrast-enhanced ct for response evaluation in metastatic breast cancer: A systematic review. Diagnostics 2019, 9, 106. [Google Scholar] [CrossRef] [Green Version]
- Chua, S.C.; Groves, A.M.; Kayani, I.; Menezes, L.; Gacinovic, S.; Du, Y.; Bomanji, J.B.; Ell, P.J. The impact of 18F-FDG PET/CT in patients with liver metastases. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1906–1914. [Google Scholar] [CrossRef]
- Avril, S.; Muzic, R.F.; Plecha, D.; Traughber, B.J.; Vinayak, S.; Avril, N. 18F-FDG PET/CT for monitoring of treatment response in breast cancer. J. Nucl. Med. 2016, 57, 34S–39S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogsen, M.; Bülow, J.; Ljungstrøm, L.; Oltmann, H.; Alamdari, T.; Naghavi-Behzad, M.; Braad, P.-E.; Gerke, O.; Hildebrandt, M. FDG-PET/CT for response monitoring in metastatic breast cancer: The feasibility and benefits of applying percist. Diagnostics 2021, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, J.S.; Vilstrup, M.H.; Holm, J.; Vogsen, M.; Bülow, J.L.; Ljungstrøm, L.; Braad, P.-E.; Gerke, O.; Hildebrandt, M.G. Interrater agreement and reliability of PERCIST and visual assessment when using 18F-FDG-PET/CT for response monitoring of metastatic breast cancer. Diagnostics 2020, 10, 1001. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; Clark, E. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swain, S.M.; Miles, D.; Kim, S.-B.; Im, Y.-H.; Im, S.-A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Ulaner, G.A. PET/CT for Patients With Breast Cancer: Where Is the Clinical Impact? Am. J. Roentgenol. 2019, 213, 254–265. [Google Scholar] [CrossRef]
- Hironaka-Mitsuhashi, A.; Calle, A.S.; Ochiya, T.; Takayama, S.; Suto, A. Towards circulating-tumor DNA-based precision medicine. J. Clin. Med. 2019, 8, 1365. [Google Scholar] [CrossRef] [Green Version]
- Wood-Bouwens, C.M.; Haslem, D.; Moulton, B.; Almeda, A.F.; Lee, H.; Heestand, G.M.; Nadauld, L.D.; Ji, H.P. therapeutic monitoring of circulating dna mutations in metastatic cancer with personalized digital PCR. J. Mol. Diagn. 2020, 22, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Dashevsky, B.Z.; Goldman, D.A.; Parsons, M.; Gonen, M.; Corben, A.D.; Jochelson, M.S.; Hudis, C.A.; Morrow, M.; Ulaner, G.A. Appearance of untreated bone metastases from breast cancer on FDG PET/CT: Importance of histologic subtype. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1666–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, M.; Goldman, D.A.; Dashevsky, B.; Riedl, C.; Gönen, M.; Osborne, J.; Jochelson, M.; Hudis, C.; Morrow, M.; Ulaner, G.A. Comparison of 18F-FDG PET/CT for Systemic staging of newly diagnosed invasive lobular carcinoma versus invasive ductal carcinoma. J. Nucl. Med. 2015, 56, 1674–1680. [Google Scholar] [CrossRef] [Green Version]
- Jambor, I.; Kuisma, A.; Ramadan, S.; Huovinen, R.; Sandell, M.; Kajander, S.; Kemppainen, J.; Kauppila, E.; Auren, J.; Merisaari, H.; et al. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 1.5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol. 2015, 55, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Kosmin, M.; Padhani, A.R.; Gogbashian, A.; Woolf, D.; Ah-See, M.-L.; Ostler, P.; Sutherland, S.; Miles, D.; Noble, J.; Koh, D.-M.; et al. Comparison of whole-body MRI, CT, and bone scintigraphy for response evaluation of cancer therapeutics in metastatic breast cancer to bone. Radiology 2020, 297, 622–629. [Google Scholar] [CrossRef]

| Characteristics a | Study Groups | P-Value | ||
|---|---|---|---|---|
| CE-CT | PET/CT | |||
| Primary tumor size (mm) | 18 (3–65) | 20 (1–80) | 0.85 | |
| Bilateral cancer | 2 (5.9) | 5 (16.1) | 0.24 | |
| Histopathology | Ductal | 26 (76.5) | 23 (74.2) | 0.59 |
| Lobular | 5 (14.7) | 3 (9.7) | ||
| Adenocarcinoma | 3 (8.8) | 3 (9.7) | ||
| Unknown | 0 (0) | 2 (6.5) | ||
| Primary surgery (lumpectomy/mastectomy) | 23 (67.6) | 27 (87.1) | 0.06 | |
| Estrogen receptor status | Positive | 31 (91.2) | 25 (80.7) | 0.43 |
| Negative | 2 (5.9) | 3 (9.7) | ||
| Unknown | 1 (2.9) | 3 (9.7) | ||
| HER-2 status | Positive | 5 (14.7) | 6 (19.4) | 0.94 |
| Negative | 21 (61.8) | 18 (58.1) | ||
| Unknown | 8 (23.5) | 7 (22.6) | ||
| Tumor grade | Grade 1 | 6 (17.7) | 6 (19.4) | 0.98 |
| Grade 2 | 14 (41.2) | 11 (35.5) | ||
| Grade 3 | 7 (20.6) | 8 (25.8) | ||
| Unknown | 7 (20.6) | 6 (19.4) | ||
| Ki-67 proliferation (%) | 20 (1–80) | 50 (10–95) | 0.16 | |
| Lymph node involvement | None | 7 (20.6) | 6 (19.4) | 0.71 |
| Micro-metastasis | 4 (11.8) | 3 (9.7) | ||
| Macro-metastasis | 13 (38.2) | 16 (51.6) | ||
| Unknown | 10 (29.4) | 6 (19.4) | ||
| Treatment protocol | Neo-adjuvant treatment | 2 (5.88) | 8 (25.8) | 0.09 |
| Endocrine treatment | 23 (67.7) | 17 (54.8) | ||
| No treatment / unknown | 9 (26.5) | 6 (19.4) | ||
| Radiotherapy (breast/breast + axilla) | 20 (58.8) | 21 (67.7) | 0.31 | |
| Characteristics | Study Groups | P-Value | ||
|---|---|---|---|---|
| CE-CT | PET/CT | |||
| Year of diagnosis | 2015 (2010–2017) | 2016 (2009–2017) | 0.02 | |
| Age at diagnosis (year) | 67.0 (31.0–84.5) | 63.4 (32.9–85.8) | 0.69 | |
| Performance status | 0 | 12 (35.3) | 13 (41.9) | 0.36 |
| 1 | 10 (29.4) | 13 (41.9) | ||
| ≥2 | 3 (8.8) | 1 (3.2) | ||
| Unknown | 9 (26.5) | 4 (12.9) | ||
| Time until relapse b (months) | 78.0 (0–271.4) | 81.8 (0–307.5) | 0.71 | |
| Histopathology | Ductal | 4 (11.8) | 5 (16.1) | 0.86 |
| Lobular | 4 (11.8) | 2 (6.5) | ||
| Adenocarcinoma | 18 (52.9) | 18 (58.1) | ||
| Unknown | 8 (23.5) | 6 (19.4) | ||
| De novo metastatic cancer | 8 (25.5) | 4 (12.9) | 0.35 | |
| Estrogen receptor status | Positive | 30 (88.2) | 28 (90.3) | 0.50 |
| Negative | 2 (5.9) | 3 (9.7) | ||
| Unknown | 2 (5.9) | 0 (0) | ||
| HER-2 status | Positive | 5 (14.7) | 6 (19.4) | 0.46 |
| Negative | 22 (64.7) | 22 (71.0) | ||
| Unknown | 7 (20.6) | 3 (9.7) | ||
| Origin of biopsy | Bone | 5 (14.7) | 13 (41.9) | 0.07 |
| Liver | 6 (17.7) | 3 (9.7) | ||
| Lung/Pleural fluid | 8 (23.5) | 7 (22.6) | ||
| Breast/lymph nodes | 15 (44.1) | 8 (25.7) | ||
| Region of metastases at baseline scan | Bone-only metastasis | 4 (11.8) | 4 (12.9) | 0.89 |
| Bone | 22 (64.7) | 21 (67.7) | 0.80 | |
| Liver | 8 (25.5) | 8 (25.8) | 0.83 | |
| Lung | 10 (29.4) | 11 (35.5) | 0.60 | |
| Regional lymph nodes | 12 (35.3) | 9 (29.0) | 0.59 | |
| Distant lymph nodes | 18 (52.9) | 17 (54.8) | 0.88 | |
| Pleura/pleural effusion | 3 (8.8) | 6 (19.4) | 0.22 | |
| Breast/local recurrence | 7 (20.6) | 6 (19.4) | 0.90 | |
| Soft tissue | 1 (2.9) | 4 (12.9) | 0.13 | |
| Others c | 2 (5.9) | 3 (9.7) | 0.57 | |
| Characteristics | Study Groups | ||
|---|---|---|---|
| CE-CT (286 scan) | FDG-PET/CT (189 scan) | ||
| Received treatments during follow-up a | Endocrine therapy | 29 (85.3) | 25 (80.6) |
| Bone-targeted therapies | 24 (70.6) | 23 (74.2) | |
| Chemotherapy | 22 (64.7) | 18 (58.1) | |
| CDK4/6 inhibitors | 19 (55.9) | 15 (48.4) | |
| Anti-HER2 therapy | 5 (14.7) | 6 (19.4) | |
| Palliative radiotherapy | 5 (14.7) | 1 (3.2) | |
| Metastatic sites during follow-up b | Bone | 166 (58.0) | 90 (47.6) |
| Liver | 55 (19.2) | 15 (7.9) | |
| Lung/plural | 110 (38.5) | 66 (34.9) | |
| Regional/distant lymph nodes | 112 (39.2) | 67 (35.4) | |
| Others c | 48 (16.8) | 28 (14.8) | |
| Treatment Actions | Response Categories a | |||||||
|---|---|---|---|---|---|---|---|---|
| Study Groups | CR/CMR | PR/PMR | SD/SMD | PD/PMD | MR/MMR | EA/EMA | ||
| CE-CT | Scans followed by progression-induced treatment change b (n = 44) | 0 (0) | 1 (2.3) | 8 (18.2) | 34 (77.3) | 0 (0) | 1 (2.3) | |
| Total scans c (N = 286) | 11 (3.8) | 24 (8.4) | 202 (70.6) | 43 (15) | 2 (0.7) | 4 (1.4) | ||
| PET/CT | Scans followe by progression-induced treatment change b (n = 22) | 0 (0) | 0 (0) | 0 (0) | 21 (95.5) | 1 (4.5) | 0 (0) | |
| Total scans c (N = 189) | 42 (22.2) | 45 (23.8) | 59 (31.2) | 35 (18.5) | 4 (2.1) | 4 (2.1) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naghavi-Behzad, M.; Oltmann, H.R.; Alamdari, T.A.; Bülow, J.L.; Ljungstrøm, L.; Braad, P.-E.; Asmussen, J.T.; Vogsen, M.; Kodahl, A.R.; Gerke, O.; et al. Clinical Impact of FDG-PET/CT Compared with CE-CT in Response Monitoring of Metastatic Breast Cancer. Cancers 2021, 13, 4080. https://doi.org/10.3390/cancers13164080
Naghavi-Behzad M, Oltmann HR, Alamdari TA, Bülow JL, Ljungstrøm L, Braad P-E, Asmussen JT, Vogsen M, Kodahl AR, Gerke O, et al. Clinical Impact of FDG-PET/CT Compared with CE-CT in Response Monitoring of Metastatic Breast Cancer. Cancers. 2021; 13(16):4080. https://doi.org/10.3390/cancers13164080
Chicago/Turabian StyleNaghavi-Behzad, Mohammad, Hjalte Rasmus Oltmann, Tural Asgharzadeh Alamdari, Jakob Lykke Bülow, Lasse Ljungstrøm, Poul-Erik Braad, Jon Thor Asmussen, Marianne Vogsen, Annette Raskov Kodahl, Oke Gerke, and et al. 2021. "Clinical Impact of FDG-PET/CT Compared with CE-CT in Response Monitoring of Metastatic Breast Cancer" Cancers 13, no. 16: 4080. https://doi.org/10.3390/cancers13164080








