Clinicopathological and Prognostic Significance of Inhibitor of Apoptosis Protein (IAP) Family Members in Lung Cancer: A Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Selection Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. RNA-Seq Data
2.6. Statistical Analysis
3. Results
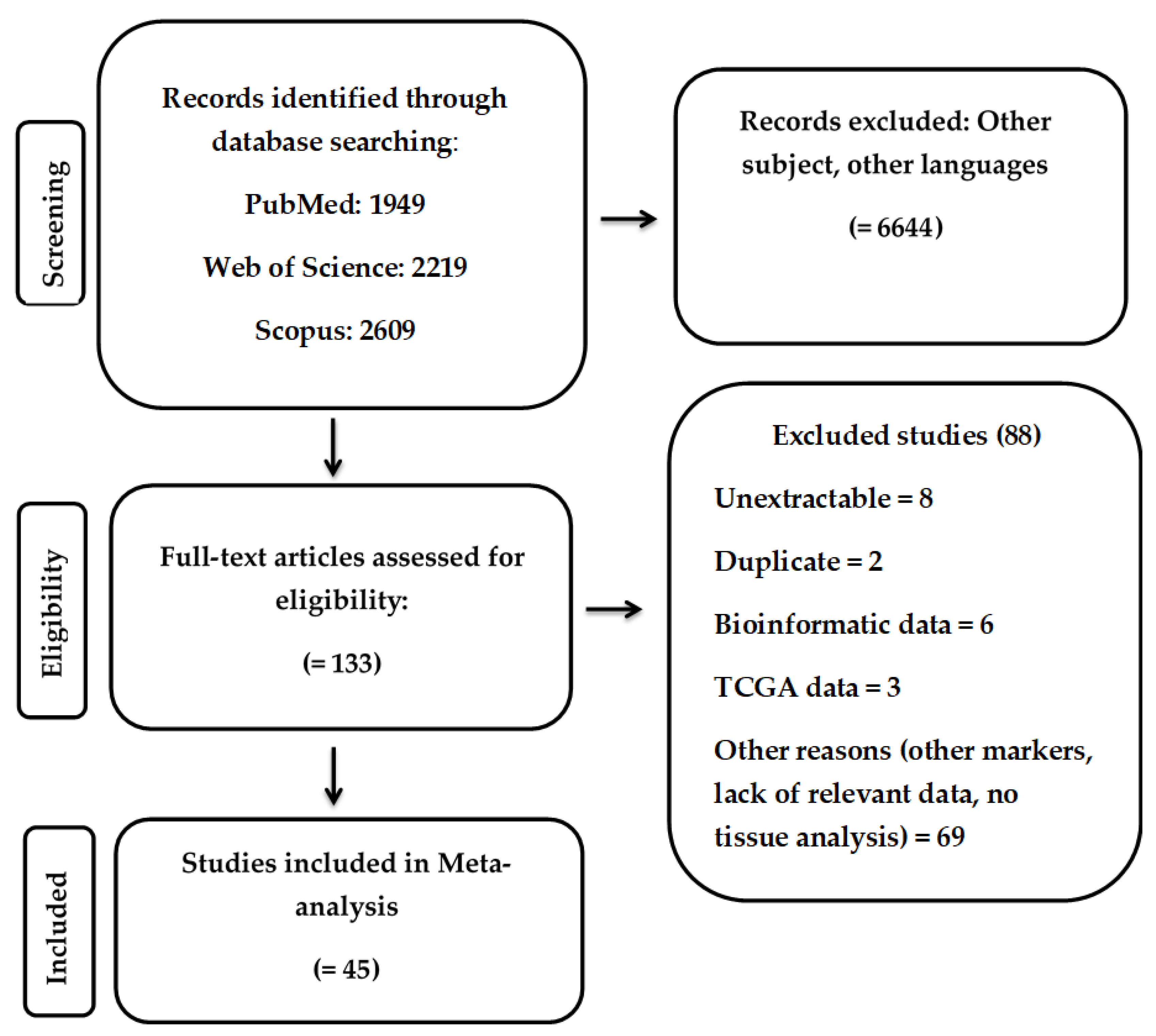
3.1. Study Selection and Characteristics
3.2. Study Quality
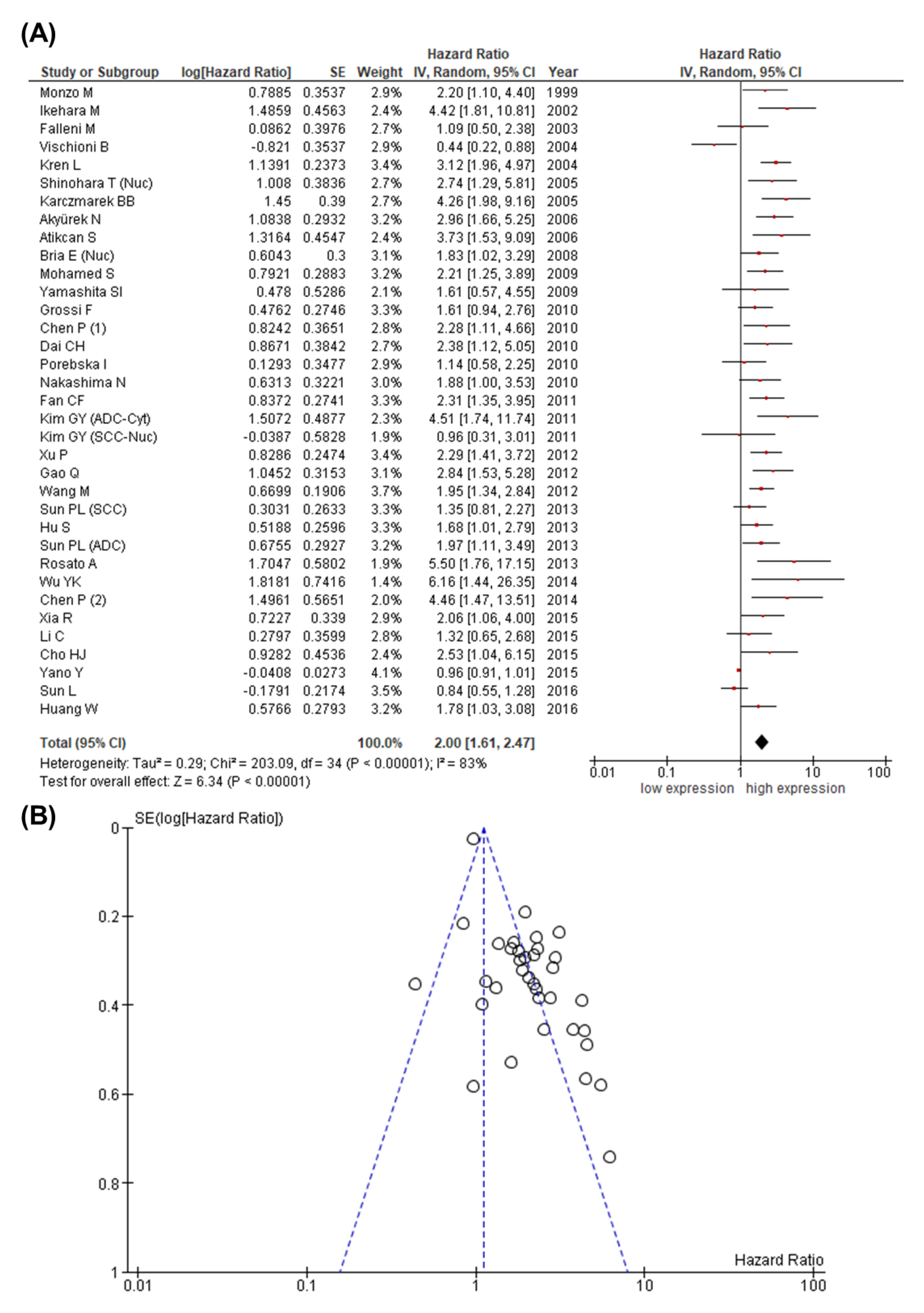
3.3. Study Results and Meta-Analysis
3.4. Validation Using the TCGA Dataset
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, A.G.; Chansky, K.; Crowley, J.; Beyruti, R.; Kubota, K.; Turrisi, A.; Eberhardt, W.E.; van Meerbeeck, J.; Rami-Porta, R. The international association for the study of lung cancer lung cancer staging project: Proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016, 11, 300–311. [Google Scholar] [CrossRef] [Green Version]
- Nagasaka, M.; Gadgeel, S.M. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev. Anticancer Ther. 2018, 18, 63–70. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef] [Green Version]
- Asamura, H.; Aokage, K.; Yotsukura, M. Wedge resection versus anatomic resection: Extent of surgical resection for stage I and II lung cancer. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 426–433. [Google Scholar] [CrossRef]
- Osmani, L.; Askin, F.; Gabrielson, E.; Li, Q.K. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin. Cancer Biol. 2018, 52, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Rusch, V.W.; Crowley, J.; Giroux, D.J.; Goldstraw, P.; Im, J.G.; Tsuboi, M.; Tsuchiya, R.; Vansteenkiste, J. The IASLC lung cancer staging project: Proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2007, 2, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Tsao, M.S.; Sakurada, A.; Cutz, J.C.; Zhu, C.Q.; Kamel-Reid, S.; Squire, J.; Lorimer, I.; Zhang, T.; Liu, N.; Daneshmand, M.; et al. Erlotinib in lung cancer—Molecular and clinical predictors of outcome. N. Engl. J. Med. 2005, 353, 133–144. [Google Scholar] [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Nilsson, M.; Goldman, J.; Reck, M.; Nakagawa, K.; Kato, T.; Ares, L.P.; Frimodt-Moller, B.; Wolff, K.; Visseren-Grul, C.; et al. Dual EGFR-VEGF pathway inhibition: A promising strategy for patients with EGFR-mutant NSCLC. J. Thorac. Oncol. 2021, 16, 205–215. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Jänne, P.A.; Yang, J.C.; Kim, D.W.; Planchard, D.; Ohe, Y.; Ramalingam, S.S.; Ahn, M.J.; Kim, S.W.; Su, W.C.; Horn, L.; et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 1689–1699. [Google Scholar] [CrossRef]
- Sharma, S.V.; Bell, D.W.; Settleman, J.; Haber, D.A. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 2007, 7, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Sequist, L.V.; Yang, J.C.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.M.; Boyer, M.; et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef] [Green Version]
- Mok, T.S.; Wu, Y.L.; Thongprasert, S.; Yang, C.H.; Chu, D.T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Buss, L.; Wayant, C.; Nobre, M.R.C. Comparison of FDA accelerated vs. regular pathway approvals for lung cancer treatments between 2006 and 2018. PLoS ONE 2020, 15, e0236345. [Google Scholar] [CrossRef]
- Lin, J.J.; Cardarella, S.; Lydon, C.A.; Dahlberg, S.E.; Jackman, D.M.; Jänne, P.A.; Johnson, B.E. Five-year survival in EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J. Thorac. Oncol. 2016, 11, 556–565. [Google Scholar] [CrossRef] [Green Version]
- Facchinetti, F.; Marabelle, A.; Rossi, G.; Soria, J.C.; Besse, B.; Tiseo, M. Moving immune checkpoint blockade in thoracic tumors beyond NSCLC. J. Thorac. Oncol. 2016, 11, 1819–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [Green Version]
- Sławiński, G.; Wrona, A.; Dąbrowska-Kugacka, A.; Raczak, G.; Lewicka, E. Immune checkpoint inhibitors and cardiac toxicity in patients treated for non-small lung cancer: A review. Int. J. Mol. Sci. 2020, 21, 7195. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, K.A.; Fitz, L.J.; Lee, J.M.; Benander, C.; George, J.A.; Wooters, J.; Qiu, Y.; Jussif, J.M.; Carter, L.L.; Wood, C.R.; et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004, 574, 37–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Pawelczyk, K.; Piotrowska, A.; Ciesielska, U.; Jablonska, K.; Gletzel-Plucinska, N.; Grzegrzolka, J.; Podhorska-Okolow, M.; Dziegiel, P.; Nowinska, K. Role of PD-L1 expression in non-small cell lung cancer and their prognostic significance according to clinicopathological factors and diagnostic markers. Int. J. Mol. Sci. 2019, 20, 824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of indications of FDA—Approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [Green Version]
- Melosky, B.; Cheema, P.K.; Brade, A.; McLeod, D.; Liu, G.; Price, P.W.; Jao, K.; Schellenberg, D.D.; Juergens, R.; Leighl, N.; et al. Prolonging survival: The role of immune checkpoint inhibitors in the treatment of extensive-stage small cell lung cancer. Oncologist 2020, 25, 981–992. [Google Scholar] [CrossRef]
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 study. J. Clin. Oncol. 2019, 37, 2518–2527. [Google Scholar] [CrossRef]
- Brueckl, W.M.; Ficker, J.H.; Zeitler, G. Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC). BMC Cancer 2020, 20, 1185. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosato, A.; Menin, C.; Boldrin, D.; Dalla Santa, S.; Bonaldi, L.; Scaini, M.C.; Del Bianco, P.; Zardo, D.; Fassan, M.; Cappellesso, R.; et al. Survivin expression impacts prognostically on NSCLC but not SCLC. Lung Cancer 2013, 79, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ikeguchi, M.; Ueda, T.; Sakatani, T.; Hirooka, Y.; Kaibara, N. Expression of survivin messenger RNA correlates with poor prognosis in patients with hepatocellular carcinoma. Diagn. Mol. Pathol. 2002, 11, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.D.; Altieri, D.C.; Tanigawa, N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res. 1998, 58, 1808–1812. [Google Scholar]
- Sarela, A.I.; Macadam, R.C.; Farmery, S.M.; Markham, A.F.; Guillou, P.J. Expression of the antiapoptosis gene, survivin, predicts death from recurrent colorectal carcinoma. Gut 2000, 46, 645–650. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Li, J.; Ge, L.P.; Dai, C.H.; Li, X.Q. Prognostic value of survivin, X-linked inhibitor of apoptosis protein and second mitochondria-derived activator of caspases expression in advanced non-small-cell lung cancer patients. Respirology 2010, 15, 501–509. [Google Scholar] [CrossRef]
- Rathore, R.; McCallum, J.E.; Varghese, E.; Florea, A.M.; Büsselberg, D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis 2017, 22, 898–919. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Park, Y.C.; Rich, R.L.; Segal, D.; Myszka, D.G.; Wu, H. Structural basis of caspase inhibition by XIAP: Differential roles of the linker versus the BIR domain. Cell 2001, 104, 781–790. [Google Scholar] [CrossRef]
- Garg, H.; Suri, P.; Gupta, J.C.; Talwar, G.P.; Dubey, S. Survivin: A unique target for tumor therapy. Cancer Cell Int. 2016, 16, 49. [Google Scholar] [CrossRef] [Green Version]
- Shinohara, E.T.; Gonzalez, A.; Massion, P.P.; Chen, H.; Li, M.; Freyer, A.S.; Olson, S.J.; Andersen, J.J.; Shyr, Y.; Carbone, D.P.; et al. Nuclear survivin predicts recurrence and poor survival in patients with resected nonsmall cell lung carcinoma. Cancer 2005, 103, 1685–1692. [Google Scholar] [CrossRef]
- Bria, E.; Visca, P.; Novelli, F.; Casini, B.; Diodoro, M.G.; Perrone-Donnorso, R.; Botti, C.; Sperduti, I.; Facciolo, F.; Milella, M.; et al. Nuclear and cytoplasmic cellular distribution of survivin as survival predictor in resected non-small-cell lung cancer. Eur. J. Surg. Oncol. 2008, 34, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Qu, Y.; Xu, X.; Xu, Q.; Geng, J.; Xu, J. Nuclear survivin and its relationship to DNA damage repair genes in non-small cell lung cancer investigated using tissue array. PLoS ONE 2013, 8, e74161. [Google Scholar] [CrossRef]
- Li, F.; Ackermann, E.J.; Bennett, C.F.; Rothermel, A.L.; Plescia, J.; Tognin, S.; Villa, A.; Marchisio, P.C.; Altieri, D.C. Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nat. Cell Biol. 1999, 1, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Dohi, T.; Okada, K.; Xia, F.; Wilford, C.E.; Samuel, T.; Welsh, K.; Marusawa, H.; Zou, H.; Armstrong, R.; Matsuzawa, S.; et al. An IAP-IAP complex inhibits apoptosis. J. Biol. Chem. 2004, 279, 34087–34090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrotra, S.; Languino, L.R.; Raskett, C.M.; Mercurio, A.M.; Dohi, T.; Altieri, D.C. IAP regulation of metastasis. Cancer Cell 2010, 17, 53–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, D.; Liu, T.; Samadashwily, G.; Li, F.; Grossman, D. Survivin repression by p53, Rb and E2F2 in normal human melanocytes. Carcinogenesis 2008, 29, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.K.; Hwang, C.S.; Choe, T.B.; Hong, S.I.; Yi, J.Y.; Hwang, S.G.; Lee, H.G.; Oh, S.T.; Lee, Y.H.; Park, I.C. Selective inhibition of histone deacetylase 2 induces p53-dependent survivin downregulation through MDM2 proteasomal degradation. Oncotarget 2015, 6, 26528–26540. [Google Scholar] [CrossRef] [PubMed]
- Tran, J.; Master, Z.; Yu, J.L.; Rak, J.; Dumont, D.J.; Kerbel, R.S. A role for survivin in chemoresistance of endothelial cells mediated by VEGF. Proc. Natl. Acad. Sci. USA 2002, 99, 4349–4354. [Google Scholar] [CrossRef] [Green Version]
- Akyürek, N.; Memiş, L.; Ekinci, O.; Köktürk, N.; Oztürk, C. Survivin expression in pre-invasive lesions and non-small cell lung carcinoma. Virchows Arch. 2006, 449, 164–170. [Google Scholar] [CrossRef]
- Karczmarek-Borowska, B.; Filip, A.; Wojcierowski, J.; Smoleń, A.; Pilecka, I.; Jabłonka, A. Survivin antiapoptotic gene expression as a prognostic factor in non-small cell lung cancer: In situ hybridization study. Folia Histochem. Cytobiol. 2005, 43, 237–242. [Google Scholar]
- Dai, C.H.; Li, J.; Shi, S.B.; Yu, L.C.; Ge, L.P.; Chen, P. Survivin and smac gene expressions but not livin are predictors of prognosis in non-small cell lung cancer patients treated with adjuvant chemotherapy following surgery. Jpn. J. Clin. Oncol. 2010, 40, 327–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Lin, D.; Low, C.; Vucic, E.A.; English, J.C.; Yee, J.; Murray, N.; Lam, W.L.; Ling, V.; Lam, S.; et al. Elevated expression of BIRC6 protein in non-small-cell lung cancers is associated with cancer recurrence and chemoresistance. J. Thorac. Oncol. 2013, 8, 161–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kren, L.; Brazdil, J.; Hermanova, M.; Goncharuk, V.N.; Kallakury, B.V.; Kaur, P.; Ross, J.S. Prognostic significance of anti-apoptosis proteins survivin and bcl-2 in non-small cell lung carcinomas: A clinicopathologic study of 102 cases. Appl. Immunohistochem. Mol. Morphol. 2004, 12, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, H.; Sun, Y.; Chen, S.; Wang, H.; Huang, R.; Zhao, X.; Fu, W.; Yang, C. miR-198-induced upregulation of Livin may be associated with the prognosis and contribute to the oncogenesis of lung adenocarcinoma. Oncol. Rep. 2017, 38, 2096–2104. [Google Scholar] [CrossRef]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, A12–A13. [Google Scholar] [CrossRef]
- Shea, B.J.; Grimshaw, J.M.; Wells, G.A.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.C.; Tugwell, P.; Moher, D.; Bouter, L.M. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Steels, E.; Paesmans, M.; Berghmans, T.; Branle, F.; Lemaitre, F.; Mascaux, C.; Meert, A.P.; Vallot, F.; Lafitte, J.J.; Sculier, J.P. Role of p53 as a prognostic factor for survival in lung cancer: A systematic review of the literature with a meta-analysis. Eur. Respir. J. 2001, 18, 705–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, Á.; Munkácsy, G.; Győrffy, B. Pancancer survival analysis of cancer hallmark genes. Sci. Rep. 2021, 11, 6047. [Google Scholar] [CrossRef]
- Lau, J.; Ioannidis, J.P.; Schmid, C.H. Quantitative synthesis in systematic reviews. Ann. Intern. Med. 1997, 127, 820–826. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188, Erratum in Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Bartoš, F.; Maier, M.; Wagenmakers, E.-J. Adjusting for publication bias in JASP—Selection models and robust bayesian meta-analysis. PsyArXiv 2020. [Google Scholar] [CrossRef]
- Yano, Y.; Otsuka, T.; Hirano, H.; Uenami, T.; Satomi, A.; Kuroyama, M.; Niinaka, M.; Yoneda, T.; Kimura, H.; Mori, M.; et al. Nuclear survivin expression in small cell lung cancer. Anticancer Res. 2015, 35, 2935–2939. [Google Scholar] [PubMed]
- Chen, P.; Zhu, J.; Liu, D.Y.; Li, H.Y.; Xu, N.; Hou, M. Over-expression of survivin and VEGF in small-cell lung cancer may predict the poorer prognosis. Med. Oncol. 2014, 31, 775. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Jung, J.H.; Lee, M.A.; Seo, K.J.; Shim, B.Y.; Kim, S.H.; Cho, D.G.; Ahn, M.I.; Kim, C.H.; Cho, K.D.; et al. Immunohistochemical analysis of non-small cell lung cancer: Correlation with clinical parameters and prognosis. J. Korean Med. Sci. 2007, 22, 318–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monzó, M.; Rosell, R.; Felip, E.; Astudillo, J.; Sánchez, J.J.; Maestre, J.; Martín, C.; Font, A.; Barnadas, A.; Abad, A. A novel anti-apoptosis gene: Re-expression of survivin messenger RNA as a prognosis marker in non-small-cell lung cancers. J. Clin. Oncol. 1999, 17, 2100–2104. [Google Scholar] [CrossRef]
- Sun, P.L.; Jin, Y.; Kim, H.; Seo, A.N.; Jheon, S.; Lee, C.T.; Chung, J.H. Survivin expression is an independent poor prognostic marker in lung adenocarcinoma but not in squamous cell carcinoma. Virchows Arch. 2013, 463, 427–436. [Google Scholar] [CrossRef]
- Xu, P.; Xu, X.L.; Huang, Q.; Zhang, Z.H.; Zhang, Y.B. CIP2A with survivin protein expressions in human non-small-cell lung cancer correlates with prognosis. Med. Oncol. 2012, 29, 1643–1647. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, S.; Kang, M.Q. Influence of survivin and Bcl-2 expression on the biological behavior of non-small cell lung cancer. Mol. Med. Rep. 2012, 5, 1409–1414. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.; Yasufuku, K.; Nakajima, T.; Hiroshima, K.; Chiyo, M.; Yoshida, S.; Suzuki, M.; Sekine, Y.; Shibuya, K.; Agamy, G.; et al. Nuclear survivin in pN2 nonsmall cell lung cancer: Prognostic and clinical implications. Eur. Respir. J. 2009, 33, 127–133. [Google Scholar] [CrossRef]
- Cho, S.; Park, T.I.; Lee, E.B.; Son, S.A. Poor prognostic factors in surgically resected stage I non-small cell lung cancer: Histopathologic and immunohistochemical analysis. Korean J. Thorac. Cardiovasc. Surg. 2012, 45, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vischioni, B.; van der Valk, P.; Span, S.W.; Kruyt, F.A.; Rodriguez, J.A.; Giaccone, G. Nuclear localization of survivin is a positive prognostic factor for survival in advanced non-small-cell lung cancer. Ann. Oncol. 2004, 15, 1654–1660. [Google Scholar] [CrossRef]
- Wang, M.; Liu, B.G.; Yang, Z.Y.; Hong, X.; Chen, G.Y. Significance of survivin expression: Prognostic value and survival in stage III non-small cell lung cancer. Exp. Ther. Med. 2012, 3, 983–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.K.; Huang, C.Y.; Yang, M.C.; Lan, C.C.; Lee, C.H.; Chan, E.C.; Chen, K.T. Nuclear survivin expression: A prognostic factor for the response to taxane-platinum chemotherapy in patients with advanced non-small cell lung cancer. Med. Oncol. 2014, 31, 79. [Google Scholar] [CrossRef]
- Yamashita, S.; Chujo, M.; Miyawaki, M.; Tokuishi, K.; Anami, K.; Yamamoto, S.; Kawahara, K. Combination of p53AIP1 and survivin expression is a powerful prognostic marker in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2009, 28, 22. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.J.; Kim, H.R.; Park, Y.S.; Kim, Y.H.; Kim, D.K.; Park, S.I. Prognostic value of survivin expression in stage III non-small cell lung cancer patients treated with platinum-based therapy. Surg. Oncol. 2015, 24, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Falleni, M.; Pellegrini, C.; Marchetti, A.; Oprandi, B.; Buttitta, F.; Barassi, F.; Santambrogio, L.; Coggi, G.; Bosari, S. Survivin gene expression in early-stage non-small cell lung cancer. J. Pathol. 2003, 200, 620–626. [Google Scholar] [CrossRef]
- Hirano, H.; Maeda, H.; Takeuchi, Y.; Susaki, Y.; Kobayashi, R.; Hayashi, A.; Ose, N.; Nakazawa, Y.; Yamaguchi, T.; Yokota, S.; et al. Association of cigarette smoking with the expression of nuclear survivin in pathological Stage IA lung adenocarcinomas. Med. Mol. Morphol. 2014, 47, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Porebska, I.; Sobańska, E.; Kosacka, M.; Jankowska, R. Apoptotic regulators: P53 and survivin expression in non-small cell lung cancer. Cancer Genom. Proteom. 2010, 7, 331–335. [Google Scholar]
- Nakashima, N.; Huang, C.L.; Liu, D.; Ueno, M.; Yokomise, H. Intratumoral Wnt1 expression affects survivin gene expression in non-small cell lung cancer. Int. J. Oncol. 2010, 37, 687–694. [Google Scholar] [CrossRef] [Green Version]
- Atikcan, S.; Unsal, E.; Demirag, F.; Köksal, D.; Yilmaz, A. Correlation between survivin expression and prognosis in non-small cell lung cancer. Respir. Med. 2006, 100, 2220–2226. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Mao, Y.; Zhan, Y.; Huang, J.; Wang, X.; Luo, P.; Li, L.I.; Mo, D.; Liu, Q.; Xu, H.; et al. Prognostic implications of survivin and lung resistance protein in advanced non-small cell lung cancer treated with platinum-based chemotherapy. Oncol. Lett. 2016, 11, 723–730. [Google Scholar] [CrossRef]
- Kim, G.Y.; Lim, S.J.; Kim, Y.W. Expression of HuR, COX-2, and survivin in lung cancers; cytoplasmic HuR stabilizes cyclooxygenase-2 in squamous cell carcinomas. Mod. Pathol. 2011, 24, 1336–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.X.; Li, N.E.; Ma, Y.; Han, Y.C.; Shi, Y. Expression of Elf-1 and survivin in non-small cell lung cancer and their relationship to intratumoral microvessel density. Chin. J. Cancer 2010, 29, 396–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.Q.; Zhao, C.L.; Li, W. Effect of hypoxia-inducible factor-1alpha on transcription of survivin in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2009, 28, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikehara, M.; Oshita, F.; Kameda, Y.; Ito, H.; Ohgane, N.; Suzuki, R.; Saito, H.; Yamada, K.; Noda, K.; Mitsuda, A. Expression of survivin correlated with vessel invasion is a marker of poor prognosis in small adenocarcinoma of the lung. Oncol. Rep. 2002, 9, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.F.; Xu, H.T.; Lin, X.Y.; Yu, J.H.; Wang, E.H. A multiple marker analysis of apoptosis-associated protein expression in non-small cell lung cancer in a Chinese population. Folia Histochem. Cytobiol. 2011, 49, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Zhang, Z.; Zhang, B.; Shu, Y.; Wu, H.; Huang, X.; Yu, Q.; Guo, R. Clinical significance of PIK3CA and survivin in primary adenosquamous lung carcinoma. Med. Oncol. 2014, 31, 983. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Zheng, L.; Zhan, X.; Xu, B.; Jiang, J.; Wu, C. SIRT1 expression is associated with poor prognosis of lung adenocarcinoma. Onco Targets Ther. 2015, 8, 977–984. [Google Scholar] [CrossRef] [Green Version]
- Grossi, F.; Spizzo, R.; Bordo, D.; Cacitti, V.; Valent, F.; Rossetto, C.; Follador, A.; Di Terlizzi, S.; Aita, M.; Morelli, A.; et al. Prognostic stratification of stage IIIA pN2 non-small cell lung cancer by hierarchical clustering analysis of tissue microarray immunostaining data: An alpe adria thoracic oncology multidisciplinary group study (ATOM 014). J. Thorac. Oncol. 2010, 5, 1354–1360. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Y.; Yao, Z.; Li, Y.; Liu, T.; Zheng, H.Y.; Zhu, C.Z.; Sun, C.Y.; Wang, A.X.; Zhao, M.; Wu, X.Y. Expression and significance of survivin mRNA in lung cancer tissue microarray detected by FISH. Chin. Med. Sci. J. 2005, 20, 214–216. [Google Scholar]
- Xia, R.; Chen, S.; Chen, Y.; Zhang, W.; Zhu, R.; Deng, A. A chromosomal passenger complex protein signature model predicts poor prognosis for non-small-cell lung cancer. OncoTargets Ther. 2015, 8, 721–726. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, C.G.; van der Valk, P.; Span, S.W.; Ludwig, I.; Smit, E.F.; Kruyt, F.A.; Pinedo, H.M.; van Tinteren, H.; Giaccone, G. Expression of X-linked inhibitor of apoptosis as a novel prognostic marker in radically resected non-small cell lung cancer patients. Clin. Cancer Res. 2001, 7, 2468–2474. [Google Scholar] [PubMed]
- Hofmann, H.S.; Simm, A.; Hammer, A.; Silber, R.E.; Bartling, B. Expression of inhibitors of apoptosis (IAP) proteins in non-small cell human lung cancer. J. Cancer Res. Clin. Oncol. 2002, 128, 554–560. [Google Scholar] [CrossRef]
- Gharabaghi, M.A. Diagnostic investigation of BIRC6 and SIRT1 protein expression level as potential prognostic biomarkers in patients with non-small cell lung cancer. Clin. Respir. J. 2018, 12, 633–638. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Yuan, H.; Burnett, J.; Pan, J.; Yang, Z.; Ran, Y.; Myers, I.; Sun, D. CPA4 is a novel diagnostic and prognostic marker for human non-small-cell lung cancer. J. Cancer 2016, 7, 1197–1204. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhang, X.J.; Zhang, Z.; Zhang, A.H.; Wang, W.; Dong, J.H. Meta-analysis: Prognostic value of survivin in patients with hepatocellular carcinoma. PLoS ONE 2013, 8, e83350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Wang, L.; Jiang, G.N.; He, W.X.; Ding, J.A. The role of survivin on overall survival of non-small cell lung cancer, a meta-analysis of published literatures. Lung Cancer 2008, 61, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Yao, X.; Wu, M. Direct interaction between survivin and Smac/DIABLO is essential for the anti-apoptotic activity of survivin during taxol-induced apoptosis. J. Biol. Chem. 2003, 278, 23130–23140. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000, 102, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Chai, J.; Du, C.; Wu, J.W.; Kyin, S.; Wang, X.; Shi, Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 2000, 406, 855–862. [Google Scholar] [CrossRef]
- Yang, C.; Wang, H.; Zhang, B.; Chen, Y.; Zhang, Y.; Sun, X.; Xiao, G.; Nan, K.; Ren, H.; Qin, S. LCL161 increases paclitaxel-induced apoptosis by degrading cIAP1 and cIAP2 in NSCLC. J. Exp. Clin. Cancer Res. 2016, 35, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolcher, A.W.; Mita, A.; Lewis, L.D.; Garrett, C.R.; Till, E.; Daud, A.I.; Patnaik, A.; Papadopoulos, K.; Takimoto, C.; Bartels, P.; et al. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J. Clin. Oncol. 2008, 26, 5198–5203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauch, A.; Hennig, D.; Schäfer, C.; Wirth, M.; Marx, C.; Heinzel, T.; Schneider, G.; Krämer, O.H. Survivin and YM155: How faithful is the liaison? Biochim. Biophys. Acta 2014, 1845, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Zatloukal, P.; Roubec, J.; Floor, K.; Musil, J.; Kuta, M.; van Klaveren, R.J.; Chaudhary, S.; Gunther, A.; Shamsili, S. Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J. Clin. Oncol. 2009, 27, 4481–4486. [Google Scholar] [CrossRef]
- Kelly, R.J.; Thomas, A.; Rajan, A.; Chun, G.; Lopez-Chavez, A.; Szabo, E.; Spencer, S.; Carter, C.A.; Guha, U.; Khozin, S.; et al. A phase I/II study of sepantronium bromide (YM155, survivin suppressor) with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer. Ann. Oncol. 2013, 24, 2601–2606. [Google Scholar] [CrossRef]
- Shimizu, T.; Nishio, K.; Sakai, K.; Okamoto, I.; Okamoto, K.; Takeda, M.; Morishita, M.; Nakagawa, K. Phase I safety and pharmacokinetic study of YM155, a potent selective survivin inhibitor, in combination with erlotinib in patients with EGFR TKI refractory advanced non-small cell lung cancer. Cancer Chemother. Pharmacol. 2020, 86, 211–219. [Google Scholar] [CrossRef]
- Sun, Y.; Giacalone, N.J.; Lu, B. Terameprocol (tetra-O-methyl nordihydroguaiaretic acid), an inhibitor of Sp1-mediated survivin transcription, induces radiosensitization in non-small cell lung carcinoma. J. Thorac. Oncol. 2011, 6, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Rödel, F.; Frey, B.; Leitmann, W.; Capalbo, G.; Weiss, C.; Rödel, C. Survivin antisense oligonucleotides effectively radiosensitize colorectal cancer cells in both tissue culture and murine xenograft models. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.B.; Fisker, N.; Westergaard, M.; Kjaerulff, L.S.; Hansen, H.F.; Thrue, C.A.; Rosenbohm, C.; Wissenbach, M.; Orum, H.; Koch, T. SPC3042: A proapoptotic survivin inhibitor. Mol. Cancer Ther. 2008, 7, 2736–2745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastian, M.; Schröder, A.; Scheel, B.; Hong, H.S.; Muth, A.; von Boehmer, L.; Zippelius, A.; Mayer, F.; Reck, M.; Atanackovic, D.; et al. A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer Immunol. Immunother. CII 2019, 68, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Li, R.; Song, H.; Geng, T.; Yang, J.; Tan, Q.; Song, L.; Wang, Y.; Xue, Y.; Li, Z.; et al. Phase I clinical trial of a novel autologous modified-DC vaccine in patients with resected NSCLC. BMC Cancer 2017, 17, 884. [Google Scholar] [CrossRef]
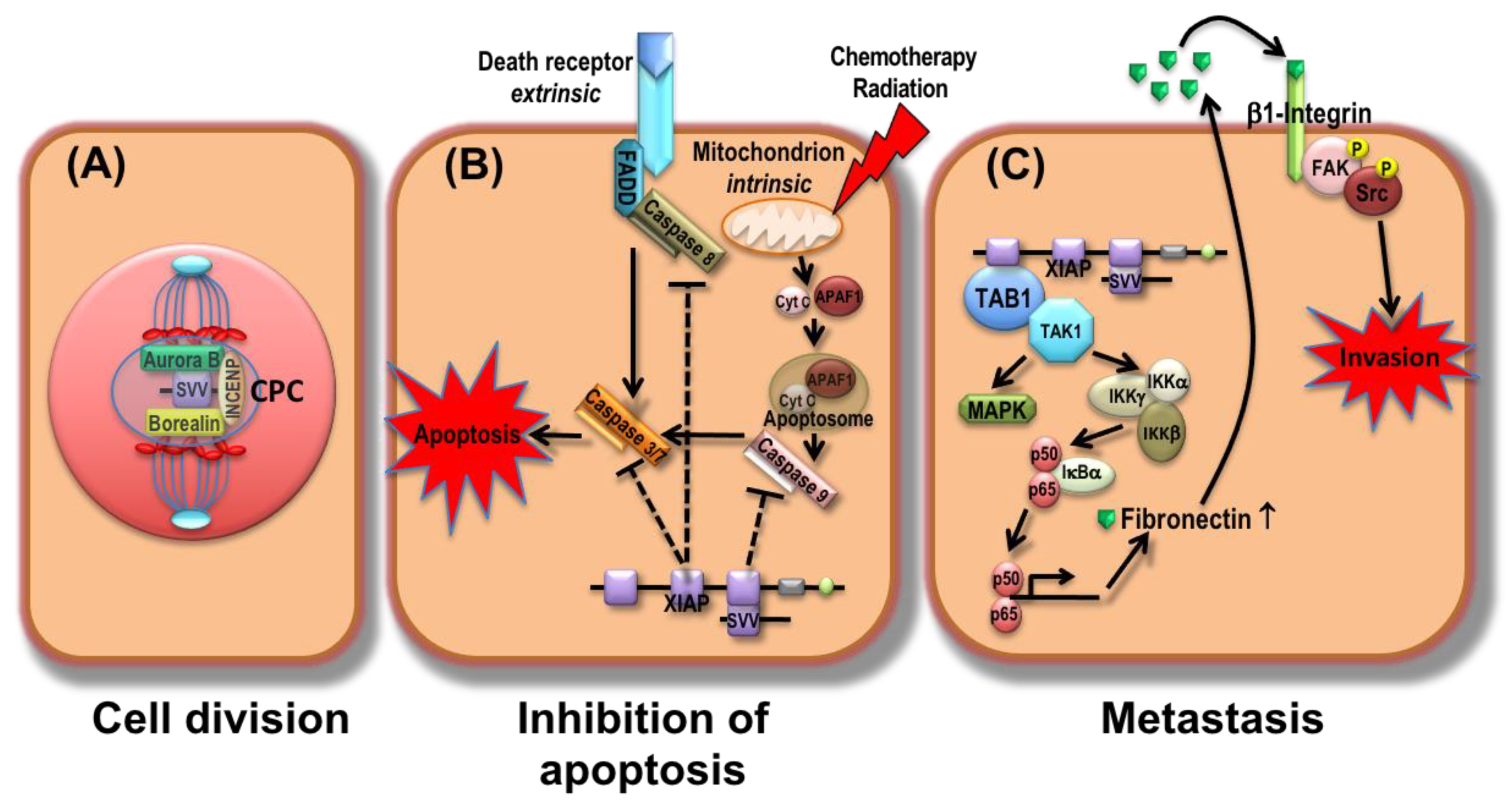


| First Author | Year | Country | Stage | Histology | Study Period | No. of Patients | Age (* Mean/# Median) | Marker | Sex (Female/Male) | Therapy/Source of Samples | * Mean/# Median Follow up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yano, Y. [65] | 2015 | Japan | I–IV | SCLC | 2003–2012 | 32 | 68 # (45–82) | SVV | 4/28 | Surgery (n = 16)/Surgery, Biopsy | NA |
| Karczmarek-Borowska, B. [51] | 2005 | Poland | II–III | NSCLC | 1993–1997 | 60 | 57 * (38–69) | SVV | 6/54 | Surgery + adj. chemotherapy | 22 # (5–60) |
| Rosato, A. [33] | 2013 | Italy | I–II I–II | NSCLC SCLC | 2002–2009 2002–2009 | 65 35 | NA NA | SVV SVV | 17/48 12/23 | Surgery NA/Biopsy | NA |
| Chen, P. (1) [37] | 2010 | China | III–IV | NSCLC | 2002–2006 | 72 | 59 # (40–77) | SVV XIAP | 21/51 | Chemotherapy/Biopsy | NA |
| Dai, C.H. [52] | 2010 | China | I–III | NSCLC | 2003–2005 | 66 | 60 # (34–78) | SVV Livin | 20/46 | Surgery + adj. chemotherapy; in 5 cases + radiation/Surgery | 52 # |
| Bria, E. [42] | 2008 | Italy | I–III | NSCLC | 1995–2001 | 116 | 60 # | SVV | 15/101 | Surgery + adj. chemotherapy (n = 45), radiochemotherapy (n = 30) | 20 # (10–129) |
| Yoo, J. [67] | 2007 | South Korea | I–III | NSCLC | NA | 219 | 67(19–89) | SVV | 51/168 | Surgery | 38.9 # (1.6–117.8) |
| Monzó, M. [68] | 1999 | Spain | I–III | NSCLC | 1995–1996 | 83 | 65 # (26–77) | SVV | 5/78 | Surgery | 18 # (2–31) |
| Sun, P.L. [69] | 2013 | South Korea | I–IV | NSCLC | 2003–2009 | 373 | 65 # (21–84) | SVV | 115/258 | Surgery + adj. chemotherapy | Range (0–80) |
| Xu, P. [70] | 2012 | China | I–III | NSCLC | 2006–2007 | 97 | 60 ND | SVV | 22/75 | Surgery | Range (4–58) |
| Chen, P. (2) [66] | 2014 | China | I–IV | SCLC | 2000–2007 | 45 | 53 # (30–76) | SVV | 8/37 | Surgery + adj. chemotherapy (n = 8) or radiochemotherapy (n = 37) | 11 # |
| Gao, Q. [71] | 2012 | China | I–IV | NSCLC | 2001–2005 | 62 | 57.8 * (35–78) | SVV | 18/44 | Surgery | Range (3–120) |
| Hu, S. [43] | 2013 | China | I–III | NSCLC | 2004–2006 | 256 | 57.7 * | SVV | 80/176 | Surgery + adj. chemotherapy (n = 217), radiotherapy (n = 92) | 64 # |
| Mohamed, S. [72] | 2009 | Japan | NA | NSCLC | 1990–1996 | 78 | 62.8 * | SVV | 20/58 | Surgery | NA |
| Shinohara, E.T. [41] | 2005 | USA | I–II | NSCLC | 1996–2002 | 144 | 67 # (34–92) | SVV | 50/94 | Surgery | NA |
| Cho, S. [73] | 2012 | South Korea | I | NSCLC | 2003–2006 | 110 | 62.3 * (41–79) | SVV | 26/84 | Surgery | 55 # (2.3−87.9) |
| Vischioni, B. [74] | 2004 | Netherlands | III–IV | NSCLC | 1993–1999 | 53 | 56 * (29–75) | SVV | 20/33 | Neoadj. chemotherapy, surgery, or radiotherapy (n = 32), palliative chemotherapy (n = 21) | 76 # |
| Wang, M. [75] | 2012 | China | III | NSCLC | 2002–2004 | 210 | 59.8 * (35–76) | SVV | 80/130 | Surgery | NA |
| Wu, Y.K. [76] | 2014 | Taiwan | III–IV | NSCLC | 2004–2009 | 48 | 59.4 * (36–83) | SVV | 16/32 | Biopsy, chemotherapy | 20.4 # (3.4–59) |
| Yamashita, S.I. [77] | 2009 | Japan | NA | NSCLC | 1997–2003 | 47 | NA | SVV | 14/33 | Surgery | 64.8 # (14.4–100.8) |
| Akyürek, N. [50] | 2006 | Turkey | I–IV | NSCLC | 1994–2001 | 78 | 63 # (39–78) | SVV | 6/72 | Surgery (n = 27), biopsy (n = 51) | 18 # (1–80) |
| Cho, H.J. [78] | 2015 | South Korea | III | NSCLC | 2000–2005 | 53 | 57.6 * | SVV | 10/43 | Neoadj. radiochemotherapy, Surgery adj. radio- (n = 25) or radiochemotherapy (n = 2) | NA |
| Falleni, M. [79] | 2003 | Italy | I | NSCLC | NA | 83 | 63.4 * (43–74) | SVV | 4/79 | Surgery | 54 # (7–94) |
| Hirano, H. [80] | 2014 | Japan | I | NSCLC | 2007–2010 | 44 | 65. 3 ND ± 1.5 | SVV | 8/36 | Surgery | 38.3 ND (5.2–58.9) |
| Kren, L. [54] | 2004 | Czech Republic | I–III | NSCLC | 1983–1994 | 102 | NA | SVV | 45/57 | Surgery | NA |
| Porebska, I. [81] | 2010 | Poland | I–IV | NSCLC | NA | 74 | 60.5 * (43–77) | SVV | 25/49 | Surgery, neoadj. therapy (n = 22), palliative therapy | NA |
| Nakashima, N. [82] | 2010 | Japan | I–III | NSCLC | 2001–2004 | 122 | NA | SVV | NA | Surgery | NA |
| Atikcan, S. [83] | 2006 | Turkey | I–III | NSCLC | 2000–2003 | 58 | 57.29 * (40–76) | SVV | 0/58 | Surgery, adj. chemotherapy (n = 9), radiotherapy (n = 13), radiochemotherapy (n = 5) | NA |
| Huang, W. [84] | 2016 | China | III–IV | NSCLC | 2006–2011 | 61 | 56 # (32–74) | SVV | 15/46 | Chemotherapy, biopsy | 7 # (2–29) |
| Kim, G.Y. [85] | 2011 | South Korea | I–IV | NSCLC ADC SCC | 1985–2005 | 244 93 151 | 62 * (35-81) NA NA | SVV | 55/189 40/53 15/136 | Surgery | NA |
| Yang, D.X. [86] | 2010 | China | I–IV | NSCLC | 2002–2004 | 60 | 53.5 # (37–71) | SVV | 20/40 | Surgery | NA |
| Chen, Y.Q. [87] | 2009 | China | I–III | NSCLC | 2005–2007 | 120 | 61 * (42–76) | SVV | 26/94 | Surgery, adj. chemotherapy | NA |
| Ikehara, M. [88] | 2002 | Japan | I–IV | NSCLC | 1992–1999 | 79 | 64 # (26–83) | SVV | 46/33 | Surgery | NA |
| Fan, C.F. [89] | 2011 | China | I–IV | NSCLC | 1998–2005 | 76 | 57.1 * (26–78) | SVV | 30/46 | Surgery | 45.6 # (3–111) |
| Yu, S. [90] | 2014 | China | I–IV | NSCLC | 2006–2013 | 32 | 60.1 * (43–82) | SVV | 9/23 | Surgery | NA |
| Li, C. [91] | 2015 | China | I–IV | NSCLC | NA | 75 | 59 # (20–84) | SVV | 36/39 | Surgery | NA |
| Grossi, F. [92] | 2010 | Italy | III | NSCLC | 1985–1997 | 87 | 62 # (35–74) | SVV | 16/71 | Surgery, adj. radiotherapy (n = 44) | 140. 4 # (61.2–214.8) |
| Wang, X.Y. [93] | 2005 | China | I–IV | NSCLC and SCLC | NA | 54 | 60 # (33–78) | SVV | 17/37 | NA | NA |
| Xia, R. [94] | 2015 | China | I–IV | NSCLC | 2004–2008 | 104 | NA | SVV | 26/78 | Surgery | 40 # (8–89) |
| Liang, Y. [55] | 2017 | China | I–IV | NSCLC | 2004–2009 | 90 | NA | Livin | 41/49 | Surgery | NA |
| Ferreira, C.G. [95] | 2001 | Netherlands | I–III | NSCLC | 1988–1995 | 144 | 65 ND | XIAP | 24/120 | Surgery | 104 # |
| Hofmann, H.S. [96] | 2002 | Germany | I–IV | NSCLC | 1999–2000 | 34 | NA | XIAP | NA | Surgery | NA |
| Dong, X. [53] | 2013 | Canada | I–IV | NSCLC | 2005–2006 | 78 | 69.09 * ± 9.52 | BIRC6 | 44/34 | Surgery | NA |
| Gharabaghi, M.A. [97] | 2016 | Iran | NA | NSCLC | 2006–2014 | 40 | NA | BIRC6 | 17/23 | Surgery | NA |
| Sun, L. [98] | 2016 | China | I–IV | NSCLC | NA | 165 | NA | SVV | 45/120 | NA | NA |
| No. of Studies | Design | Laboratory Methodology | Generalizability | Results Analysis | Global Score (%) | |
|---|---|---|---|---|---|---|
| All studies | 45 | 6.00 | 6.00 | 4 | 6 | 55.26 |
| Survival data | 40 | 7 | 6 | 4 | 6 | 60.53 |
| No survival data | 5 | 5 | 4 | 4 | NA | 34.21 |
| p | <0.001 | 0.26 | 0.37 | NA | 0.001 | |
| UV | 13 | 6 | 6 | 4 | 4 | 55.26 |
| MV | 27 | 7 | 5 | 5 | 6 | 63.16 |
| p | 0.15 | 0.43 | 0.07 | <0.001 | 0.009 | |
| IHC | 36 | 7 | 6 | 4 | 6 | 60.53 |
| RNA-based | 9 | 6 | 4 | 4 | 6 | 50 |
| p | 0.11 | 0.002 | 0.24 | 0.97 | 0.01 | |
| Asian | 32 | 6 | 6 | 4 | 6 | 55.26 |
| Other regions | 13 | 7 | 5 | 6 | 6 | 60.53 |
| p | 0.39 | 0.92 | 0.88 | 0.73 | 0.39 |
| Pooled Data (Random) | Test for Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup | No. of Studies | Cases | HR | 95% CI | p-Value | Chi2 | p-Value | I2 (%) |
| Method | ||||||||
| IHC | 26 | 2892 | 1.92 | 1.52–2.42 | <0.00001 | 170.44 | <0.00001 | 84 |
| PCR | 6 | 451 | 2.09 | 1.45–3.00 | <0.0001 | 5.89 | 0.32 | 15 |
| FISH | 1 | 60 | 4.26 | 1.98–9.16 | NA | NA | NA | NA |
| Survival analysis | ||||||||
| Kaplan–Meier | 9 | 758 | 2.21 | 1.69–2.88 | <0.00001 | 13.7 | 0.09 | 42 |
| UV | 4 | 353 | 1.54 | 1.10–2.16 | 0.01 | 1.44 | 0.7 | 0 |
| MV | 21 | 2292 | 2.01 | 1.52–2.65 | <0.00001 | 130.19 | <0.00001 | 84 |
| Global Quality Score | ||||||||
| ≥57.89 | 19 | 2013 | 2.14 | 1.75–2.63 | <0.00001 | 38.56 | 0.005 | 51 |
| <57.89 | 14 | 1390 | 1.76 | 1.30–2.37 | 0.0002 | 64.48 | <0.00001 | 78 |
| Cases (N) | ||||||||
| ≥78 | 18 | 2521 | 1.99 | 1.64 | <0.00001 | 38.4 | 0.005 | 51 |
| <78 | 15 | 882 | 1.96 | 1.36–2.81 | 0.0003 | 83.24 | <0.00001 | 83 |
| UICC | ||||||||
| I-IV | 12 | 1407 | 1.83 | 1.32–2.54 | 0.0003 | 75.84 | <0.00001 | 83 |
| I-III | 8 | 900 | 2.03 | 1.60–2.58 | <0.00001 | 12.9 | 0.12 | 38 |
| w/o IV | 15 | 1602 | 2.21 | 1.87–2.62 | <0.00001 | 15.89 | 0.32 | 12 |
| NA | 2 | 125 | 2.05 | 1.25–3.37 | 0.004 | 0.27 | 0.6 | 0 |
| Histological Type | ||||||||
| NSCLC | 31 | 3326 | 1.99 | 1.68–2.36 | <0.00001 | 70.97 | <0.0001 | 55 |
| SCLC | 2 | 77 | 1.87 | 0.42–8.30 | 0.41 | 7.38 | 0.007 | 86 |
| Country | ||||||||
| Asian | 23 | 2501 | 2.04 | 1.60–2.62 | <0.00001 | 141.66 | <0.00001 | 83 |
| Caucasian | 10 | 902 | 1.87 | 1.23–2.84 | 0.003 | 34.72 | <0.0001 | 74 |
| Pooled Data (Random) | Test for Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinicopathological Variable | No. of Studies | Cases | OR | 95% CI | p-Value | Chi2 | p-Value | I2 (%) |
| Sex (female/male) | 22 | 2387 | 0.92 | 0.70–1.20 | 0.52 | 32.63 | 0.05 | 36 |
| UICC stage (I + II/III + IV) | 18 | 2160 | 2.24 | 1.56–3.21 | <0.0001 | 36.09 | 0.004 | 53 |
| T stage (T1 + 2/T3 + 4) | 12 | 1515 | 1.57 | 1.14–2.18 | 0.006 | 7.22 | 0.78 | 0 |
| Differentiation (well + moderate/poor) | 17 | 1778 | 1.66 | 1.20–2.29 | 0.002 | 25.21 | 0.07 | 37 |
| Lymph node metastasis | 23 | 2687 | 1.95 | 1.36–2.78 | 0.0003 | 64.26 | <0.00001 | 66 |
| Distant metastasis | 5 | 484 | 0.84 | 0.47–1.51 | 0.56 | 4.14 | 0.39 | 3 |
| Smoking | 8 | 969 | 1.11 | 0.84–1.46 | 0.73 | 4.82 | 0.68 | 0 |
| Age | 16 | 2016 | 0.99 | 0.78–1.25 | 0.91 | 19.3 | 0.2 | 22 |
| Tumor size | 7 | 859 | 0.98 | 0.59–1.61 | 0.93 | 13.21 | 0.04 | 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fung, S.; Knoefel, W.T.; Krieg, A. Clinicopathological and Prognostic Significance of Inhibitor of Apoptosis Protein (IAP) Family Members in Lung Cancer: A Meta-Analysis. Cancers 2021, 13, 4098. https://doi.org/10.3390/cancers13164098
Fung S, Knoefel WT, Krieg A. Clinicopathological and Prognostic Significance of Inhibitor of Apoptosis Protein (IAP) Family Members in Lung Cancer: A Meta-Analysis. Cancers. 2021; 13(16):4098. https://doi.org/10.3390/cancers13164098
Chicago/Turabian StyleFung, Stephen, Wolfram Trudo Knoefel, and Andreas Krieg. 2021. "Clinicopathological and Prognostic Significance of Inhibitor of Apoptosis Protein (IAP) Family Members in Lung Cancer: A Meta-Analysis" Cancers 13, no. 16: 4098. https://doi.org/10.3390/cancers13164098






