Deterioration of Intended Target Volume Radiation Dose Due to Anatomical Changes in Patients with Head-and-Neck Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
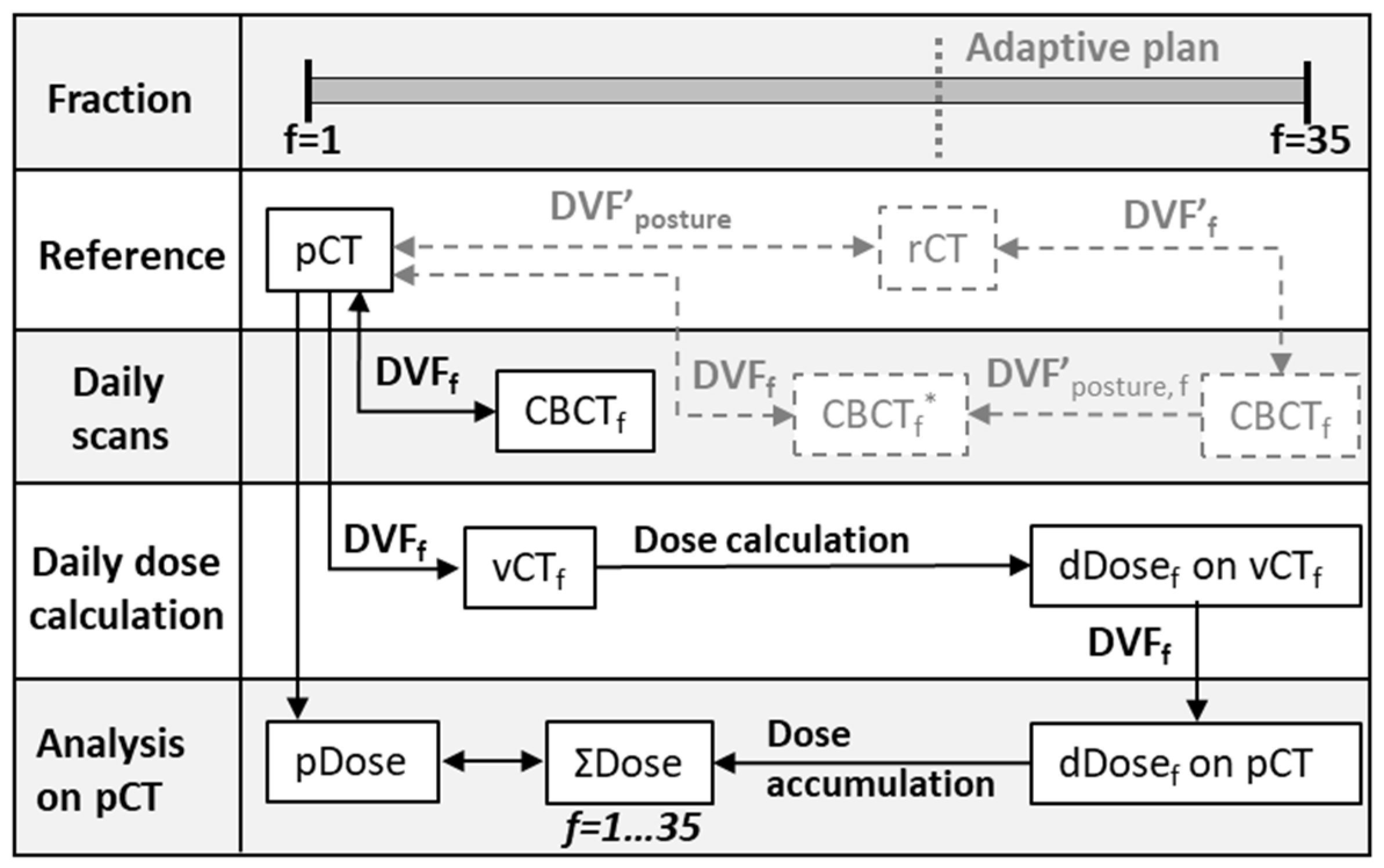
2. Materials and Methods
Statistical Analysis
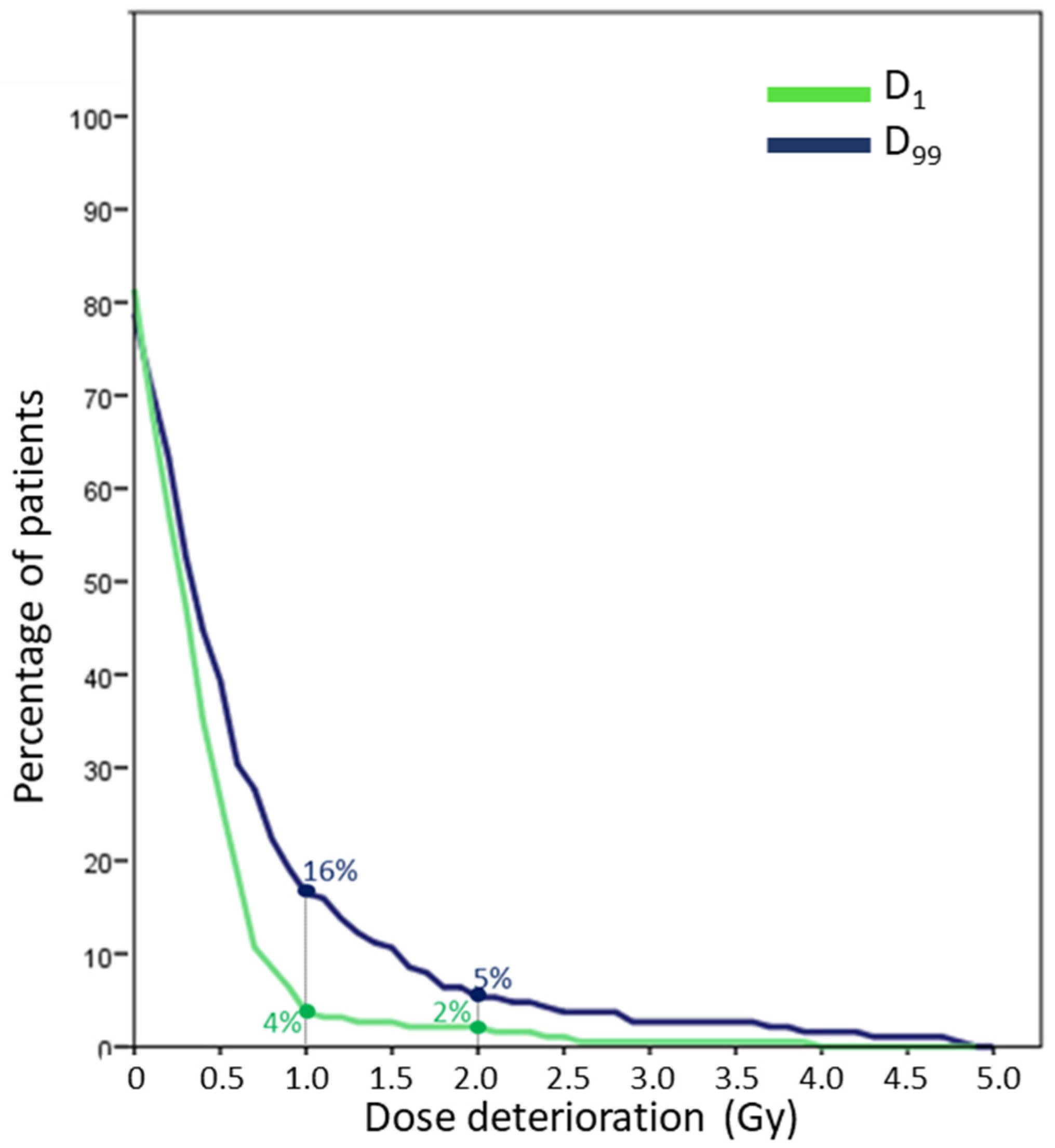
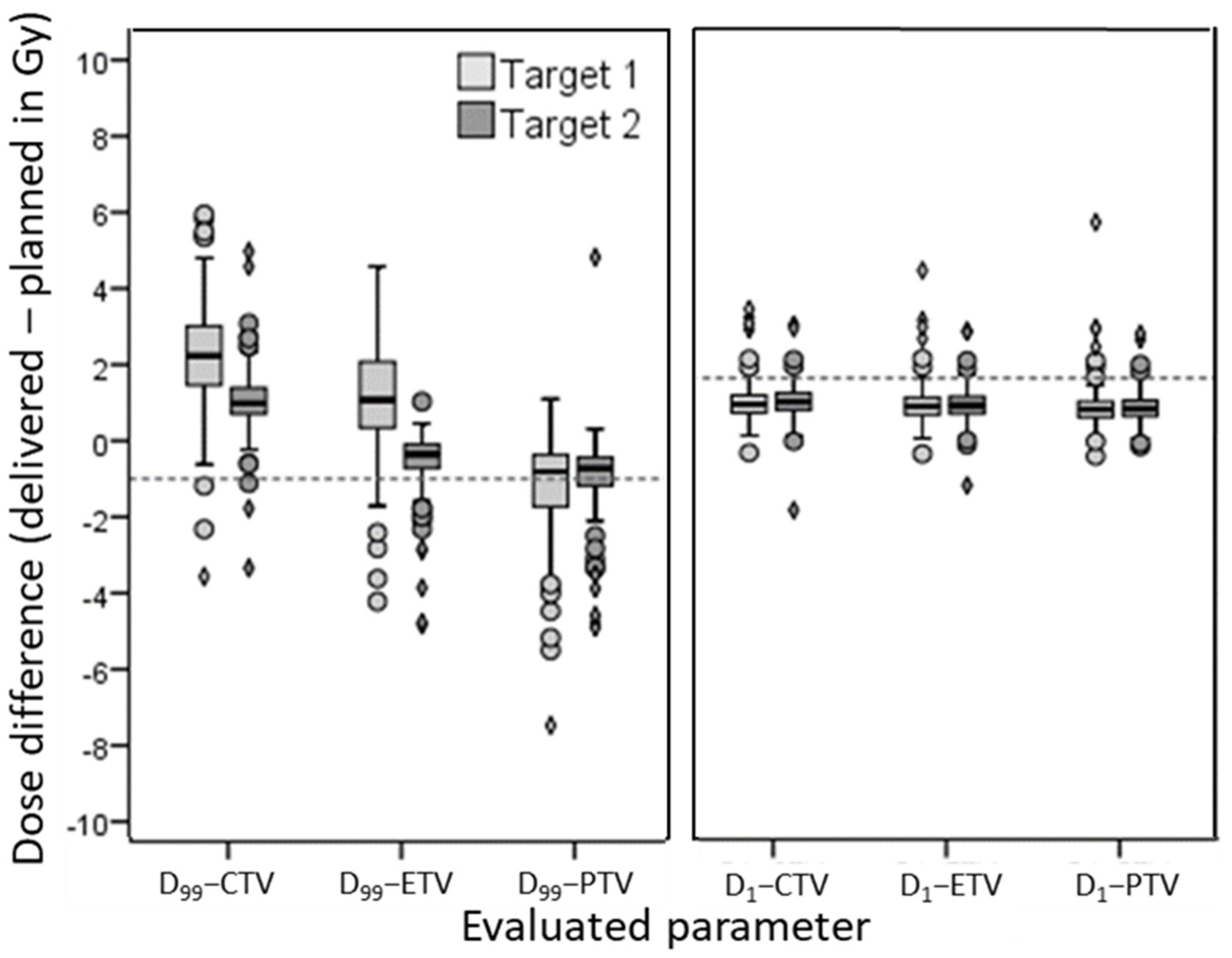
3. Results
3.1. Minimum Dose (D99)
3.2. Maximum Dose (D1)
3.3. Clinical Selected Patients for ART
3.4. Reference and Evaluated Volumes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gregoire, V.; Jeraj, R.; Lee, J.A.; O’Sullivan, B. Radiotherapy for head and neck tumors in 2012 and beyond: Conformal, tailored, and adaptive? Lancet Oncol. 2012, 13, e292–e300. [Google Scholar] [CrossRef]
- Ahn, P.H.; Chen, C.C.; Ahn, A.I.; Hong, L.; Scripes, P.G.; Shen, J.; Lee, C.-C.; Miller, E.; Kalnicki, S.; Garget, M.K. Adaptive planning in intensity-modulated radiotherapy for head and neck cancers: Single institution experience and clinical implications. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 677–685. [Google Scholar] [CrossRef]
- Bhide, S.A.; Davies, M.; Burke, K.; McNair, H.A.; Hansen, V.; Barbachano, Y.; El-Hariry, I.A.; Newbold, K.; Harrington, K.J.; Nutting, C.M. Weekly volume and dosimetric changes during chemoradiotherapy with intensity-modulated radiotherapy for head and neck cancer: A prospective observational study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1360–1368. [Google Scholar] [CrossRef]
- Castelli, J.; Simon, A.; Rigaud, B.; Chajon, E.; Thariat, J.; Benezery, K.; Vauleon, E.; Jegoux, F.; Henry, O.; Lafond, C.; et al. Adaptive radiotherapy in head and neck cancer is required to avoid tumor underdose. Acta Oncol. 2018, 57, 1267–1270. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Fei, Z.; Chen, L.; Bai, P.; Lin, X.; Pan, J. Will weight loss cause significant dosimetric changes of target volumes and organs at risk in nasopharyngeal carcinoma treated with intensity-modulated radiotherapy? Med. Dosim. 2014, 39, 34–37. [Google Scholar] [CrossRef]
- Hansen, E.K.; Bucci, M.K.; Quivey, J.M.; Weinberg, V.; Xia, P. Repeat CT imaging and replanning during the course of IMRT for head and neck cancer. Int. J. Radiat. Oncol. Biol. 2006, 64, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.L.; Garden, A.S.; Shah, S.J.; Chronowski, G.; Seipal, S.; Rosenthal, D.I.; Chen, Y.; Zhang, Y.; Zhang, L.; Wong, P.F.; et al. Adaptive radiotherapy for head and neck cancer—Dosimetric results from a prospective clinical trial. Radiother. Oncol. 2013, 106, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chi, Y.; Chen, P.Y.; Krauss, D.J.; Yan, D.; Martinez, A. Adaptive replanning strategies accounting for shrinkage in head and neck IMRT. Int. J. Radiat. Oncol. Bio. Phys. 2009, 75, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.; Thomas, C.; Michaelidou, A.; James, D.; Lynn, R.; Lei, M.; Guerrero Urbano, T. Co-registration of cone beam CT and planning CT in head and neck IMRT dose estimation: A feasible adaptive radiotherapy strategy. Br. J. Radiol. 2014, 87, 20130532. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, M.; Cao, J.; Luo, J.W.; Xu, G.Z.; Gao, L.; Yi, J.; Huang, X.; Xiao, J.; Li, S.; et al. Dosimetric variations of target volumes and organs at risk in nasopharyngeal carcinoma intensity modulated radiotherapy. Br. J. Radiol. 2012, 85, e506–e513.3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Height, R.; Khoo, V.; Lawford, C.; Cox, J.; Joon, D.L.; Rolfo, A.; Wada, M. The dosimetric consequences of anatomical changes in head and neck radiotherapy patients. J. Med. Imaging Radiat. Oncol. 2010, 54, 497–504. [Google Scholar] [CrossRef]
- Marzi, S.; Pinnaro, P.; D’Alessio, D.; Strigari, L.; Bruzzaniti, V.; Giordano, C.; Giovinazzo, G.; Marucci, L. Anatomical and dose changes of gross tumour volume and parotid glands for head and neck cancer patients during intensity-modulated radiotherapy: Effect on the probability of xerostomia incidence. Clin. Oncol. 2012, 24, e54–e62. [Google Scholar] [CrossRef]
- Van Beek, S.; van Kranen, S.; Mencarelli, A.; Remeijer, P.; Rasch, C.; van Herk, M.; Sonke, J.J. First clinical experience with a multiple region of interest registration and correction method in radiotherapy of head and neck cancer patients. Radiother. Oncol. 2010, 94, 213–217. [Google Scholar] [CrossRef]
- Van Kranen, S.; Mencarelli, A.; van Beek, S.; Rasch, C.; van Herk, M.; Sonke, J.J. Adaptive radiotherapy with an average anatomy model: Evaluation and quantification of residual deformations in head and neck cancer patients. Radiother. Oncol. 2013, 109, 463–468. [Google Scholar] [CrossRef]
- Rueckert, D.; Sonoda, L.I.; Hayes, C.; Hill, D.L.; Leach, M.O.; Hawkes, D.J. Nonrigid registration using free-form deformations: Application to breast MR images. IEEE Trans. Med. Imaging 1999, 18, 712–721. [Google Scholar] [CrossRef]
- Mandrekar, J. Receiver operating characteristic curve in diagnostic test assessment. Biostat. Clin. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olteanu, L.A.M.; Duprez, F.; De Neve, W.; Berwouts, D.; Vercauteren, T.; Bauters, W.; Deron, P.; Huvenne, W.; Bonte, K.; Goethals, I.; et al. Late mucosal ulcers in dose-escalated adaptive dose-painting treatments for head and neck cancer. Acta Oncol. 2018, 57, 262–268. [Google Scholar] [CrossRef]
- Yang, H.; Hu, W.; Wang, W.; Chen, P.; Ding, W.; Luo, W. Replanning during intensity modulated radiation therapy improved quality of life in patients with nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, e47–e54. [Google Scholar] [CrossRef]
- Chen, A.M.; Daly, M.E.; Cui, J.; Mathai, M.; Benedict, S.; Purdy, J.A. Clinical outcomes among patients with head and neck cancer treated by intensity-modulated radiotherapy with and without replanning. Head Neck 2014, 36, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Kataria, T.; Gupta, D.; Goyal, S.; Bisht, S.S.; Basu, T.; Abhishek, A.; Narang, K.; Banerjee, S.; Nasreen, S.; Sambasivamet, S.; et al. Clinical outcomes of adaptive radiotherapy in head and neck cancers. Br. J. Radiol. 2016, 89, 20160085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, D.L.; Garden, A.S.; Thomas, J.; Chen, Y.; Zhang, Y.; Lewin, J.; Chambers, M.S.; Dong, L. Adaptive radiotherapy for head and neck cancer: Initial clinical outcomes from a prospective trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 986–993. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.; Owen, R.; Harden, F.; Mengersen, K.; Oestreich, K.; Houghton, W.; Poulsen, M.; Harris, S.; Lin, C.; Porceddu, S. Predicting the need for adaptive radiotherapy in head and neck cancer. Radiother. Oncol. 2015, 16, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickress, J.R.; Battista, J.; Barnett, R.; Yartsev, S. Online daily assessment of dose change in head and neck radiotherapy without dose-recalculation. J. Appl. Clin. Med. Phys. 2018, 19, 659–665. [Google Scholar] [CrossRef]
- Mencarelli, A.; van Kranen, S.R.; Hamming-Vrieze, O.; van Beek, S.; Rasch, C.R.; van Herk, M.; Sonke, J.J. Deformable image registration for adaptive radiation therapy of head and neck cancer: Accuracy and precision in the presence of tumor changes. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 680–687. [Google Scholar] [CrossRef]
- Qin, A.; Lonascu, D.; Liang, J.; Han, X.; O’Connell, N.; Yan, D. The evaluation of hybrid biomechanical deformable registration method on a multistage physical phantom with reproducible deformation. Radiat. Oncol. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Zhong, H.; Chetty, I.J. Caution must be exercised when performing deformable dose accumulation for tumors undergoing mass changes during fractionated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, J.A.; Lambin, P.; de Ruysscher, D.; Widder, J.; Bos, M.; Verheij, M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: The model based approach. Radiother. Oncol. 2013, 107, 267–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bibault, J.E.; Zapletal, E.; Rance, B.; Giraud, P.; Burgun, A. Labeling for Big Data in radiation oncology: The Radiation Oncology Structures. PLoS ONE 2018, 13, e0191263. [Google Scholar] [CrossRef] [PubMed]

| Patient and Treatment Characteristics (n = 188) | |
|---|---|
| Age (years, (SD)) | 61.1 (10.8) |
| Gender (n, (%)) | |
| Male | 133 (71) |
| Female | 55 (29) |
| Weight (kg, (SD)) | 76.3 (17.9) |
| BMI at start (kg/m2, (SD)) | 24.9 (5.5) |
| Location (n, (%)) | |
| Nasopharynx | 31 (16) |
| Oroph/oral cavity | 86 (46) |
| Larynx/Hypoph | 43 (23) |
| Other | 28 (15) |
| Pathology (n, (%)) | |
| Squamous cell | 174 (93) |
| Other | 14 (7) |
| HPV | |
| No | 64 (34) |
| Yes | 47 (25) |
| Unknown | 77 (41) |
| T stage (n, (%)) * | |
| Tx/T1/T2 | 109 (58) |
| T3/T4 | 79 (42) |
| N stage (n, (%)) * | |
| N0 | 66 (35) |
| N1-3 | 122 (65) |
| N side (n, (%)) | |
| Unilateral | 66 (54) |
| Bilateral | 56 (46) |
| N largest size (cm, (SD)) | 2.3 (1.6) |
| N largest size (n, (%)) | |
| <3 cm | 82 (67) |
| ≥3 cm | 40 (33) |
| Dahanca (n, (%)) | |
| No | 94 (50) |
| Yes | 94 (50) |
| Concurrent chemotherapy (n, (%)) | |
| No | 96 (51) |
| Yes | 92 (49) |
| PTV margin (n, (%)) | |
| 5 mm | 90 (48) |
| 3 mm | 98 (52) |
| Tube feeding (n, (%)) | |
| No | 124 (66) |
| Yes | 64 (34) |
| Clinical ART (n, (%)) | |
| No | 164 (87) |
| Yes | 24 (13) |
| Evaluated | Significant | Category | OR | 95% CI | p | AUC | 95% CI |
|---|---|---|---|---|---|---|---|
| D99_TV1 (15 out of 188 had a threshold violation) | Weight ∆ week 2 | <−2 kg | 0.72 | 0.06–8.36 | 0.796 | ||
| −2 to −1 kg | 0.72 | 0.10–5.35 | 0.752 | ||||
| −1 to 0 kg | 1.00 | ||||||
| >0 kg | 6.46 | 1.27–32.81 | 0.024 | 0.74 | 0.58–0.89 | ||
| Weight ∆ week 3 | <−3 kg | 0.73 | 0.06–8.34 | 0.800 | |||
| −3 to −1 kg | 3.40 | 0.62–18.30 | 0.158 | ||||
| −1 to 0 kg | 1.00 | ||||||
| >0 kg | 5.40 | 1.02–28.44 | 0.047 | 0.70 | 0.56–0.84 | ||
| Weight ∆ week 4 | <−4 kg | 1.06 | 0.14–7.90 | 0.958 | |||
| −4 to −2 kg | 1.54 | 0.24–9.75 | 0.646 | ||||
| −2 to 0 kg | 1.00 | ||||||
| >0 kg | 3.40 | 0.68–16.87 | 0.138 | ||||
| PTV margin | 3 mm | n.a. * | |||||
| 5 mm | |||||||
| D99_TV2 (23 out of 177 had a threshold violation) | Weight | Per kg | 1.03 | 1.01–1.06 | 0.005 | 0.67 | 0.55–0.79 |
| BMI | Per kg/m2 | 1.10 | 1.02–1.18 | 0.010 | 0.64 | 0.52–0.76 | |
| Weight ∆ week 4 | <−4 kg | 1.50 | 0.43–5.22 | 0.530 | |||
| −4 to −2 kg | 1.40 | 0.40–4.88 | 0.597 | ||||
| −2 to 0 kg | 1.00 | ||||||
| >0 kg | 0.41 | 0.09–1.83 | 0.242 | ||||
| PTV margin | 3 mm | 7.40 | 2.11–25.92 | 0.002 | 0.70 | 0.60–0.80 | |
| 5 mm | 1.00 | ||||||
| D1_TV1 (7 out of 188 had a threshold violation) | Age | Per year | 1.06 | 0.98–1.14 | 0.144 | ||
| Weight | Per kg | 1.04 | 1.00–1.08 | 0.037 | 0.74 | 0.59–0.89 | |
| BMI | Per kg/m2 | 1.11 | 0.99–1.24 | 0.056 | |||
| T-category | T1/2 | 1.00 | |||||
| T3/4 | 8.88 | 1.05–75.28 | 0.045 | 0.73 | 0.56–0.89 | ||
| D1_TV2 (7 out of 177 had a threshold violation) | Age | Per year | 1.06 | 0.98–1.14 | 0.132 | ||
| Weight | Per kg | 1.04 | 1.00–1.08 | 0.040 | 0.74 | 0.59–0.89 | |
| BMI | Per kg/m2 | 1.11 | 0.99–1.24 | 0.056 | |||
| T-category | T1/2 | 1.00 | |||||
| T3/4 | 8.78 | 1.03–74.57 | 0.046 | 0.73 | 0.56–0.89 |
| Threshold 1 Gy | Dosimetrical Yes | Dosimetrical No | Total |
|---|---|---|---|
| Clinical Yes | 8 (4%) | 16 (9%) | 24 (13%) |
| Clinical No | 28 (15%) | 136 (72%) | 164 (87%) |
| Total | 36 (19%) | 152 (81%) | 188 (100%) |
| Threshold 2 Gy | |||
| Clinical Yes | 4 (2%) | 20 (11%) | 24 (13%) |
| Clinical No | 10 (5%) | 154 (82%) | 164 (87%) |
| Total | 14 (7%) | 174 (93%) | 188 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamming-Vrieze, O.; van Kranen, S.; Walraven, I.; Navran, A.; Al-Mamgani, A.; Tesselaar, M.; van den Brekel, M.; Sonke, J.-J. Deterioration of Intended Target Volume Radiation Dose Due to Anatomical Changes in Patients with Head-and-Neck Cancer. Cancers 2021, 13, 4253. https://doi.org/10.3390/cancers13174253
Hamming-Vrieze O, van Kranen S, Walraven I, Navran A, Al-Mamgani A, Tesselaar M, van den Brekel M, Sonke J-J. Deterioration of Intended Target Volume Radiation Dose Due to Anatomical Changes in Patients with Head-and-Neck Cancer. Cancers. 2021; 13(17):4253. https://doi.org/10.3390/cancers13174253
Chicago/Turabian StyleHamming-Vrieze, Olga, Simon van Kranen, Iris Walraven, Arash Navran, Abrahim Al-Mamgani, Margot Tesselaar, Michiel van den Brekel, and Jan-Jakob Sonke. 2021. "Deterioration of Intended Target Volume Radiation Dose Due to Anatomical Changes in Patients with Head-and-Neck Cancer" Cancers 13, no. 17: 4253. https://doi.org/10.3390/cancers13174253






