Mechanism of Differential Susceptibility of Two (Canine Lung Adenocarcinoma) Cell Lines to 5-Aminolevulinic Acid-Mediated Photodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Establishment of Primary Canine Lung Adenocarcinoma Cell Lines
2.3. Intracellular PpIX Concentration in Canine Primary Lung Adenocarcinoma Cell Lines
2.4. Fluorescence Microscopic Imaging
2.5. Evaluation of the Cytotoxic Effects of Different 5-ALA Doses on Canine Primary Lung Adenocarcinoma Cell Lines
2.6. Analysis of Apoptosis and Reactive Oxygen Species Induced by 5-ALA-Mediated PDT
2.7. Determination of Cellular Glutathione Peroxidase Activity
2.8. Effect of Glutathione Peroxidase 4 Inhibitor on 5-ALA-Mediated PDT-Induced Cell Death
2.9. Analysis of Nitric Oxide-Positive Cells
2.10. Effect of NO Donors on 5-ALA-Mediated PDT-Induced Cell Death
2.11. Statistical Analysis
3. Results
3.1. Intracellular PpIX Concentration in Canine Primary Lung Adenocarcinoma Cell Lines
3.2. Fluorescence Microscopic Imaging
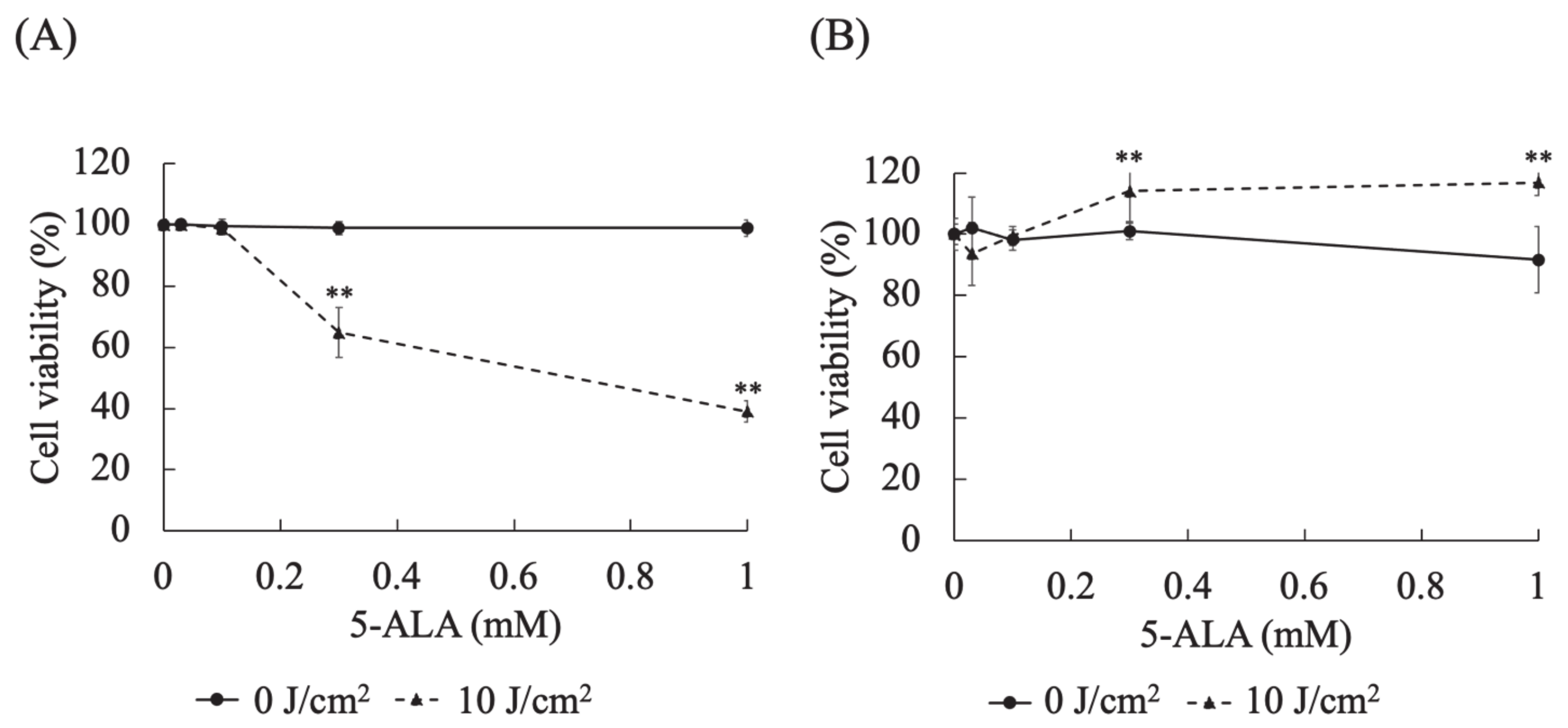
3.3. Evaluation of the Cytotoxic Effects of Different 5-ALA Doses on Canine Primary Lung Adenocarcinoma Cell Lines
3.4. Analysis of Apoptosis and Reactive Oxygen Species Induced by 5-ALA-Mediated PDT
3.5. Determination of Cellular Glutathione Peroxidase Activity
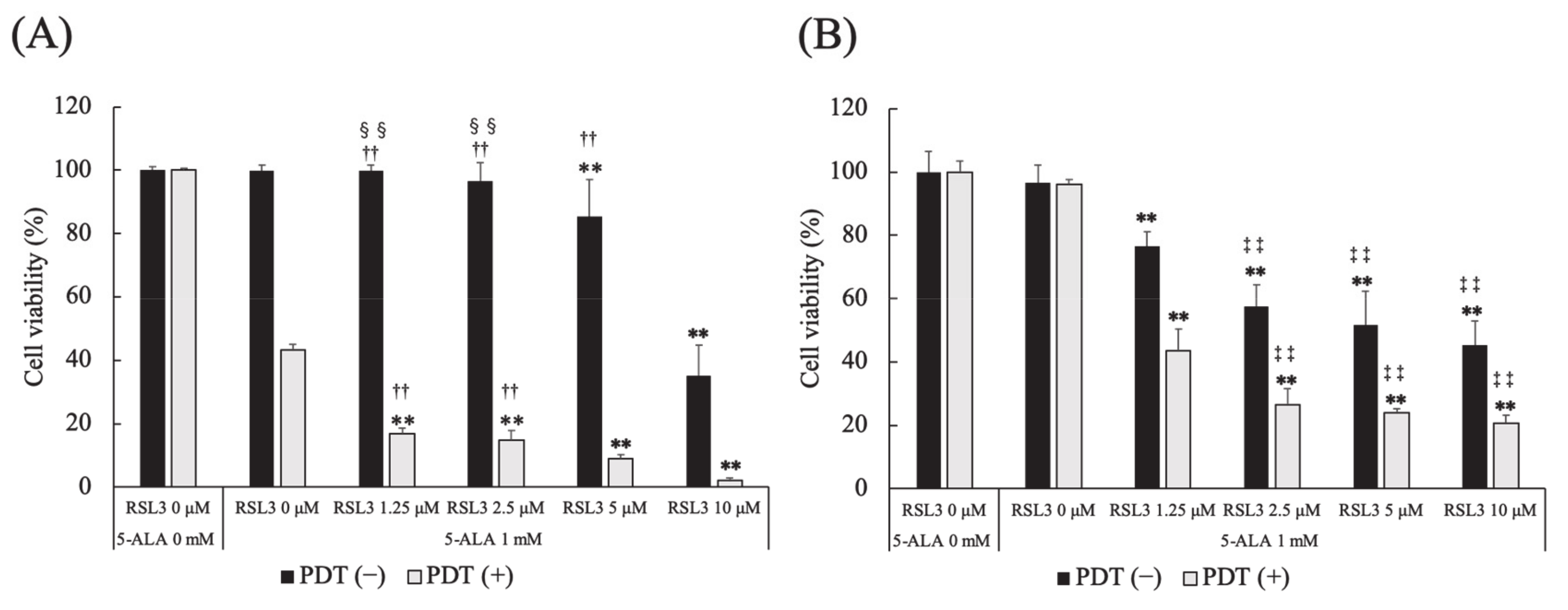
3.6. Effect of Glutathione Peroxidase 4 Inhibitor on 5-ALA-Mediated PDT-Induced Cell Death
3.7. Analysis of Nitric Oxide-Positive Cells
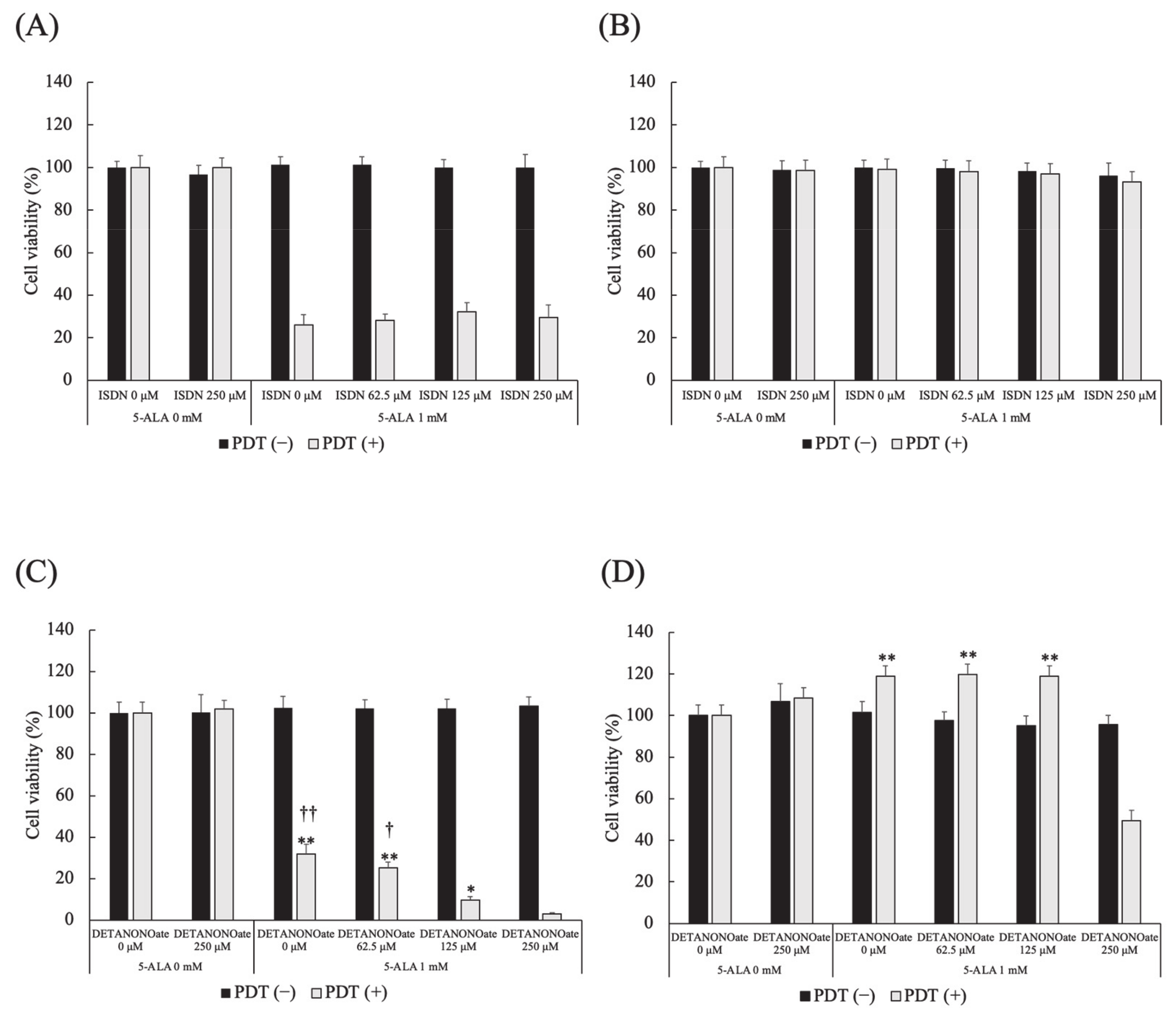
3.8. Effect of NO Donors on 5-ALA-Mediated PDT-Induced Cell Death
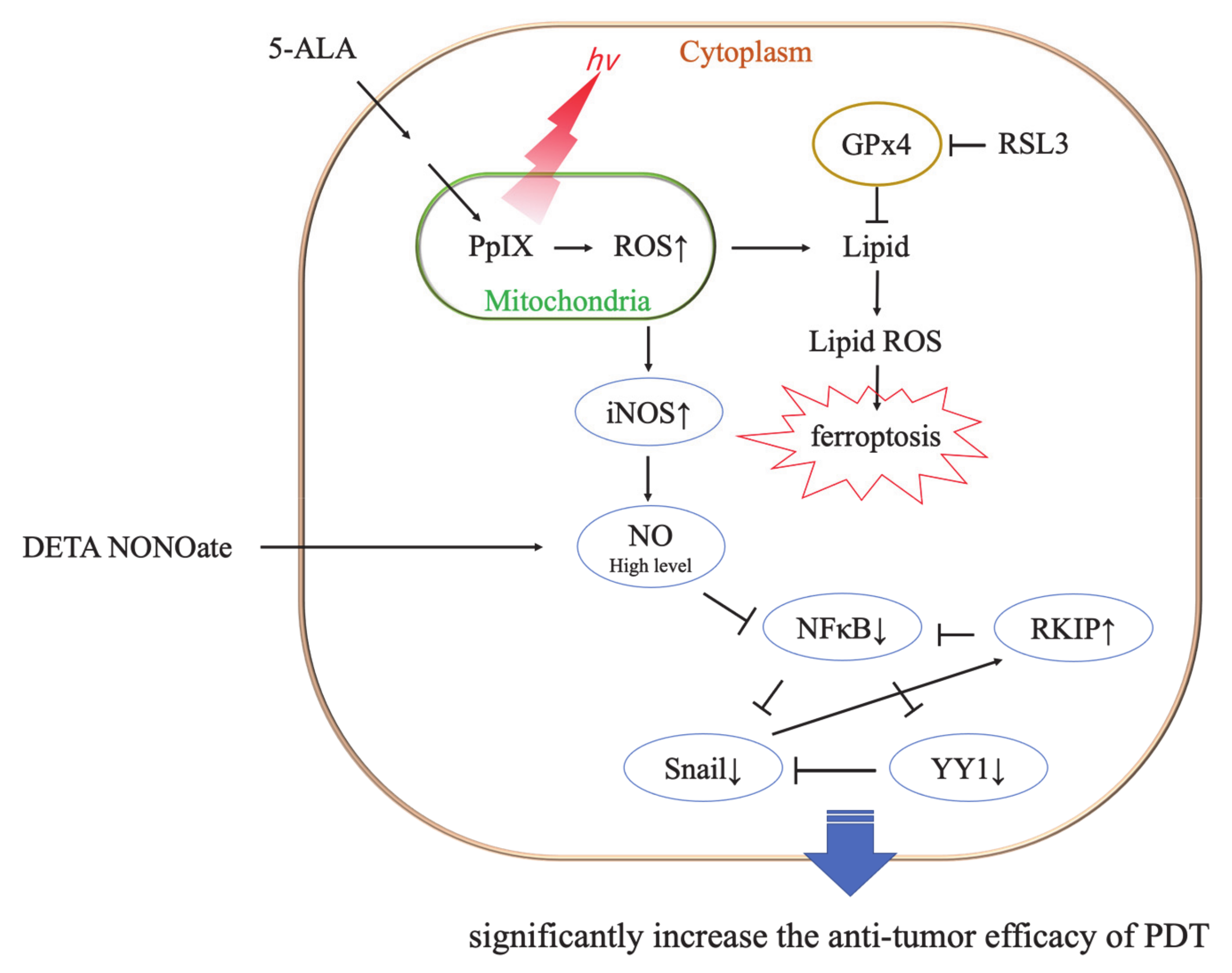
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bacellar, I.O.; Tsubone, T.M.; Pavani, C.; Baptista, M.S. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. Int. J. Mol. Sci. 2015, 16, 20523–20559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triesscheijn, M.; Baas, P.; Schellens, J.H.; Stewart, F.A. Photodynamic therapy in oncology. Oncologist 2006, 11, 1034–1044. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Mehraban, N.; Freeman, H.S. Developments in PDT Sensitizers for Increased Selectivity and Singlet Oxygen Production. Materials 2015, 8, 4421–4456. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [Green Version]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, e1900132. [Google Scholar] [CrossRef]
- Ji, Z.; Yang, G.; Vasovic, V.; Cunderlikova, B.; Suo, Z.; Nesland, J.M.; Peng, Q. Subcellular localization pattern of protoporphyrin IX is an important determinant for its photodynamic efficiency of human carcinoma and normal cell lines. J. Photochem. Photobiol. B 2006, 84, 213–220. [Google Scholar] [CrossRef]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wanczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef] [Green Version]
- Nokes, B.; Apel, M.; Jones, C.; Brown, G.; Lang, J.E. Aminolevulinic acid (ALA): Photodynamic detection and potential therapeutic applications. J. Surg. Res. 2013, 181, 262–271. [Google Scholar] [CrossRef]
- Kawai, N.; Hirohashi, Y.; Ebihara, Y.; Saito, T.; Murai, A.; Saito, T.; Shirosaki, T.; Kubo, T.; Nakatsugawa, M.; Kanaseki, T.; et al. ABCG2 expression is related to low 5-ALA photodynamic diagnosis (PDD) efficacy and cancer stem cell phenotype, and suppression of ABCG2 improves the efficacy of PDD. PLoS ONE. 2019, 14, e0216503. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Fujita, H.; Katase, N.; Inoue, K.; Nagatsuka, H.; Utsumi, K.; Sasaki, J.; Ohuchi, H. Improvement of the efficacy of 5-aminolevulinic acid-mediated photodynamic treatment in human oral squamous cell carcinoma HSC-4. Acta Med. Okayama 2013, 67, 153–164. [Google Scholar] [PubMed]
- El-Khatib, M.; Tepe, C.; Senger, B.; Dibué-Adjei, M.; Riemenschneider, M.J.; Stummer, W.; Steiger, H.J.; Cornelius, J.F. Aminolevulinic acid-mediated photodynamic therapy of human meningioma: An in vitro study on primary cell lines. Int. J. Mol. Sci. 2015, 16, 9936–9948. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Shigeto, T.; Miura, R.; Kobayashi, A.; Mizunuma, M.; Yamauchi, A.; Futagami, M.; Mizunuma, H. Differences in the sensitivity of ovarian cancer to photodynamic therapy and the mechanisms for those differences. Oncol. Lett. 2017, 13, 4933–4938. [Google Scholar] [CrossRef] [Green Version]
- Osaki, T.; Yokoe, I.; Sunden, Y.; Ota, U.; Ichikawa, T.; Imazato, H.; Ishii, T.; Takahashi, K.; Ishizuka, M.; Tanaka, T.; et al. Efficacy of 5-Aminolevulinic Acid in Photodynamic Detection and Photodynamic Therapy in Veterinary Medicine. Cancers 2019, 11, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theodossiou, T.A.; Olsen, C.E.; Jonsson, M.; Kubin, A.; Hothersall, J.S.; Berg, K. The diverse roles of glutathione-associated cell resistance against hypericin photodynamic therapy. Redox Biol. 2017, 12, 191–197. [Google Scholar] [CrossRef]
- Girotti, A.W.; Fahey, J.M.; Korytowski, W. Negative effects of tumor cell nitric oxide on anti-glioblastoma photodynamic therapy. J. Cancer Metastasis Treat. 2020, 6, 52. [Google Scholar]
- Bonnett, R.; Martinez, G. Photobleaching of sensitisers used in photodynamic therapy. Tetrahedron 2001, 57, 9513–9547. [Google Scholar] [CrossRef]
- Konig, K.; Wyss-Desserich, M.; Tadir, Y.; Haller, U.; Tromberg, B.; Berns, M.W.; Wyss, P. Modifications of protoporphyrin IX fluorescence during ALA-based photodynamic therapy of endometriosis. Med. Laser Appl. 2006, 21, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.J.; de Bruijn, H.S.; van der Veen, N.; Stringer, M.R.; Brown, S.B.; Star, W.M. Fluorescence photobleaching of ALA-induced protoporphyrin IX during photodynamic therapy of normal hairless mouse skin: The effect of light dose and irradiance and the resulting biological effect. Photochem. Photobiol. 1998, 67, 140–149. [Google Scholar] [CrossRef]
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef]
- Mailloux, R.J.; McBride, S.L.; Harper, M.E. Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics. Trends Biochem. Sci. 2013, 38, 592–602. [Google Scholar] [CrossRef]
- Bermingham, E.N.; Hesketh, J.E.; Sinclair, B.R.; Koolaard, J.P.; Roy, N.C. Selenium-enriched foods are more effective at increasing glutathione peroxidase (GPx) activity compared with selenomethionine: A meta-analysis. Nutrients 2014, 6, 4002–4031. [Google Scholar] [CrossRef]
- Cole-Ezea, P.; Swan, D.; Shanley, D.; Hesketh, J. Glutathione peroxidase 4 has a major role in protecting mitochondria from oxidative damage and maintaining oxidative phosphorylation complexes in gut epithelial cells. Free Radic. Biol. Med. 2012, 53, 488–497. [Google Scholar] [CrossRef]
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Molecular mechanisms of ferroptosis and its role in cancer therapy. J. Cell Mol. Med. 2019, 23, 4900–4912. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [Green Version]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wang, K. The induction of ferroptosis by impairing STAT3/Nrf2/GPx4 signaling enhances the sensitivity of osteosarcoma cells to cisplatin. Cell Biol. Int. 2019, 43, 1245–1256. [Google Scholar] [CrossRef]
- Fahey, J.M.; Girotti, A.W. Nitric Oxide Antagonism to Anti-Glioblastoma Photodynamic Therapy: Mitigation by Inhibitors of Nitric Oxide Generation. Cancers 2019, 11, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girotti, A.W. Modulation of the Anti-Tumor Efficacy of Photodynamic Therapy by Nitric Oxide. Cancers 2016, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Rapozzi, V.; Della Pietra, E.; Bonavida, B. Dual roles of nitric oxide in the regulation of tumor cell response and resistance to photodynamic therapy. Redox Biol. 2015, 6, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeves, K.J.; Reed, M.W.; Brown, N.J. Is nitric oxide important in photodynamic therapy? J. Photochem. Photobiol. B 2009, 95, 141–147. [Google Scholar] [CrossRef]
- Girotti, A.W. Nitric Oxide-Mediated Resistance to Antitumor Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 500–505. [Google Scholar] [CrossRef]
- Rapozzi, V.; Della Pietra, E.; Zorzet, S.; Zacchigna, M.; Bonavida, B.; Xodo, L.E. Nitric oxide-mediated activity in anti-cancer photodynamic therapy. Nitric Oxide 2013, 30, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Münzel, T. Organic Nitrate Therapy, Nitrate Tolerance, and Nitrate-Induced Endothelial Dysfunction: Emphasis on Redox Biology and Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 899–942. [Google Scholar] [CrossRef] [Green Version]
- Divakaran, S.; Loscalzo, J. The Role of Nitroglycerin and Other Nitrogen Oxides in Cardiovascular Therapeutics. J. Am. Coll. Cardiol. 2017, 70, 2393–2410. [Google Scholar] [CrossRef]
- Alimoradi, H.; Greish, K.; Gamble, A.B.; Giles, G.I. Controlled Delivery of Nitric Oxide for Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 279–303. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osaki, T.; Kunisue, N.; Ota, U.; Imazato, H.; Ishii, T.; Takahashi, K.; Ishizuka, M.; Tanaka, T.; Okamoto, Y. Mechanism of Differential Susceptibility of Two (Canine Lung Adenocarcinoma) Cell Lines to 5-Aminolevulinic Acid-Mediated Photodynamic Therapy. Cancers 2021, 13, 4174. https://doi.org/10.3390/cancers13164174
Osaki T, Kunisue N, Ota U, Imazato H, Ishii T, Takahashi K, Ishizuka M, Tanaka T, Okamoto Y. Mechanism of Differential Susceptibility of Two (Canine Lung Adenocarcinoma) Cell Lines to 5-Aminolevulinic Acid-Mediated Photodynamic Therapy. Cancers. 2021; 13(16):4174. https://doi.org/10.3390/cancers13164174
Chicago/Turabian StyleOsaki, Tomohiro, Narumi Kunisue, Urara Ota, Hideo Imazato, Takuya Ishii, Kiwamu Takahashi, Masahiro Ishizuka, Tohru Tanaka, and Yoshiharu Okamoto. 2021. "Mechanism of Differential Susceptibility of Two (Canine Lung Adenocarcinoma) Cell Lines to 5-Aminolevulinic Acid-Mediated Photodynamic Therapy" Cancers 13, no. 16: 4174. https://doi.org/10.3390/cancers13164174
APA StyleOsaki, T., Kunisue, N., Ota, U., Imazato, H., Ishii, T., Takahashi, K., Ishizuka, M., Tanaka, T., & Okamoto, Y. (2021). Mechanism of Differential Susceptibility of Two (Canine Lung Adenocarcinoma) Cell Lines to 5-Aminolevulinic Acid-Mediated Photodynamic Therapy. Cancers, 13(16), 4174. https://doi.org/10.3390/cancers13164174





