Options to Determine Pathological Response of Axillary Lymph Node Metastasis after Neoadjuvant Chemotherapy in Advanced Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Techniques to Optimize Removal of Targeted or Clipped Positive Node
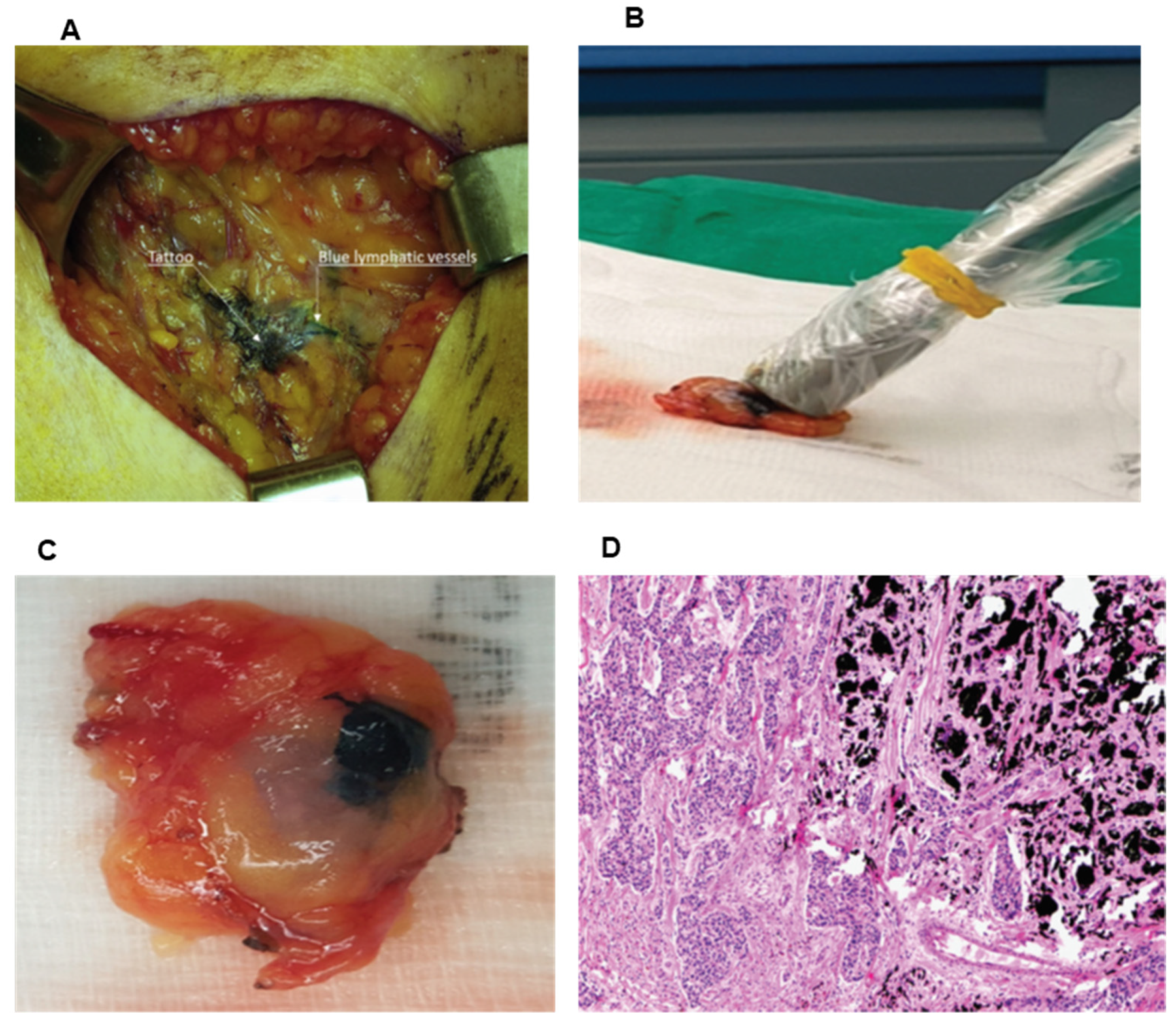
2.1. Carbon Suspension/Charcoal Tattoo
2.2. Wire Localization
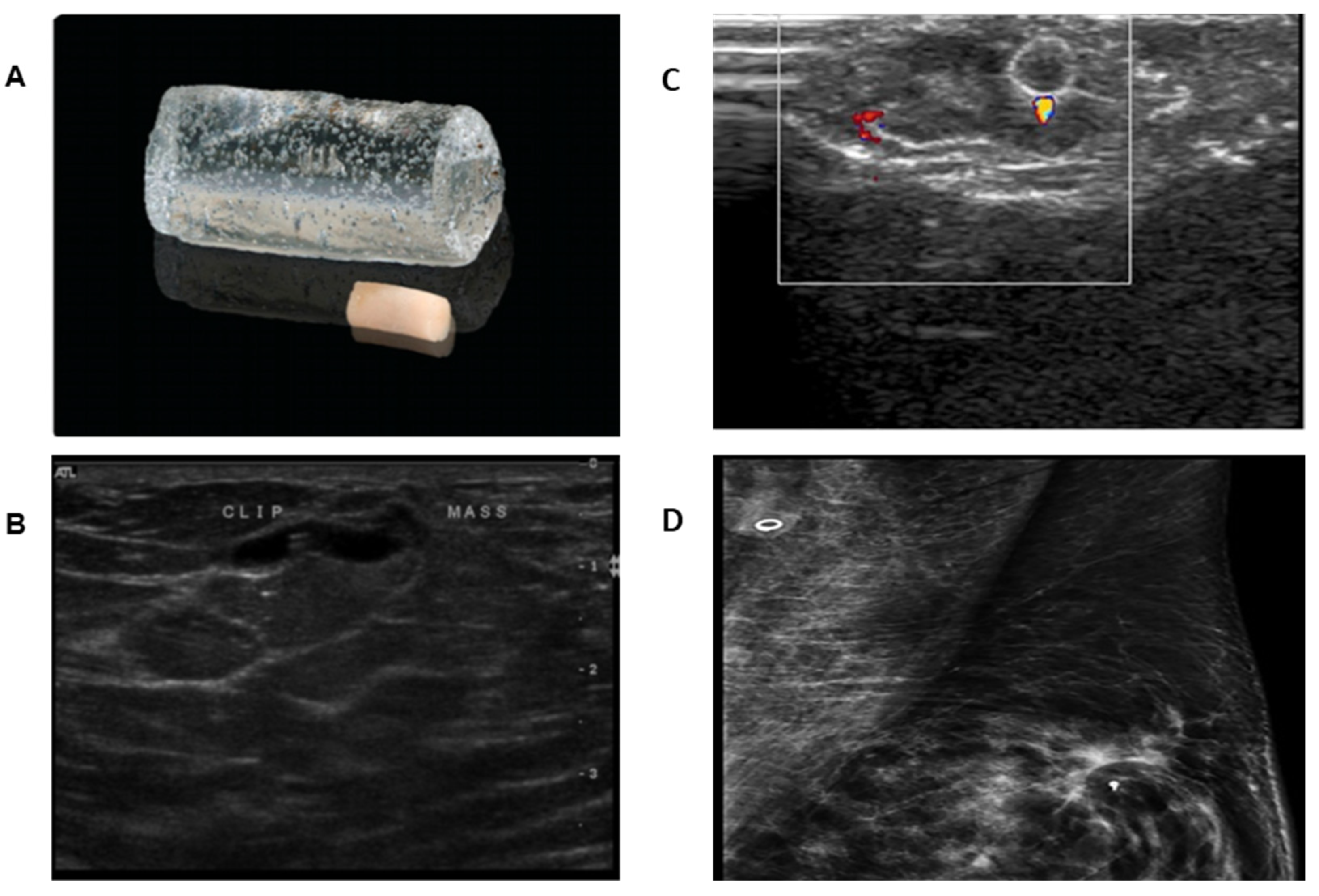
2.3. Ultrasound Visible Clip
2.4. Magnetic Seed Localization (MSL)
2.5. I125 Radioactive Seed Localization (RSL)
2.6. SAVI SCOUT
3. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Kuerer, H.M.; Sahin, A.A.; Hunt, K.K.; Newman, L.A.; Breslin, T.M.; Ames, F.C.; Ross, M.I.; Buzdar, A.U.; Hortobagyi, G.N.; Singletary, S.E. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann. Surg. 1999, 230, 72–78. [Google Scholar] [CrossRef]
- Vila, J.; Mittendorf, E.A.; Farante, G.; Bassett, R.L.; Veronesi, P.; Galimberti, V.; Peradze, N.; Stauder, M.C.; Chavez-MacGregor, M.; Litton, J.F.; et al. Nomograms for Predicting Axillary Response to Neoadjuvant Chemotherapy in Clinically Node-Positive Patients with Breast Cancer. Ann. Surg. Oncol. 2016, 23, 3501–3509. [Google Scholar] [CrossRef] [PubMed]
- Rouzier, R.; Perou, C.M.; Symmans, W.F.; Ibrahim, N.; Cristofanilli, M.; Anderson, K.; Hess, K.R.; Stec, J.; Ayers, M.; Wagner, P.; et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 2005, 11, 5678–5685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, N.S.; Decker, D.; Severson, D.; Schell, S.; Vicini, F.; Margolis, J.; Dekhne, N.S. Molecular classification system identifies invasive breast carcinoma patients who are most likely and those who are least likely to achieve a complete pathologic response after neoadjuvant chemotherapy. Cancer 2007, 110, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, M.; Gandhi, S.; Ishikawa, T.; Takabe, K. Neoadjuvant Chemotherapy for Breast Cancer: Past, Present, and Future. Breast Cancer 2020, 14, 1178223420980377. [Google Scholar] [CrossRef] [PubMed]
- Mamounas, E.P.; Brown, A.; Anderson, S.; Smith, R.; Julian, T.; Miller, B.; Bear, H.D.; Caldwell, C.B.; Walker, A.P.; Mikkelson, W.M.; et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: Results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J. Clin. Oncol. 2005, 23, 2694–2702. [Google Scholar] [CrossRef]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Kuerer, H.M.; Bowling, M.; Flippo-Morton, T.S.; et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013, 310, 1455–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Classe, J.M.; Loaec, C.; Gimbergues, P.; Alran, S.; de Lara, C.T.; Dupre, P.F.; Rouzier, R.; Faure, C.; Paillocher, N.; Chauvet, M.P.; et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: The GANEA 2 study. Breast Cancer Res. Treat. 2019, 173, 343–352. [Google Scholar] [CrossRef]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hausschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; von Minckwitz, G.; Nekljudova, V.; et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet. Oncol. 2013, 14, 609–618. [Google Scholar] [CrossRef]
- Boileau, J.F.; Poirier, B.; Basik, M.; Holloway, C.M.; Gaboury, L.; Sideris, L.; Meterissian, S.; Arnaout, A.; Brackstone, M.; McCready, D.R.; et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: The SN FNAC study. J. Clin. Oncol. 2015, 33, 258–264. [Google Scholar] [CrossRef]
- Cao, S.; Liu, X.; Cui, J.; Liu, X.; Zhong, J.; Yang, Z.; Sun, D.; Wei, W. Feasibility and reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients with positive axillary nodes at initial diagnosis: An up-to date meta-analysis of 3,578 patients. Breast 2021, 59, 256–269. [Google Scholar] [CrossRef]
- Damin, A.P.; Zancan, M.; Melo, M.P.; Biazus, J.V. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with node-positive breast cancer: Guiding a more selective axillary approach. Breast Cancer Res. Treat. 2021, 186, 527–534. [Google Scholar] [CrossRef]
- García-Novoa, A.; Acea-Nebril, B.; Díaz Carballada, C.; Bouzón Alejandro, A.; Conde, C.; Cereijo Garea, C.; Varela, J.R.; Santiago Freijanes, P.; Antolín Novoa, S.; Calvo Martínez, L.; et al. Combining Wire Localization of Clipped Nodes with Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Node-Positive Breast Cancer: Preliminary Results from a Prospective Study. Ann. Surg. Oncol. 2021, 28, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Tee, S.R.; Devane, L.A.; Evoy, D.; Rothwell, J.; Geraghty, J.; Prichard, R.S.; McDermott, E.W. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br. J. Surg. 2018, 105, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Morrow, M.; Dang, C.T. Sentinel node biopsy after neoadjuvant chemotherapy: A new standard for patients with axillary metastases? JAMA 2013, 310, 1449–1450. [Google Scholar] [CrossRef] [PubMed]
- Surgical Axillary Staging; Version 6.2021 BINV-D; National Comprehensive Cancer Network (NCCN); Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 16 August 2021).
- Swarnkar, P.K.; Tayeh, S.; Michell, M.J.; Mokbel, K. The Evolving Role of Marked Lymph Node Biopsy (MLNB) and Targeted Axillary Dissection (TAD) after Neoadjuvant Chemotherapy (NACT) for Node-Positive Breast Cancer: Systematic Review and Pooled Analysis. Cancers 2021, 13, 1539. [Google Scholar] [CrossRef] [PubMed]
- Shatz, B.A.; Weinstock, L.B.; Swanson, P.E.; Thyssen, E.P. Long-term safety of India ink tattoos in the colon. Gastrointest. Endosc. 1997, 45, 153–156. [Google Scholar] [CrossRef]
- Svane, G. A stereotaxic technique for preoperative marking of non-palpable breast lesions. Acta Radiol. Diagn. 1983, 24, 145–151. [Google Scholar] [CrossRef]
- Choy, N.; Lipson, J.; Porter, C.; Ozawa, M.; Kieryn, A.; Pal, S.; Kao, J.; Trinh, L.; Wheeler, A.; Ikeda, D.; et al. Initial results with preoperative tattooing of biopsied axillary lymph nodes and correlation to sentinel lymph nodes in breast cancer patients. Ann. Surg. Oncol. 2015, 22, 377–382. [Google Scholar] [CrossRef]
- Park, S.; Koo, J.S.; Kim, G.M.; Sohn, J.; Kim, S.I.; Cho, Y.U.; Park, B.W.; Park, V.Y.; Yoon, J.H.; Moon, H.J.; et al. Feasibility of Charcoal Tattooing of Cytology-Proven Metastatic Axillary Lymph Node at Diagnosis and Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Breast Cancer Patients. Cancer Res. Treat. 2018, 50, 801–812. [Google Scholar] [CrossRef] [Green Version]
- Allweis, T.M.; Menes, T.; Rotbart, N.; Rapson, Y.; Cernik, H.; Bokov, I.; Diment, J.; Magen, A.; Golan, O.; Levi-Bendet, N.; et al. Ultrasound guided tattooing of axillary lymph nodes in breast cancer patients prior to neoadjuvant therapy, and identification of tattooed nodes at the time of surgery. Eur. J. Surg. Oncol. 2020, 46, 1041–1045. [Google Scholar] [CrossRef]
- Patel, R.; MacKerricher, W.; Tsai, J.; Choy, N.; Lipson, J.; Ikeda, D.; Pal, S.; De Martini, W.; Allison, K.H.; Wapnir, I.L. Pretreatment Tattoo Marking of Suspicious Axillary Lymph Nodes: Reliability and Correlation with Sentinel Lymph Node. Ann. Surg. Oncol. 2019, 26, 2452–2458. [Google Scholar] [CrossRef]
- Ruiz-Delgado, M.L.; López-Ruiz, J.A.; Sáiz-López, A. Abnormal mammography and sonography associated with foreign-body giant-cell reaction after stereotactic vacuum-assisted breast biopsy with carbon marking. Acta Radiol. 2008, 49, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.; Morgan, C.; Shaari, E.; Kovacs, T.; Pinder, S.E.; Hamed, H.; Sever, A.R.; Kothari, A. Wire guided localisation for targeted axillary node dissection is accurate in axillary staging in node positive breast cancer following neoadjuvant chemotherapy. Eur. J. Surg. Oncol. 2020, 46, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Plecha, D.; Bai, S.; Patterson, H.; Thompson, C.; Shenk, R. Improving the Accuracy of Axillary Lymph Node Surgery in Breast Cancer with Ultrasound-Guided Wire Localization of Biopsy Proven Metastatic Lymph Nodes. Ann. Surg. Oncol. 2015, 22, 4241–4246. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Reimer, T.; Gerber, B.; Stubert, J.; Stengel, B.; Stachs, A. Wire localization of clip-marked axillary lymph nodes in breast cancer patients treated with primary systemic therapy. Eur. J. Surg. Oncol. 2018, 44, 1307–1311. [Google Scholar] [CrossRef]
- Trinh, L.; Miyake, K.K.; Dirbas, F.M.; Kothary, N.; Horst, K.C.; Lipson, J.A.; Carpenter, C.; Thompson, A.C.; Ikeda, D.M. CT-Guided Wire Localization for Involved Axillary Lymph Nodes after Neo-adjuvant Chemotherapy in Patients with Initially Node-Positive Breast Cancer. Breast J. 2016, 22, 390–396. [Google Scholar] [CrossRef]
- Alarcón, M.; Buch, E.; Julve, A.; Hernandorena, M.; Tajahuerce, M.; Rodríguez, H.; Bermejo, B.; Ramírez, J.; Burgués, O.; Díaz, S.; et al. Sentinel lymph node BIOPSY after neoadjuvant therapy in breast cancer patients with lymph node involvement at diagnosis. Could wire localization of clipped node improve our results? Surgeon 2021, in press. [Google Scholar] [CrossRef]
- Martinez, S.R.; Gelfand, M.; Hourani, H.S.; Sorrento, J.J.; Mohan, E.P. Cardiac injury during needle localized surgical breast biopsy. J. Surg. Oncol. 2003, 82, 261–265. [Google Scholar] [CrossRef]
- Bristol, J.B.; Jones, P.A. Transgression of localizing wire into the pleural cavity prior to mammography. Br. J. Radiol. 1981, 54, 139–140. [Google Scholar] [CrossRef]
- Klein, R.L.; Mook, J.A.; Euhus, D.M.; Rao, R.; Wynn, R.T.; Eastman, A.B.; Leitch, A.M. Evaluation of a hydrogel based breast biopsy marker (HydroMARK®) as an alternative to wire and radioactive seed localization for non-palpable breast lesions. J. Surg. Oncol. 2012, 105, 591–594. [Google Scholar] [CrossRef]
- Woods, R.W.; Camp, M.S.; Durr, N.J.; Harvey, S.C. A review of options for localization of axillary lymph nodes in the treatment of invasive breast cancer. Acad. Radiol. 2019, 26, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.P.; Bi, Z.; Ong, E.M.W. The ‘twinkle’ artifact—A novel method of clip identification to facilitate targeted axillary surgery following neoadjuvant chemotherapy in breast cancer patients. Clin. Imaging 2020, 68, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Siso, C.; de Torres, J.; Esgueva-Colmenarejo, A.; Espinosa-Bravo, M.; Rus, N.; Cordoba, O.; Rodriguez, R.; Peg, V.; Rubio, I.T. Intraoperative Ultrasound-Guided Excision of Axillary Clip in Patients with Node-Positive Breast Cancer Treated with Neoadjuvant Therapy (ILINA Trial): A New Tool to Guide the Excision of the Clipped Node After Neoadjuvant Treatment. Ann. Surg. Oncol. 2018, 25, 784–791. [Google Scholar] [CrossRef]
- Samreen, N.; Bhatt, A.A.; Adler, K.; Zingula, S.; Glazebrook, K.N. Best MRI sequences for identifying axillary lymph node markers in patients with metastatic breast cancer: An inter-reader observational study. Eur. Radiol. Exp. 2020, 4, 34. [Google Scholar] [CrossRef]
- Dashevsky, B.Z.; Altman, A.; Abe, H.; Jaskowiak, N.; Bao, J.; Schacht, D.V.; Sheth, D.; Kulkarni, K. Lymph node wire localization post-chemotherapy: Towards improving the false negative sentinel lymph node biopsy rate in breast cancer patients. Clin. Imaging 2018, 48, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Hieken, T.J.; Glazebrook, K.N.; Boughey, J.C. Localizing the Clipped Node in Patients with Node-Positive Breast Cancer Treated with Neoadjuvant Chemotherapy: Early Learning Experience and Challenges. Ann. Surg. Oncol. 2017, 24, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Simons, J.M.; Scoggins, M.E.; Kuerer, H.M.; Krishnamurthy, S.; Yang, W.T.; Sahin, A.A.; Shen, Y.; Lin, H.; Bedrosian, I.; Mittendorf, E.A.; et al. Prospective Registry Trial Assessing the Use of Magnetic Seeds to Locate Clipped Nodes after Neoadjuvant Chemotherapy for Breast Cancer Patients. Ann. Surg. Oncol. 2021, 28, 4277–4283. [Google Scholar] [CrossRef]
- Greenwood, H.I.; Wong, J.M.; Mukhtar, R.A.; Alvarado, M.D.; Price, E.R. Feasibility of Magnetic Seeds for Preoperative Localization of Axillary Lymph Nodes in Breast Cancer Treatment. AJR Am. J. Roentgenol. 2019, 213, 953–957. [Google Scholar] [CrossRef]
- Laws, A.; Dillon, K.; Kelly, B.N.; Kantor, O.; Hughes, K.S.; Gadd, M.A.; Smith, B.L.; Lamb, L.R.; Specht, M. Node-Positive Patients Treated with Neoadjuvant Chemotherapy Can Be Spared Axillary Lymph Node Dissection with Wireless Non-Radioactive Localizers. Ann. Surg. Oncol. 2020, 27, 4819–4827. [Google Scholar] [CrossRef]
- Miller, M.E.; Patil, N.; Li, P.; Freyvogel, M.; Greenwalt, I.; Rock, L.; Simpson, A.; Teresczuk, M.; Carlisle, S.; Peñuela, M.; et al. Hospital System Adoption of Magnetic Seeds for Wireless Breast and Lymph Node Localization. Ann. Surg. Oncol. 2021, 28, 3223–3229. [Google Scholar] [CrossRef] [PubMed]
- A Prospective Open Label Sudy the Use of Magseed Markers® and Sentimag® to Localize Axillary Lymph Nodes with Biopen Metastases in Breast Cancer Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT03796559 (accessed on 20 October 2020).
- Straver, M.E.; Loo, C.E.; Alderliesten, T.; Rutgers, E.J.; Vrancken Peeters, M.T. Marking the axilla with radioactive iodine seeds (MARI procedure) may reduce the need for axillary dissection after neoadjuvant chemotherapy for breast cancer. Br. J. Surg. 2010, 97, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Donker, M.; Straver, M.E.; Wesseling, J.; Loo, C.E.; Schot, M.; Drukker, C.A.; van Tinteren, H.; Sonke, G.S.; Rutgers, E.J.; Vrancken Peeters, M.J. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: The MARI procedure. Ann. Surg. 2015, 261, 378–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Nijnatten, T.J.A.; Simons, J.M.; Smidt, M.L.; van der Pol, C.C.; van Diest, P.J.; Jager, A.; van Klaveren, D.; Kam, B.L.R.; Lobbes, M.B.I.; de Boer, M.; et al. A Novel Less-invasive Approach for Axillary Staging after Neoadjuvant Chemotherapy in Patients with Axillary Node-positive Breast Cancer by Combining Radioactive Iodine Seed Localization in the Axilla with the Sentinel Node Procedure (RISAS): A Dutch Prospective Multicenter Validation Study. Clin. Breast Cancer 2017, 17, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Caudle, A.S.; Yang, W.T.; Krishnamurthy, S.; Mittendorf, E.A.; Black, D.M.; Gilcrease, M.Z.; Bedrosian, I.; Hobbs, B.P.; DeSnyder, S.M.; Hwang, R.F.; et al. Improved Axillary Evaluation following Neoadjuvant Therapy for Patients with Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J. Clin. Oncol. 2016, 34, 1072–1078. [Google Scholar] [CrossRef] [Green Version]
- Choudhery, S. CT-Guided Seed Localization in the Breast and Axilla. AJR Am. J. Roentgenol. 2020, 214, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Custodio Rebollo Aguirre, A.; Sánchez Sánchez, R.; González Jiménez, A.D.; Culiañez Casas, M.; Mendoza Arnau, I.; Rashki, M.; Rudolphi Solero, T.; Martínez Meca, S. Combined procedure of marking axillary positive node with iodine-125 seed and sentinel lymph node biopsy in breast cancer patients treated with neoadjuvant chemotherapy. Rev. Esp. Med. Nucl. Imagen Mol. 2020, 39, 75–83. [Google Scholar] [CrossRef]
- Jeffries, D.O.; Dossett, L.A.; Jorns, J.M. Localization for Breast Surgery: The Next Generation. Arch. Pathol. Lab Med. 2017, 141, 1324–1329. [Google Scholar] [CrossRef]
- Sun, J.; Henry, D.A.; Carr, M.J.; Yazdankhahkenary, A.; Laronga, C.; Lee, M.C.; Hoover, S.J.; Sun, W.; Czerniecki, B.J.; Khakpour, N.; et al. Feasibility of Axillary Lymph Node Localization and Excision Using Radar Reflector Localization. Clin. Breast Cancer 2020, 21, e189–e193. [Google Scholar] [CrossRef]
- Cheang, E.; Ha, R.; Thornton, C.M.; Mango, V.L. Innovations in image-guided preoperative breast lesion localization. Br. J. Radiol. 2018, 91, 20170740. [Google Scholar] [CrossRef]
- Mango, V.L.; Wynn, R.T.; Feldman, S.; Friedlander, L.; Desperito, E.; Patel, S.N.; Gomberawalla, A.; Ha, R. Beyond Wires and Seeds: Reflector-guided Breast Lesion Localization and Excision. Radiology 2017, 284, 365–371. [Google Scholar] [CrossRef] [PubMed]




| Trial Name [Ref. Number] | False Negative Rate (%) |
|---|---|
| SENTINA [9] | 14.2% |
| SN-FNAC [10] | 8.4% |
| Z1071 [7] | 12.6% |
| GANEA2 [8] | 11.9% |
| Procedure | Cost (US$) | Advantages | Disadvantages |
|---|---|---|---|
| Carbon suspension/Charcoal tattoo | $100 per 5 mL vial | 100% identification in node Seen up to 6–8 months after injection No side effects | Color misidentification Possibility of some ink migration to non-tattooed node or surrounding tissue One report of foreign body granulomas |
| Wire Localization | $25–30 per wire | Cost effective | Same day as surgery Pt discomfort, pain, hematoma, adjacent tissue injury High chance of migration due to arm movement, muscle contraction |
| Ultrasound visible clip | $60 | Small in size 3 mm Sonographically visible up to 12–15 months Newer ones UltraCor, O-Twist not water absorbing | Will need intraop ultrasound to detect the clip Needs instrument confidence by the surgeon Hygroscopic, absorbs water and subject to migration Decreased visibility over time |
| Magnetic seed localization | $500 per seed $55,000 for probe and Console | Can be placed up to 30 days prior to surgery | Depth limitation 3.5 cm Cost Calibration required and susceptible to other magnetic fields If done prior to MRI breast, artifact in the axilla |
| I 125 radioactive seed localization | $17–60 per seed $15,000 for probe $30,000 for Console | Small size 4 × 0.8 mm Long half-life (60 days) Detectable up to 4 months post NAC by Gamma probe | Strict Nuclear Regulatory Commission protocol →Complete chain important from insertion to removal to retrieval of all seeds Migration chances more intraop than preop |
| SAVI SCOUT | $400 for Single use reflector $40,000 for probe | Up to 6–8 cm depth detection Operating room efficiency Long term placement up to 30 days before surgery | One report of migration due to hematoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murthy, V.; Young, J.; Tokumaru, Y.; Quinn, M.; Edge, S.B.; Takabe, K. Options to Determine Pathological Response of Axillary Lymph Node Metastasis after Neoadjuvant Chemotherapy in Advanced Breast Cancer. Cancers 2021, 13, 4167. https://doi.org/10.3390/cancers13164167
Murthy V, Young J, Tokumaru Y, Quinn M, Edge SB, Takabe K. Options to Determine Pathological Response of Axillary Lymph Node Metastasis after Neoadjuvant Chemotherapy in Advanced Breast Cancer. Cancers. 2021; 13(16):4167. https://doi.org/10.3390/cancers13164167
Chicago/Turabian StyleMurthy, Vijayashree, Jessica Young, Yoshihisa Tokumaru, Marie Quinn, Stephen B. Edge, and Kazuaki Takabe. 2021. "Options to Determine Pathological Response of Axillary Lymph Node Metastasis after Neoadjuvant Chemotherapy in Advanced Breast Cancer" Cancers 13, no. 16: 4167. https://doi.org/10.3390/cancers13164167
APA StyleMurthy, V., Young, J., Tokumaru, Y., Quinn, M., Edge, S. B., & Takabe, K. (2021). Options to Determine Pathological Response of Axillary Lymph Node Metastasis after Neoadjuvant Chemotherapy in Advanced Breast Cancer. Cancers, 13(16), 4167. https://doi.org/10.3390/cancers13164167







