Is the Morphological Subtype of Extra-Pulmonary Neuroendocrine Carcinoma Clinically Relevant?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
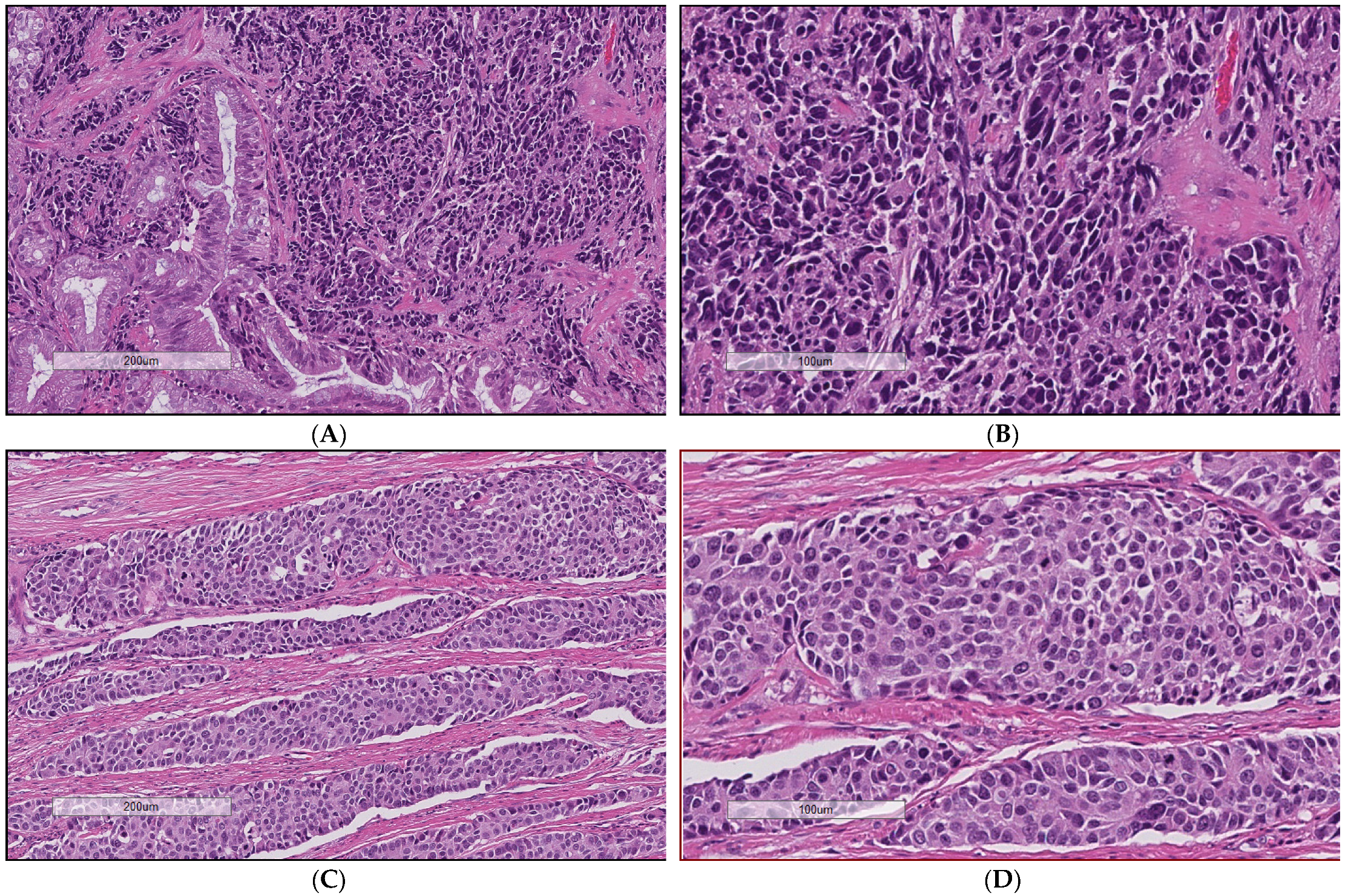
3. Results
3.1. “Potentially Curable” Subgroup
3.2. “Advanced” Subgroup
3.2.1. Treatment Response in the “Advanced” Subgroup
3.2.2. Survival Outcomes in the “Advanced” Subgroup
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours. Digestive System Tumours, 5th ed.; International Agency for Research in Cancer (IARC): Lyon, France, 2019. [Google Scholar]
- Dasari, A.; Mehta, K.; Byers, L.A.; Sorbye, H.; Yao, J.C. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer 2018, 124, 807–815. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Capdevila, J.; Crespo-Herrero, G.; Diaz-Perez, J.A.; Del Prado, M.P.; Alonso Orduna, V.; Sevilla-Garcia, I.; Villabona-Artero, C.; Beguiristain-Gomez, A.; Llanos-Munoz, M.; et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): Results from the National Cancer Registry of Spain (RGETNE). Ann. Oncol. 2010, 21, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Strosberg, J.; Baudin, E.; Klimstra, D.S.; Yao, J.C. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer 2014, 120, 2814–2823. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Sorbye, H.; Baudin, E.; Raymond, E.; Wiedenmann, B.; Niederle, B.; Sedlackova, E.; Toumpanakis, C.; Anlauf, M.; Cwikla, J.B.; et al. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology 2016, 103, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamara, M.G.; Frizziero, M.; Jacobs, T.; Lamarca, A.; Hubner, R.A.; Valle, J.W.; Amir, E. Second-line treatment in patients with advanced extra-pulmonary poorly differentiated neuroendocrine carcinoma: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020, 12, 1758835920915299. [Google Scholar] [CrossRef] [PubMed]
- Girardi, D.M.; Silva, A.C.B.; Rego, J.F.M.; Coudry, R.A.; Riechelmann, R.P. Unraveling molecular pathways of poorly differentiated neuroendocrine carcinomas of the gastroenteropancreatic system: A systematic review. Cancer Treat. Rev. 2017, 56, 28–35. [Google Scholar] [CrossRef]
- Sorbye, H.; Welin, S.; Langer, S.W.; Vestermark, L.W.; Holt, N.; Osterlund, P.; Dueland, S.; Hofsli, E.; Guren, M.G.; Ohrling, K.; et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann. Oncol. 2013, 24, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Milione, M.; Maisonneuve, P.; Spada, F.; Pellegrinelli, A.; Spaggiari, P.; Albarello, L.; Pisa, E.; Barberis, M.; Vanoli, A.; Buzzoni, R.; et al. The Clinicopathologic Heterogeneity of Grade 3 Gastroenteropancreatic Neuroendocrine Neoplasms: Morphological Differentiation and Proliferation Identify Different Prognostic Categories. Neuroendocrinology 2017, 104, 85–93. [Google Scholar] [CrossRef]
- Busico, A.; Maisonneuve, P.; Prinzi, N.; Pusceddu, S.; Centonze, G.; Garzone, G.; Pelligrinelli, A.; Giacomelli, L.; Mangogna, A.; Paolino, C.; et al. Gastroenteropancreatic High-Grade Neuroendocrine Neoplasms (H-NENs): Histology and molecular analysis, two sides of the same coin. Neuroendocrinology 2019, 110, 616–629. [Google Scholar] [CrossRef]
- Sahnane, N.; Furlan, D.; Monti, M.; Romualdi, C.; Vanoli, A.; Vicari, E.; Solcia, E.; Capella, C.; Sessa, F.; La Rosa, S. Microsatellite unstable gastrointestinal neuroendocrine carcinomas: A new clinicopathologic entity. Endocr. Relat. Cancer 2015, 22, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Lantuejoul, S.; Fernandez-Cuesta, L.; Damiola, F.; Girard, N.; McLeer, A. New molecular classification of large cell neuroendocrine carcinoma and small cell lung carcinoma with potential therapeutic impacts. Transl. Lung Cancer Res. 2020, 9, 2233–2244. [Google Scholar] [CrossRef]
- Walter, T.; Tougeron, D.; Baudin, E.; Le Malicot, K.; Lecomte, T.; Malka, D.; Hentic, O.; Manfredi, S.; Bonnet, I.; Guimbaud, R.; et al. Poorly differentiated gastro-entero-pancreatic neuroendocrine carcinomas: Are they really heterogeneous? Insights from the FFCD-GTE national cohort. Eur. J. Cancer 2017, 79, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Shia, J.; Tang, L.H.; Weiser, M.R.; Brenner, B.; Adsay, N.V.; Stelow, E.B.; Saltz, L.B.; Qin, J.; Landmann, R.; Leonard, G.D.; et al. Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity? Am. J. Surg. Pathol. 2008, 32, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2015. [Google Scholar]
- Amin, M.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M.; Statistics Subcommittee of the N C I Eortc Working Group on Cancer Diagnostics. Reporting recommendations for tumor marker prognostic studies (REMARK). J. Natl. Cancer Inst. 2005, 97, 1180–1184. [Google Scholar] [CrossRef] [Green Version]
- de M Rêgo, J.F.; de Medeiros, R.S.S.; Braghiroli, M.I.; Galvão, B.; Neto, J.E.B.; Munhoz, R.R.; Guerra, J.; Nonogaki, S.; Kimura, L.; Pfiffer, T.E.; et al. Expression of ERCC1, Bcl-2, Lin28a, and Ki-67 as biomarkers of response to first-line platinum-based chemotherapy in patients with high-grade extrapulmonary neuroendocrine carcinomas or small cell lung cancer. Ecancermedicalscience 2017, 11, 767. [Google Scholar] [CrossRef] [Green Version]
- Jesinghaus, M.; Konukiewitz, B.; Keller, G.; Kloor, M.; Steiger, K.; Reiche, M.; Penzel, R.; Endris, V.; Arsenic, R.; Hermann, G.; et al. Colorectal mixed adenoneuroendocrine carcinomas and neuroendocrine carcinomas are genetically closely related to colorectal adenocarcinomas. Mod. Pathol. 2017, 30, 610–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woischke, C.; Schaaf, C.W.; Yang, H.M.; Vieth, M.; Veits, L.; Geddert, H.; Markl, B.; Stommer, P.; Schaeffer, D.F.; Frolich, M.; et al. In-depth mutational analyses of colorectal neuroendocrine carcinomas with adenoma or adenocarcinoma components. Mod. Pathol. 2017, 30, 95–103. [Google Scholar] [CrossRef]
- Venizelos, A.; Elvebakken, H.; Perren, A.; Hjortland, G.; Sundlov, A.; Svensson, J.B.; Lothe, I.; Detlefsen, S.; Garresori, H.; Kersten, C.; et al. Mutational landscape of 109 High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms G3. Neuroendocrinology 2020, 110 (Suppl. S1), 53. [Google Scholar]
- Gerard, L.; Garcia, J.; Gauthier, A.; Lopez, J.; Durand, A.; Hervieu, V.; Lemelin, A.; Chardon, L.; Landel, V.; Gibert, B.; et al. ctDNA in neuroendocrine carcinoma of gastroenteropancreatic origin or of unknown primary: The CIRCAN-NEC pilot study. Neuroendocrinology 2020. [Google Scholar] [CrossRef]
- McNamara, M.G.; Scoazec, J.Y.; Walter, T. Extrapulmonary poorly differentiated NECs, including molecular and immune aspects. Endocr. Relat. Cancer 2020, 27, R219–R238. [Google Scholar] [CrossRef]
- Lamarca, A.; Walter, T.; Pavel, M.; Borbath, I.; Freis, P.; Nunez, B.; Childs, A.; McNamara, M.G.; Hubner, R.A.; Garcia-Carbonero, R.; et al. Design and Validation of the GI-NEC Score to Prognosticate Overall Survival in Patients with High-Grade Gastrointestinal Neuroendocrine Carcinomas. J. Natl. Cancer Inst. 2017, 109, djw277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.J.; Ueberroth, B.E.; McGarrah, P.W.; Buckner Petty, S.A.; Kendi, A.T.; Starr, J.; Hobday, T.J.; Halfdanarson, T.R.; Sonbol, M.B. Treatment Outcomes of Well-Differentiated High-Grade Neuroendocrine Tumors. Oncologist 2021, 26, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Uccella, S.; La Rosa, S.; Metovic, J.; Marchiori, D.; Scoazec, J.Y.; Volante, M.; Mete, O.; Papotti, M. Genomics of High-Grade Neuroendocrine Neoplasms: Well-Differentiated Neuroendocrine Tumor with High-Grade Features (G3 NET) and Neuroendocrine Carcinomas (NEC) of Various Anatomic Sites. Endocr. Pathol. 2021, 32, 192–210. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Baudin, E.; Borbath, I.; Caplin, M.; Chen, J.; Cwikla, J.B.; Frilling, A.; Grossman, A.; Kaltsas, G.; Scarpa, A.; et al. Unmet Needs in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms (WHO G3). Neuroendocrinology 2019, 108, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Malka, D.; Hentic, O.; Lombard-Bohas, C.; Le Malicot, K.; Smith, D.; Ferru, A.; Assenat, E.; Cadiot, G.; Lievre, A.; et al. Evaluating bevacizumab in combination with FOLFIRI after the failure of platinum-etoposide regimen in patients with advanced poorly differentiated neuroendocrine carcinoma: The PRODIGE 41-BEVANEC randomized phase II study. Dig. Liver Dis. 2018, 50, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Craig, Z.; Swain, J.; Batman, E.; Wadsley, J.; Reed, N.; Faluyi, O.; Cave, J.; Sharma, R.; Chau, I.; Wall, L.; et al. NET-02 trial protocol: A multicentre, randomised, parallel group, open-label, phase II, single-stage selection trial of liposomal irinotecan (nal-IRI) and 5-fluorouracil (5-FU)/folinic acid or docetaxel as second-line therapy in patients with progressive poorly differentiated extrapulmonary neuroendocrine carcinoma (NEC). BMJ Open 2020, 10, e034527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capdevila, J.; Arques, O.; Hernandez Mora, J.R.; Matito, J.; Caratu, G.; Mancuso, F.M.; Landolfi, S.; Barriuso, J.; Jimenez-Fonseca, P.; Lopez Lopez, C.; et al. Epigenetic EGFR gene repression confers sensitivity to therapeutic BRAFV600E blockade in colon neuroendocrine carcinomas. Clin. Cancer Res. 2019. [Google Scholar] [CrossRef] [Green Version]
- Dizdar, L.; Werner, T.A.; Drusenheimer, J.C.; Mohlendick, B.; Raba, K.; Boeck, I.; Anlauf, M.; Schott, M.; Goring, W.; Esposito, I.; et al. BRAF(V600E) mutation: A promising target in colorectal neuroendocrine carcinoma. Int. J. Cancer 2019, 144, 1379–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltran, H.; Rickman, D.S.; Park, K.; Chae, S.S.; Sboner, A.; MacDonald, T.Y.; Wang, Y.; Sheikh, K.L.; Terry, S.; Tagawa, S.T.; et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011, 1, 487–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Yang, Z.; Shao, F.; Cheng, H.; Wen, Y.; Sun, S.; Guo, W.; Li, Z.; Zhang, F.; Xue, L.; et al. Multi-omics profiling of primary small cell carcinoma of the esophagus reveals RB1 disruption and additional molecular subtypes. Nat. Commun. 2021, 12, 3785. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Jing, Y.; Zhang, R.; Cai, M.C.; Ma, P.; Chen, H.; Zhuang, G. Comprehensive genomic profiling of neuroendocrine bladder cancer pinpoints molecular origin and potential therapeutics. Oncogene 2018, 37, 3039–3044. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Zheng, G.; Schoolmeester, J.K.; Li, Z.; Pallavajjala, A.; Haley, L.; Conner, M.G.; Vang, R.; Hung, C.F.; Wu, T.C.; et al. Next-generation Sequencing Reveals Recurrent Somatic Mutations in Small Cell Neuroendocrine Carcinoma of the Uterine Cervix. Am. J. Surg. Pathol. 2018, 42, 750–760. [Google Scholar] [CrossRef] [PubMed]



| Whole Population (n = 170) | Small Cell (n = 77) | Non-Small Cell (n = 93) | p-Value | ||
|---|---|---|---|---|---|
| Characteristics | n (Frequency) | n (Frequency) | n (Frequency) | ||
| Gender | |||||
| Male | 112 (65.9%) | 53 (68.8%) | 59 (63.4%) | p = 0.461 | |
| Female | 58 (34.1%) | 24 (31.2%) | 34 (36.5%) | ||
| Age at an initial diagnosis | |||||
| Median (range) (yrs) | 62.5 (26.1–90.9) | 58.8 (32.7–82.5) | 64.8 (26.1–90.9) | ||
| Mean (StDev) | 61.1 (12.7) | 59.4 (11.5) | 62.3 (13.4) | p = 0.140 | |
| Site of origin | |||||
| Pancreas | 41 (24.1%) | 19 (24.7%) | 22 (23.7%) | Oesophagus/OGJ vs. others (p = 0.001) Rectum vs. others (p = 0.068) | |
| CUP | 39 (22.9%) | 14 (18.2%) | 25 (26.9%) | ||
| Colon | 21 (12.4%) | 7 (9.1%) | 14 (15.1%) | ||
| Stomach | 20 (11.8%) | 6 (7.8%) | 14 (15.1%) | ||
| Oesophagus/OGJ | 12 (7.3%) | 11 (14.3%) | 1 (1.1%) | ||
| Rectum | 11 (6.5%) | 8 (10.4%) | 3 (3.2%) | ||
| Biliary tract | 11 (6.5%) | 6 (7.8%) | 5 (5.4%) | ||
| Small bowel | 5 (2.9%) | 2 (2.6%) | 3 (3.2%) | ||
| Anus | 3 (1.8%) | 1 (1.3%) | 2 (2.2%) | ||
| Bladder | 2 (1.2%) | 1 (1.3%) | 1 (1.1%) | ||
| Head & Neck | 2 (1.2%) | 1 (1.3%) | 1 (1.1%) | ||
| Prostate | 1 (0.6%) | 1 (1.3%) | 0 (0.0%) | ||
| Ovary | 1 (0.6%) | 0 (0.0%) | 1 (1.1%) | ||
| Appendix | 1 (0.6%) | 0 (0.0%) | 1 (1.1%) | ||
| GEP | 125 (73.5%) | 60 (77.9%) | 65 (69.9%) | GEP vs. CUP + others (p = 0.238) CUP vs. GEP + others (p = 0.179) | |
| CUP | 39 (22.9%) | 14 (18.2%) | 25 (26.9%) | ||
| Others | 6 (3.5%) | 3 (3.9%) | 3 (3.2%) | ||
| Disease stage at diagnosis # | |||||
| Stage II | 6 (3.5%) | 2 (2.6%) | 4 (4.3%) | ||
| Stage III | 27 (15.9%) | 8 (10.4%) | 19 (20.4%) | ||
| Stage IV | 131 (77.1%) | 64 (83.1%) | 67 (72.0%) | ||
| Potentially curable n.o.s. | 6 (3.5%) | 3 (3.9%) | 3 (3.2%) | ||
| Potentially curable | 39 (22.9%) | 13 (16.9%) | 26 (28.0%) | p = 0.087 | |
| Incurable | 131 (77.1%) | 64 (83.1%) | 67 (72.0%) | ||
| Ki-67 index | |||||
| Median (range) expressed in % | 80.0 (25–100) | 80.0 (25–100) | 77.5 (25–100) | ||
| Mean (StDev)expressed in % | 73.2 (19.9) | 78.7 (18.5) | 69.3 (20.1) | p = 0.002 | |
| <55% | 30/150 * (20%) | 7/62 * (11.3%) | 23/88 * (26.1%) | p = 0.025 | |
| ≥55% | 120/150 * (80%) | 55/62 * (88.7%) | 65/88 * (73.9%) | ||
| Unknown | 20 (11.8%) | ||||
| Smoking history | |||||
| Active/former smoker | 72/115 * (62.6%) | 31/54 * (57.4%) | 41/61 * (67.2%) | p = 0.278 | |
| Never smoker | 43/115 * (37.4%) | 23/54 * (42.6%) | 20/61 * (32.8%) | ||
| Unknown | 55 (23.4%) |
| PFS | OS | ||||
|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | ||
| HR, p-Value | HR, p-Value | HR, p-Value | HR, p-Value | ||
| n = 146 | n = 160 | ||||
| Age at diagnosis # | Continuous | 1.00, p = 0.566 | 1.00, p = 0.838 | ||
| Gender | Male vs. Female | 0.83, p = 0.317 | 0.88, p = 0.485 | ||
| ECOG PS | ≥2 vs. 0–1 | 2.62, p < 0.005 | 1.99, p = 0.006 | 2.83, p < 0.005 | 2.36, p = 0.001 |
| Ki-67 index | ≥55% vs. <55% | 1.67, p = 0.040 | 1.80, p = 0.028 | 1.54, p = 0.091 | 1.67, p = 0.063 |
| Site of origin | CUP vs. GEP | 1.01, p = 0.959 | 1.21, p = 0.576 | ||
| Others vs. GEP | 0.89, p = 0.821 | 0.92, p = 0.851 | |||
| Morphological subtype | non-SC vs. SC | 1.27, p = 0.180 | 1.62, p = 0.016 | 1.35, p = 0.095 | 1.64, p = 0.015 |
| Interaction Ki-67/morphological subtype | 0.54, p = 0.300 * | 0.79, p = 0.699 * | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frizziero, M.; Durand, A.; Taboada, R.G.; Zaninotto, E.; Luchini, C.; Chakrabarty, B.; Hervieu, V.; Claro, L.C.L.; Zhou, C.; Cingarlini, S.; et al. Is the Morphological Subtype of Extra-Pulmonary Neuroendocrine Carcinoma Clinically Relevant? Cancers 2021, 13, 4152. https://doi.org/10.3390/cancers13164152
Frizziero M, Durand A, Taboada RG, Zaninotto E, Luchini C, Chakrabarty B, Hervieu V, Claro LCL, Zhou C, Cingarlini S, et al. Is the Morphological Subtype of Extra-Pulmonary Neuroendocrine Carcinoma Clinically Relevant? Cancers. 2021; 13(16):4152. https://doi.org/10.3390/cancers13164152
Chicago/Turabian StyleFrizziero, Melissa, Alice Durand, Rodrigo G. Taboada, Elisa Zaninotto, Claudio Luchini, Bipasha Chakrabarty, Valérie Hervieu, Laura C. L. Claro, Cong Zhou, Sara Cingarlini, and et al. 2021. "Is the Morphological Subtype of Extra-Pulmonary Neuroendocrine Carcinoma Clinically Relevant?" Cancers 13, no. 16: 4152. https://doi.org/10.3390/cancers13164152






