The Impact of the Human Papillomavirus Vaccine on High-Grade Cervical Lesions in Urban and Rural Areas: An Age–Period–Cohort Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Population
- an HPV screening examination: International Classification of Diseases 9th revision (ICD-9) code V73.81 or International Classification of Diseases 10th revision (ICD-10) code Z11.51; or
- a Papanicolaou test: ICD-9 codes V72.31, V72.32, V76.2, V76.47, 795.06, 91.46, or ICD-10 codes Z01.411, Z01.419, Z01.42, Z12.4, Z12.72, R87.614, or Current Procedural Terminology (CPT) codes 88141-88145, 88147-88148, 88150-88158, 88164-88167, 88174-88175, or Healthcare Common Procedure Coding System codes P3000-P3001, G0101, G0123-G0124, G0141, G0143-G0145, G0147-G0148, Q0091; or
- an HPV deoxyribonucleic acid (DNA) test: ICD-9 codes 795.05, 795.09, or ICD-10 codes R87.10, R87.820, or CPT codes 87620-87622, 87623-87625.
2.2. Incident CIN2+ Event Definition
- relevant diagnoses: CIN2+ tissue, non-specific CIN, high-grade squamous intraepithelial lesion cytology, CIN grade 1 tissue, low-grade squamous intraepithelial lesion cytology, and atypical squamous cells of undetermined significance; and/or
- cervical screening: HPV screening examination, Papanicolaou test, and HPV DNA test; and/or
- relevant procedures: cervical treatment procedures and cervical or vaginal biopsies.
2.3. Denominator and Rates
2.4. Joinpoint Trend Analyses
2.5. Age–Period–Cohort Analyses
3. Results
3.1. Age-Specific Trends in CIN2+ Incidence
3.2. Descriptive Age, Period, Cohort Effects
3.3. Age, Period, and Cohort Effects in Poisson Regression Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Sanjosé, S.; Serrano, B.; Tous, S.; Alejo, M.; Lloveras, B.; Quirós, B.; Clavero, O.; Vidal, A.; Ferrándiz-Pulido, C.; Pavón, M.Á.; et al. Burden of Human Papillomavirus (HPV)-Related Cancers Attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI Cancer Spectr. 2019, 2. [Google Scholar] [CrossRef] [Green Version]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of Incidence and Mortality of Cervical Cancer in 2018: A Worldwide Analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [Green Version]
- Drolet, M.; Bénard, É.; Boily, M.-C.; Ali, H.; Baandrup, L.; Bauer, H.; Beddows, S.; Brisson, J.; Brotherton, J.M.L.; Cummings, T.; et al. Population-Level Impact and Herd Effects Following Human Papillomavirus Vaccination Programmes: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2015, 15, 565–580. [Google Scholar] [CrossRef] [Green Version]
- Curtis, C.R.; Dorell, C.; Yankey, D.; Jeyarajah, J.; Chesson, H.; Saraiya, M.; Gold, R.; Dunne, E.F.; Stokley, S. Centers for Disease Control and Prevention (CDC) National Human Papillomavirus Vaccination Coverage among Adolescents Aged 13–17 Years-National Immunization Survey--Teen, United States, 2011. MMWR Suppl. 2014, 63, 61–70. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/su6302a10.htm (accessed on 14 July 2020).
- Henry, K.A.; Stroup, A.M.; Warner, E.L.; Kepka, D. Geographic Factors and Human Papillomavirus (HPV) Vaccination Initiation among Adolescent Girls in the United States. Cancer Epidemiol. Biomark. Prev. 2016, 25, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Monnat, S.M.; Rhubart, D.C.; Wallington, S.F. Differences in Human Papillomavirus Vaccination among Adolescent Girls in Metropolitan versus Non-Metropolitan Areas: Considering the Moderating Roles of Maternal Socioeconomic Status and Health Care Access. Matern. Child Health J. 2016, 20, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Walker, T.Y.; Elam-Evans, L.D.; Williams, C.L.; Fredua, B.; Yankey, D.; Markowitz, L.E.; Stokley, S. Trends in Human Papillomavirus (HPV) Vaccination Initiation among Adolescents Aged 13–17 by Metropolitan Statistical Area (MSA) Status, National Immunization Survey–Teen, 2013–2017. Hum. Vaccin. Immunother. 2020, 16, 554–561. [Google Scholar] [CrossRef]
- Lee, M.; Gerend, M.A.; Boakye, E.A. Rural–Urban Differences in Human Papillomavirus Vaccination among Young Adults in 8 U.S. States. Am. J. Prev. Med. 2020, 60, 298–299. [Google Scholar] [CrossRef]
- Elam-Evans, L.D. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years—United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Vanderpool, R.C.; Stradtman, L.R.; Brandt, H.M. Policy Opportunities to Increase HPV Vaccination in Rural Communities. Hum. Vaccin. Immunother. 2019, 15, 1527–1532. [Google Scholar] [CrossRef]
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV Vaccination Introduction Worldwide and WHO and UNICEF Estimates of National HPV Immunization Coverage 2010-2019. Prev. Med. 2021, 144, 106399. [Google Scholar] [CrossRef]
- Niccolai, L.M.; Julian, P.J.; Meek, J.I.; McBride, V.; Hadler, J.L.; Sosa, L.E. Declining Rates of High-Grade Cervical Lesions in Young Women in Connecticut, 2008-2011. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1446–1450. [Google Scholar] [CrossRef] [Green Version]
- Shing, J.Z.; Hull, P.C.; Zhu, Y.; Gargano, J.W.; Markowitz, L.E.; Cleveland, A.A.; Pemmaraju, M.; Park, I.U.; Whitney, E.; Mitchel, E.F.; et al. Trends in Anogenital Wart Incidence among Tennessee Medicaid Enrollees, 2006–2014: The Impact of Human Papillomavirus Vaccination. Papillomavirus Res. 2019, 141–149. [Google Scholar] [CrossRef]
- Smith, M.A.; Liu, B.; McIntyre, P.; Menzies, R.; Dey, A.; Canfell, K. Trends in Genital Warts by Socioeconomic Status after the Introduction of the National HPV Vaccination Program in Australia: Analysis of National Hospital Data. BMC Infect. Dis. 2016, 16. [Google Scholar] [CrossRef] [Green Version]
- Thompson, L.H.; Nugent, Z.; Blanchard, J.F.; Ens, C.; Yu, B.N. Increasing Incidence of Anogenital Warts with an Urban–Rural Divide among Males in Manitoba, Canada, 1990–2011. BMC Public Health 2016, 16. [Google Scholar] [CrossRef] [Green Version]
- US Census Bureau Geography 2010 Geographic Terms and Concepts-Core Based Statistical Areas and Related Statistical Areas. Available online: https://www.census.gov/geo/reference/gtc/gtc_cbsa.html (accessed on 3 December 2017).
- Shing, J.Z.; Griffin, M.R.; Nguyen, L.D.; Slaughter, J.C.; Mitchel, E.F.; Pemmaraju, M.; Rentuza, A.B.; Hull, P.C. Improving Cervical Precancer Surveillance: Validity of Claims-Based Prediction Models in ICD-9 and ICD-10 Eras. JNCI Cancer Spectrum. 2020. [Google Scholar] [CrossRef]
- Average Annual Percent Change (AAPC)—Joinpoint Help System 4.5.0.1. Available online: https://surveillance.cancer.gov/help/joinpoint/setting-parameters/method-and-parameters-tab/average-annual-percent-change-aapc (accessed on 4 December 2017).
- Clayton, D.; Schifflers, E. Models for Temporal Variation in Cancer Rates. II: Age-Period-Cohort Models. Stat. Med. 1987, 6, 469–481. [Google Scholar] [CrossRef]
- Carstensen, B. Age-Period-Cohort Models for the Lexis Diagram. Stat. Med. 2007, 26, 3018–3045. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 2007, 56, 1–24. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr56e312a1.htm (accessed on 11 June 2018).
- Committee on Practice Bulletins—Gynecology. ACOG Committee Opinion No. 431: Routine Pelvic Examination and Cervical Cytology Screening. Obstet. Gynecol. 2009, 113, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Saslow, D.; Solomon, D.; Lawson, H.W.; Killackey, M.; Kulasingam, S.; Cain, J.; Garcia, F.A.R.; Moriarty, A.; Waxman, A.; Wilbur, D.; et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology Screening Guidelines for the Prevention and Early Detection of Cervical Cancer. CA Cancer J. Clin. 2012, 62, 147–172. [Google Scholar] [CrossRef] [Green Version]
- Boersma, P.; Black, L.I. Human Papillomavirus Vaccination among Adults Aged 18–26, 2013–2018. NCHS Data Brief. 2020, 354, 1–8. Available online: https://www.cdc.gov/nchs/data/databriefs/db354-h.pdf (accessed on 3 December 2020).
- Sabeena, S.; Bhat, P.V.; Kamath, V.; Bhat, S.K.; Nair, S.; N, R.; Chandrabharani, K.; Arunkumar, G. Community-Based Prevalence of Genital Human Papilloma Virus (HPV) Infection: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2017, 18, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Sabik, L.M.; Dahman, B.; Vichare, A.; Bradley, C.J. Breast and Cervical Cancer Screening among Medicaid Beneficiaries: The Role of Physician Payment and Managed Care. Med. Care Res. Rev. 2020, 77, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, L.M.; Julian, P.J.; Bilinski, A.; Mehta, N.R.; Meek, J.I.; Zelterman, D.; Hadler, J.L.; Sosa, L. Geographic Poverty and Racial/Ethnic Disparities in Cervical Cancer Precursor Rates in Connecticut, 2008–2009. Am. J. Public Health 2013, 103, 156–163. [Google Scholar] [CrossRef]
- Benard, V.B.; Castle, P.E.; Jenison, S.A.; Hunt, W.C.; Kim, J.J.; Cuzick, J.; Lee, J.-H.; Du, R.; Robertson, M.; Norville, S.; et al. Population-Based Incidence Rates of Cervical Intraepithelial Neoplasia in the Human Papillomavirus Vaccine Era. JAMA Oncol. 2017, 3, 833–837. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention Human Papillomavirus Vaccine Impact Monitoring Project (HPV-IMPACT). Available online: https://www.cdc.gov/ncird/surveillance/hpvimpact/overview.html (accessed on 9 February 2019).
- World Health Organization Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/336583/9789240014107-eng.pdf (accessed on 2 July 2021).
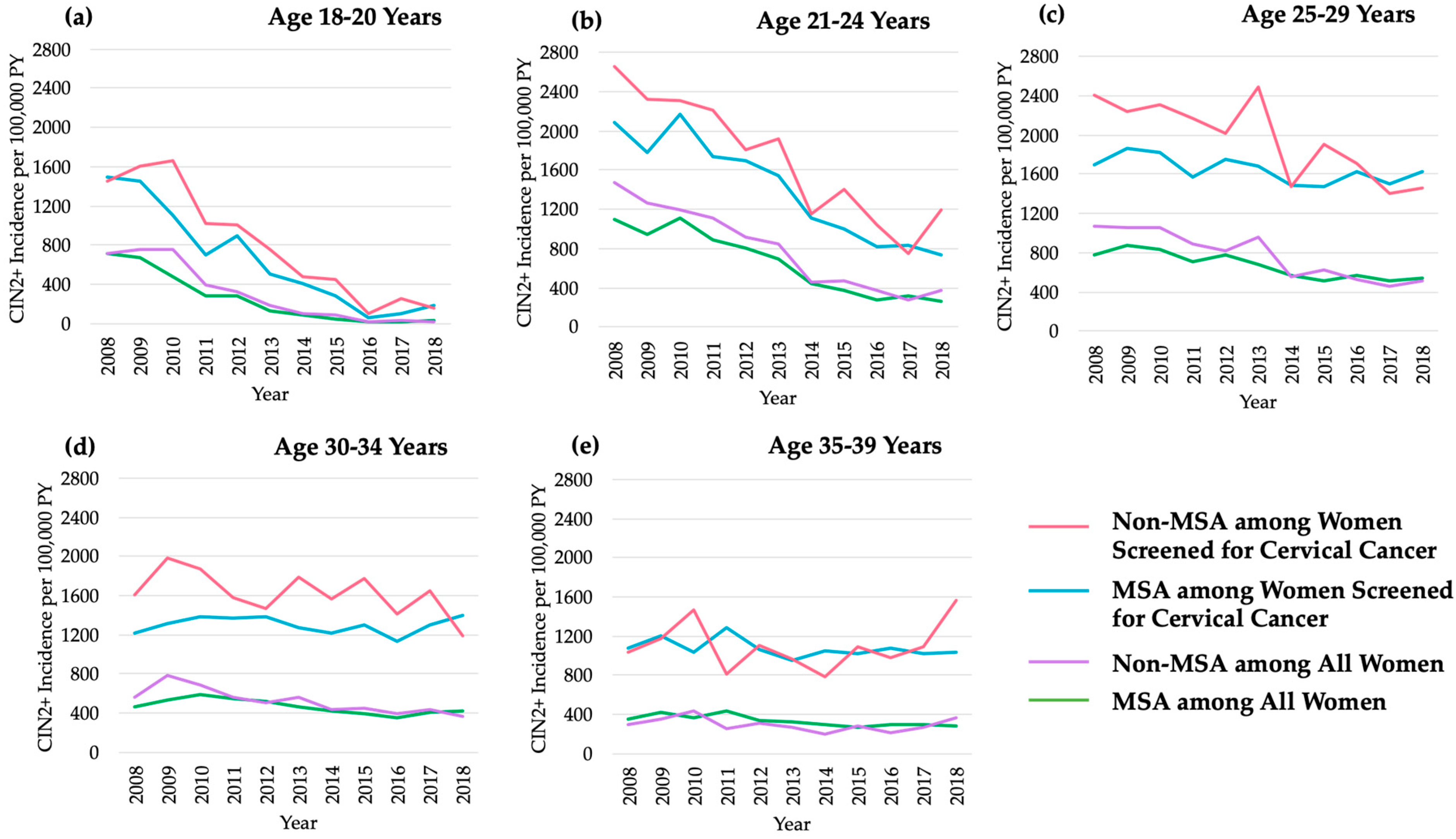
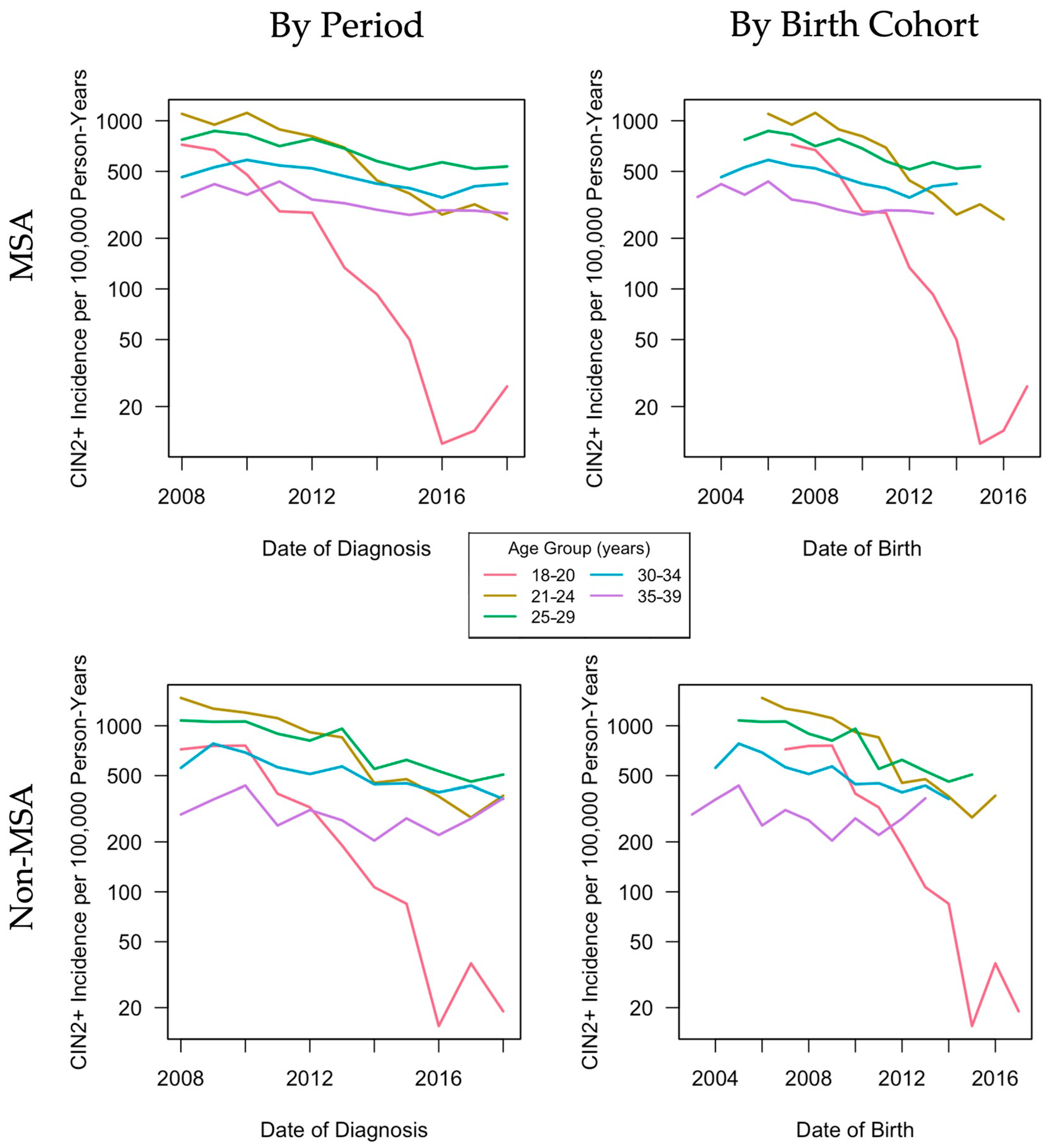


| Age (yrs) | MSA Residence | Non-MSA Residence | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 18–20 | 21–24 | 25–29 | 30–34 | 35–39 | 18–20 | 21–24 | 25–29 | 30–34 | 35–39 | |
| Among All Women | ||||||||||
| Total PY | 279,140 | 308,959 | 412,173 | 380,147 | 318,804 | 109,144 | 114,431 | 148,471 | 138,304 | 121,270 |
| CIN2+ Events | 640 | 1909 | 2664 | 1713 | 1030 | 312 | 847 | 1083 | 690 | 355 |
| Year | ||||||||||
| 2008 | 720.5 | 1098.7 | 771.7 | 462.4 | 352.2 | 720.5 | 1471.7 | 1076.1 | 557.1 | 292.7 |
| 2009 | 669.3 | 946.9 | 869.1 | 529.2 | 420.0 | 755.5 | 1267.8 | 1056.5 | 779.7 | 360.1 |
| 2010 | 477.8 | 1113.3 | 827.0 | 584.6 | 362.9 | 758.7 | 1200.4 | 1061.4 | 689.1 | 436.0 |
| 2011 | 289.1 | 887.2 | 706.2 | 542.1 | 435.4 | 390.8 | 1110.6 | 895.0 | 561.9 | 251.2 |
| 2012 | 284.0 | 808.0 | 779.5 | 521.1 | 340.0 | 323.4 | 913.9 | 813.5 | 511.4 | 311.3 |
| 2013 | 133.7 | 692.3 | 683.4 | 468.2 | 323.2 | 190.6 | 851.0 | 959.4 | 568.1 | 270.1 |
| 2014 | 92.7 | 441.5 | 574.8 | 422.4 | 295.5 | 106.7 | 452.3 | 549.0 | 443.8 | 203.7 |
| 2015 | 49.9 | 369.2 | 513.7 | 397.5 | 275.4 | 84.7 | 476.1 | 622.0 | 450.1 | 277.4 |
| 2016 | 12.0 | 276.6 | 566.1 | 349.2 | 293.4 | 15.5 | 376.1 | 532.6 | 398.3 | 220.1 |
| 2017 | 14.4 | 318.4 | 518.8 | 407.6 | 291.9 | 37.0 | 281.0 | 460.1 | 435.4 | 276.2 |
| 2018 | 26.3 | 259.2 | 534.5 | 422.5 | 281.1 | 19.0 | 380.1 | 507.5 | 363.3 | 366.6 |
| Among Women Screened for Cervical Cancer | ||||||||||
| Total PY | 75,181 | 123,842 | 153,229 | 122,514 | 88,209 | 29,593 | 44,909 | 51,356 | 40,178 | 30,174 |
| CIN2+ Events | 616 | 1798 | 2521 | 1591 | 944 | 305 | 783 | 1001 | 648 | 333 |
| Year | ||||||||||
| 2008 | 1497.6 | 2081.3 | 1702.6 | 1221.0 | 1080.7 | 1456.2 | 2660.4 | 2411.1 | 1601.1 | 1035.2 |
| 2009 | 1451.8 | 1787.5 | 1867.3 | 1317.5 | 1206.8 | 1603.8 | 2319.1 | 2239.1 | 1979.6 | 1170.1 |
| 2010 | 1106.1 | 2170.3 | 1823.2 | 1377.9 | 1033.4 | 1663.3 | 2313.6 | 2315.5 | 1872.1 | 1462.7 |
| 2011 | 696.1 | 1734.7 | 1569.5 | 1373.9 | 1281.5 | 1015.4 | 2216.6 | 2169.7 | 1583.3 | 815.7 |
| 2012 | 892.1 | 1695.8 | 1758.4 | 1385.4 | 1060.7 | 1011.2 | 1814.1 | 2023.1 | 1470.2 | 1104.3 |
| 2013 | 506.9 | 1547.3 | 1683.0 | 1277.9 | 955.4 | 760.8 | 1914.8 | 2497.7 | 1787.3 | 959.9 |
| 2014 | 401.7 | 1118.5 | 1495.3 | 1223.7 | 1054.9 | 472.2 | 1151.0 | 1475.1 | 1562.5 | 782.5 |
| 2015 | 288.5 | 999.2 | 1474.9 | 1300.1 | 1016.9 | 452.7 | 1409.1 | 1899.6 | 1770.4 | 1088.4 |
| 2016 | 58.5 | 822.7 | 1626.2 | 1137.2 | 1079.2 | 107.4 | 1046.9 | 1718.0 | 1418.0 | 980.7 |
| 2017 | 99.9 | 835.8 | 1500.3 | 1298.8 | 1016.1 | 258.1 | 748.3 | 1409.6 | 1651.7 | 1089.7 |
| 2018 | 178.8 | 739.8 | 1627.1 | 1393.0 | 1041.0 | 159.1 | 1193.5 | 1453.7 | 1191.6 | 1561.0 |
| MSA Residence | Non-MSA Residence | |||
|---|---|---|---|---|
| Age, Years | AAPC 1 | 95% CI | AAPC 1 | 95% CI |
| Among All Women | ||||
| 18–20 | −30.4 * | −35.4, −25.0 | −30.9 * | −36.8, −24.5 |
| 21–24 | −14.8 * | −18.1, −11.3 | −15.1 * | −17.9, −12.2 |
| 25–29 | −5.3 * | −7.1, −3.6 | −8.8 * | −11.3, −6.3 |
| 30–34 | −0.8 | −2.6, 1.1 | −6.2 * | −8.5, −3.8 |
| 35–39 | −3.9 * | −5.8, −1.9 | −1.5 | −6.1, 3.2 |
| Among Women Screened for Cervical Cancer | ||||
| 18–20 | −21.1 * | −26.1, −15.8 | −19.8 * | −26.5, −12.4 |
| 21–24 | −10.4 * | −13.2, −7.6 | −10.0 * | −12.7, −7.1 |
| 25–29 | −2.6 * | −2.9, −0.2 | −4.9 * | −7.5, −2.3 |
| 30–34 | 1.3 | −2.4, 5.2 | −2.5 | −5.0, 0.1 |
| 35–39 | −1.1 | −2.7, 0.5 | 1.1 | −3.4, 5.8 |
| MSA Residence | Non-MSA Residence | |||||||
|---|---|---|---|---|---|---|---|---|
| Inflection Year | Time Period | APC 1 | 95% CI | Inflection Year | Time Period | APC 1 | 95% CI | |
| Among All Women | ||||||||
| Age, Years | ||||||||
| 18–20 | -- | 2008–2018 | −30.4 * | −35.4, −25.0 | 2010 | 2008–2010 | 0.2 | −29.4, 42.4 |
| 2010–2018 | −37.0 * | −43.4, −29.9 | ||||||
| 21–24 | -- | 2008–2018 | −14.8 * | −18.1, −11.3 | -- | 2008–2018 | −15.1 * | −17.9, −12.2 |
| 25–29 | -- | 2008–2018 | −5.3 * | −7.1, −3.6 | -- | 2008–2018 | −8.8 * | −11.3, −6.3 |
| 30–34 | 2010 | 2008–2010 | 13.3 * | 1.5, 26.4 | -- | 2008–2018 | −6.2 * | -8.5, −3.8 |
| 2016 | 2010–2016 | −8.0 * | −9.9, −6.1 | |||||
| 2016–2018 | 9.0 | −0.2, 19.2 | ||||||
| 35–39 | -- | 2008–2018 | −3.9 * | −5.8, −1.9 | -- | 2008–2018 | −1.5 | −6.1, 3.2 |
| Among Women Screened for Cervical Cancer | ||||||||
| Age, Years | ||||||||
| 18–20 | -- | 2008–2018 | −21.1 * | −26.1, −15.8 | 2010 | 2008–2010 | 4.5 | −26.2, 47.8 |
| 2010–2018 | −24.9 * | −32.5, −16.5 | ||||||
| 21–24 | -- | 2008–2018 | −10.4 * | −13.2, −7.6 | -- | 2008–2018 | −10.0 * | −12.7, −7.1 |
| 25–29 | -- | 2008–2018 | −2.6 * | −2.9, −0.2 | -- | 2008–2018 | −4.9 * | −7.5, −2.3 |
| 30–34 | 2010 | 2008–2010 | 7.5 | −13.3, 33.3 | -- | 2008–2018 | −2.5 | −5.0, 0.1 |
| 2016 | 2010–2016 | −2.8 | −6.7, 1.2 | |||||
| 2016–2018 | 8.4 | −9.4, 29.7 | ||||||
| 35–39 | -- | 2008–2016 | −1.1 | −2.7, 0.5 | -- | 2008–2018 | 1.1 | −3.4, 5.8 |
| Goodness-of-Fit | Model Comparison | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Residual df | Residual Deviance | p-Value | Model Comparison | Interpretation | Change in df | Change in Deviance | p-Value | EAPC (95% CI) | |
| Among All Women | |||||||||
| MSA | 0.90(0.90, 0.91) | ||||||||
| 1. Age | 238 | 2006.75 | -- | -- | -- | -- | |||
| 2. Age–Drift | 237 | 1295.52 | <0.001 | 2 versus 1 | Trend (drift) | 1 | 711.23 | <0.001 | |
| 3. Age–Cohort | 234 | 733.25 | <0.001 | 3 versus 2 | Nonlinear cohort effect | 3 | 562.27 | <0.001 | |
| 4. Age–Period | 234 | 1244.25 | <0.001 | 4 versus 2 | Nonlinear period effect | 3 | 51.27 | <0.001 | |
| 5. Age–Period–Cohort | 231 | 662.64 | <0.001 | 5 versus 3 | Period effect adjusted for cohort | 3 | 70.60 | <0.001 | |
| 5 versus 4 | Cohort effect adjusted for period | 3 | 581.60 | <0.001 | |||||
| Non-MSA | 0.89(0.88, 0.90) | ||||||||
| 1. Age | 238 | 1080.50 | -- | -- | -- | -- | |||
| 2. Age–Drift | 237 | 639.10 | <0.001 | 2 versus 1 | Trend (drift) | 1 | 441.40 | <0.001 | |
| 3. Age–Cohort | 234 | 435.57 | <0.001 | 3 versus 2 | Nonlinear cohort effect | 3 | 203.53 | <0.001 | |
| 4. Age–Period | 234 | 619.29 | <0.001 | 4 versus 2 | Nonlinear period effect | 3 | 19.81 | <0.001 | |
| 5. Age–Period–Cohort | 231 | 410.92 | <0.001 | 5 versus 3 | Period effect adjusted for cohort | 3 | 24.66 | <0.001 | |
| 5 versus 4 | Cohort effect adjusted for period | 3 | 208.37 | <0.001 | |||||
| Among Women Screened for Cervical Cancer | |||||||||
| MSA | 0.96(0.95, 0.96) | ||||||||
| 1. Age | 238 | 891.14 | -- | -- | -- | -- | |||
| 2. Age–Drift | 237 | 729.89 | <0.001 | 2 versus 1 | Trend (drift) | 1 | 161.25 | <0.001 | |
| 3. Age–Cohort | 234 | 463.58 | <0.001 | 3 versus 2 | Nonlinear cohort effect | 3 | 266.30 | <0.001 | |
| 4. Age–Period | 234 | 714.29 | <0.001 | 4 versus 2 | Nonlinear period effect | 3 | 15.60 | 0.001 | |
| 5. Age–Period–Cohort | 231 | 436.61 | <0.001 | 5 versus 3 | Period effect adjusted for cohort | 3 | 26.97 | <0.001 | |
| 5 versus 4 | Cohort effect adjusted for period | 3 | 277.68 | <0.001 | |||||
| Non-MSA | 0.94(0.93, 0.96) | ||||||||
| 1. Age | 238 | 499.55 | -- | -- | -- | -- | |||
| 2. Age–Drift | 237 | 383.80 | <0.001 | 2 versus 1 | Trend (drift) | 1 | 1115.75 | <0.001 | |
| 3. Age–Cohort | 234 | 301.08 | 0.002 | 3 versus 2 | Nonlinear cohort effect | 3 | 82.72 | <0.001 | |
| 4. Age–Period | 234 | 380.60 | <0.001 | 4 versus 2 | Nonlinear period effect | 3 | 3.20 | 0.362 | |
| 5. Age–Period–Cohort | 231 | 296.86 | 0.002 | 5 versus 3 | Period effect adjusted for cohort | 3 | 4.21 | 0.239 | |
| 5 versus 4 | Cohort effect adjusted for period | 3 | 83.73 | <0.001 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shing, J.Z.; Beeghly-Fadiel, A.; Griffin, M.R.; Chang, R.S.; Sudenga, S.L.; Slaughter, J.C.; Pemmaraju, M.; Mitchel, E.F.; Hull, P.C. The Impact of the Human Papillomavirus Vaccine on High-Grade Cervical Lesions in Urban and Rural Areas: An Age–Period–Cohort Analysis. Cancers 2021, 13, 4215. https://doi.org/10.3390/cancers13164215
Shing JZ, Beeghly-Fadiel A, Griffin MR, Chang RS, Sudenga SL, Slaughter JC, Pemmaraju M, Mitchel EF, Hull PC. The Impact of the Human Papillomavirus Vaccine on High-Grade Cervical Lesions in Urban and Rural Areas: An Age–Period–Cohort Analysis. Cancers. 2021; 13(16):4215. https://doi.org/10.3390/cancers13164215
Chicago/Turabian StyleShing, Jaimie Z., Alicia Beeghly-Fadiel, Marie R. Griffin, Rachel S. Chang, Staci L. Sudenga, James C. Slaughter, Manideepthi Pemmaraju, Edward F. Mitchel, and Pamela C. Hull. 2021. "The Impact of the Human Papillomavirus Vaccine on High-Grade Cervical Lesions in Urban and Rural Areas: An Age–Period–Cohort Analysis" Cancers 13, no. 16: 4215. https://doi.org/10.3390/cancers13164215






