CD47-SIRPα Checkpoint Inhibition Enhances Neutrophil-Mediated Killing of Dinutuximab-Opsonized Neuroblastoma Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Neutrophil Gene Signature Enrichment in Neuroblastoma Samples
2.2. mRNA Data Analysis for CD47 Expression
2.3. Neutrophil Isolation and Stimulation
2.4. Cell Culture
2.5. Generation of Genetically Modified Cells
2.6. Flow Cytometry Staining
2.7. ADCC
2.8. Statistical Analysis
3. Results
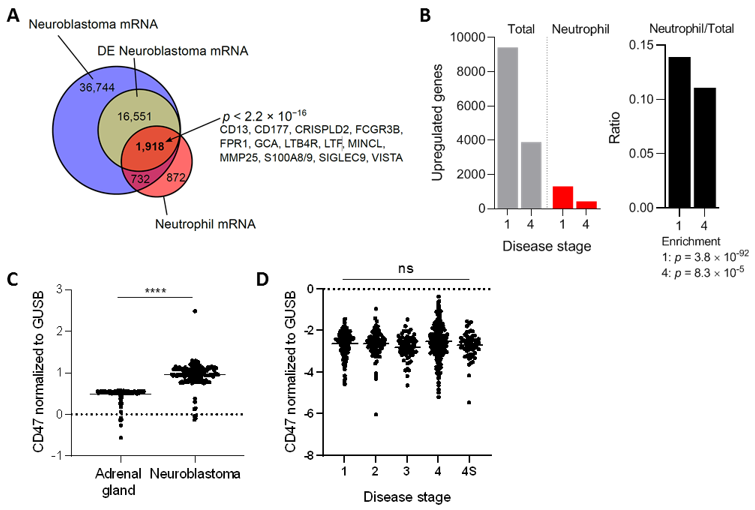
3.1. Neuroblastoma Tumors Contain Neutrophil mRNA Signatures and Upregulate CD47 Expression
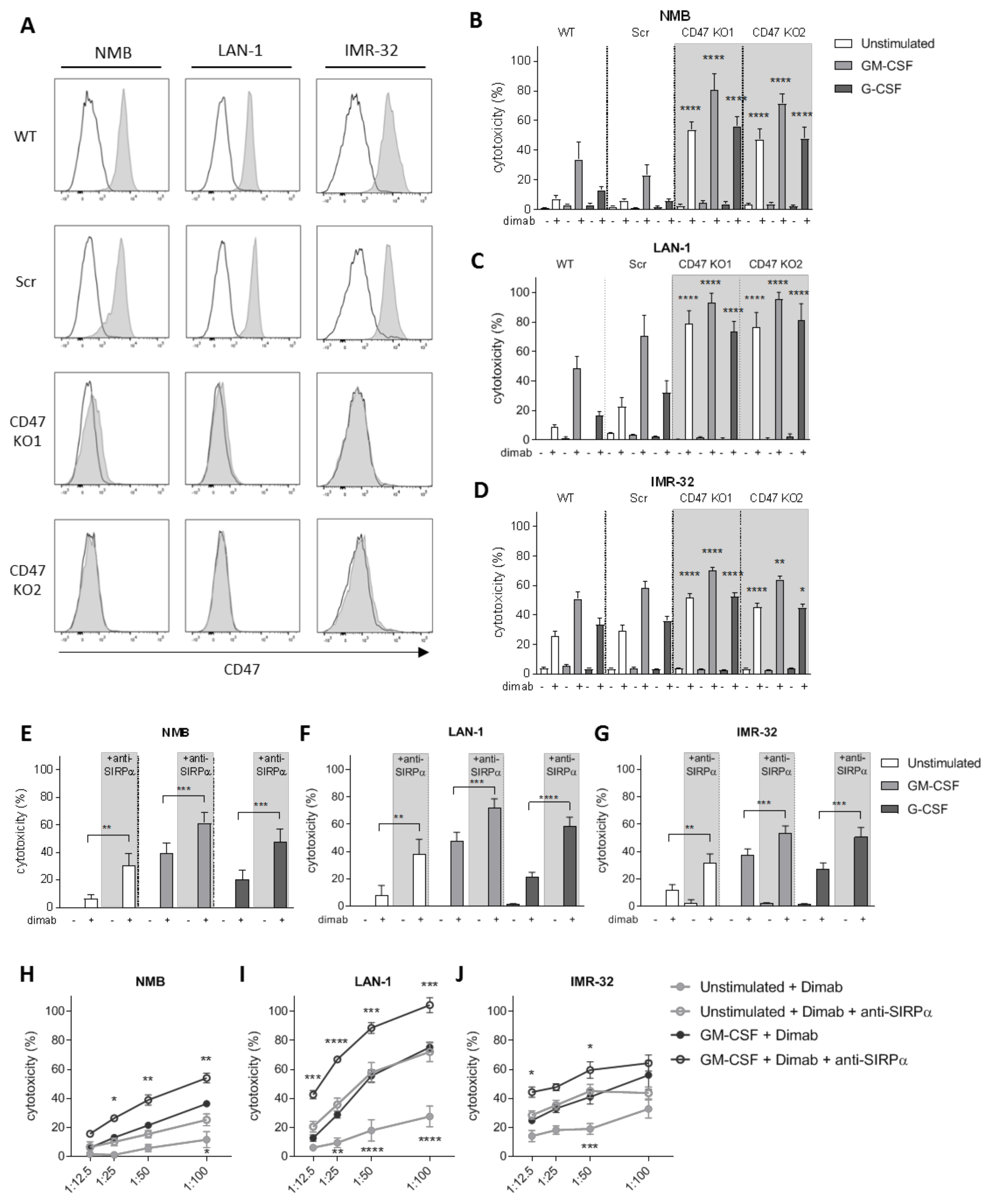
3.2. CD47-SIRPα Disruption Potentiates Neutrophil-Dependent Antitumor Activity towards Neuroblastoma Cells
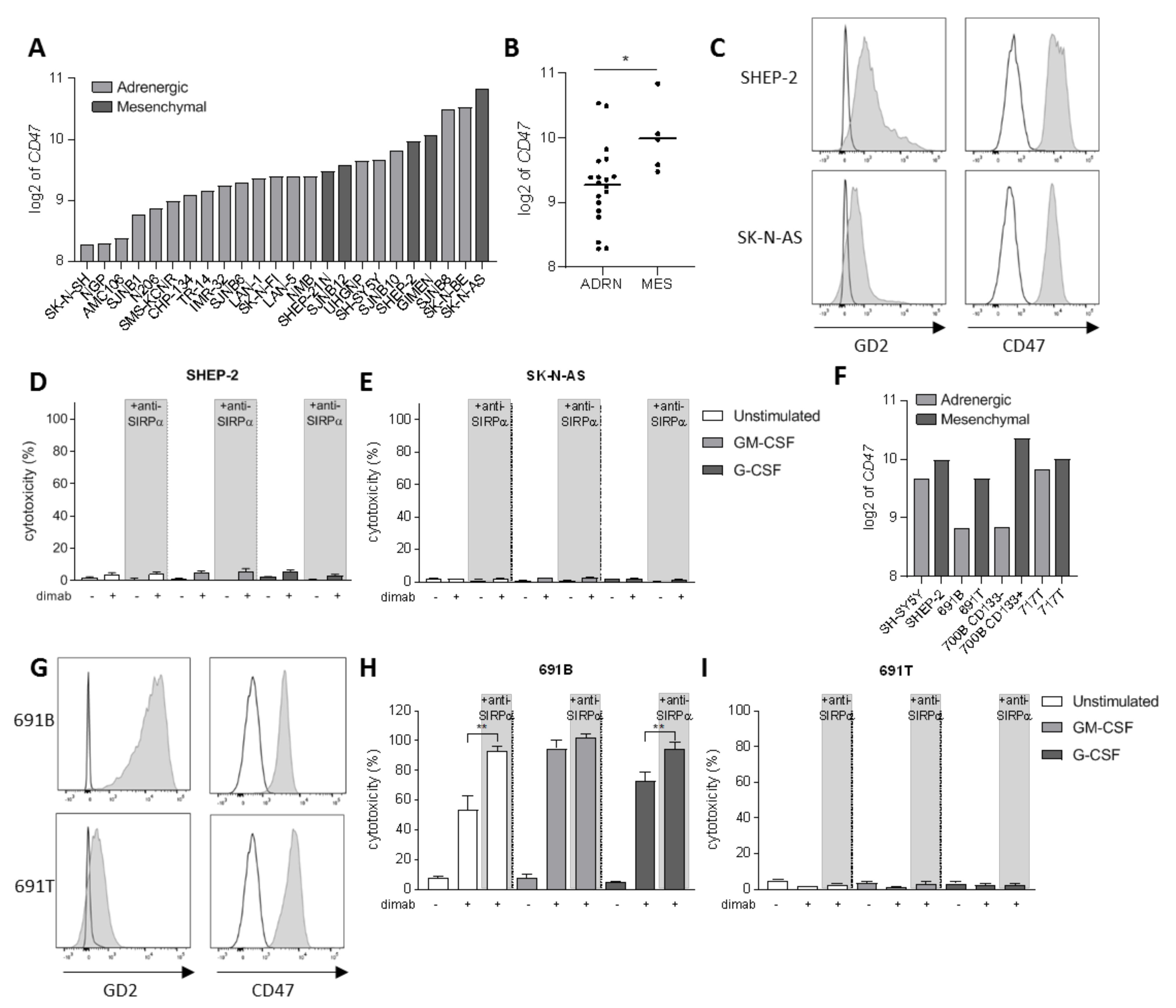
3.3. Tumor Cell Opsonization Determines Anti-SIRPα Treatment Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCC | antibody-dependent cellular cytotoxicity |
| ADRN | adrenergic |
| ANOVA | analysis of variance |
| CD | cluster of differentiation |
| CPM | counts per minute |
| DE | differentially expressed |
| Dimab | dinutuximab |
| DMEM | Dulbecco’s modified Eagle medium |
| FACS | fluorescence-activated cell sorting |
| FCS | fetal calf serum |
| G-CSF | granulocyte colony-stimulating factor |
| GD2 | disialoganglioside |
| GEO | gene expression omnibus |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GTEx | Genotype-Tissue Expression project |
| IFNγ | interferon-gamma |
| IMDM | Iscove’s modified Dulbecco’s medium |
| KO | knock out |
| MES | mesenchymal |
| MFI | mean fluorescence intensity |
| Scr | scrambles |
| SIRPα | signal regulatory protein alpha |
| TARGET | Therapeutically Applicable Research to Generate Effective Treatment program |
| T:E | target:effector |
| WT | wildtype |
References
- Darvin, P.; Toor, S.M.; Nair, V.S.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Park, J.A.; Cheung, N.-K.V. Limitations and opportunities for immune checkpoint inhibitors in pediatric malignancies. Cancer Treat. Rev. 2017, 58, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Majzner, R.G.; Heitzeneder, S.; Mackall, C.L. Harnessing the Immunotherapy Revolution for the Treatment of Childhood Cancers. Cancer Cell 2017, 31, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nat. Cell Biol. 2013, 499, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, M.F.; Denton, N.L.; Chen, C.-Y.; Cripe, T.P. Pediatric Cancer Immunotherapy: Opportunities and Challenges. Pediatr. Drugs 2018, 20, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Gröbner, S.N.; Project, I.P.-S.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef]
- Raffaghello, L.; Prigione, I.; Airoldi, I.; Camoriano, M.; Morandi, F.; Bocca, P.; Gambini, C.; Ferrone, S.; Pistoia, V. Mechanisms of immune evasion of human neuroblastoma. Cancer Lett. 2005, 228, 155–161. [Google Scholar] [CrossRef]
- Wienke, J.; Dierselhuis, M.P.; Tytgat, G.A.; Künkele, A.; Nierkens, S.; Molenaar, J.J. The immune landscape of neuroblastoma: Challenges and opportunities for novel therapeutic strategies in pediatric oncology. Eur. J. Cancer 2021, 144, 123–150. [Google Scholar] [CrossRef]
- Van Egmond, M.; Bakema, J.E. Neutrophils as effector cells for antibody-based immunotherapy of cancer. Semin. Cancer Biol. 2013, 23, 190–199. [Google Scholar] [CrossRef]
- Treffers, L.W.; Hiemstra, I.H.; Kuijpers, T.W.; Berg, T.K.V.D.; Matlung, H.L. Neutrophils in cancer. Immunol. Rev. 2016, 273, 312–328. [Google Scholar] [CrossRef]
- Furumaya, C.; Martinez-Sanz, P.; Bouti, P.; Kuijpers, T.W.; Matlung, H.L. Plasticity in Pro- and Anti-tumor Activity of Neutrophils: Shifting the Balance. Front. Immunol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Gale, R.P.; Zighelboim, J. Polymorphonuclear leukocytes in antibody-dependent cellular cytotoxicity. J. Immunol. 1975, 114. [Google Scholar]
- Albanesi, M.; Mancardi, D.A.; Jönsson, F.; Iannascoli, B.; Fiette, L.; Di Santo, J.; Lowell, C.A.; Bruhns, P. Neutrophils mediate antibody-induced antitumor effects in mice. Blood 2013, 122, 3160–3164. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 Antibody with GM-CSF, Interleukin-2, and Isotretinoin for Neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- McGinty, L.; Kolesar, J. Dinutuximab for maintenance therapy in pediatric neuroblastoma. Am. J. Health Pharm. 2017, 74, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.-K.V.; Dyer, M.A. Neuroblastoma: Developmental biology, cancer genomics and immunotherapy. Nat. Rev. Cancer 2013, 13, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.-K.V.; Sowers, R.; Vickers, A.; Cheung, I.Y.; Kushner, B.H.; Gorlick, R. FCGR2A Polymorphism Is Correlated With Clinical Outcome After Immunotherapy of Neuroblastoma With Anti-GD2 Antibody and Granulocyte Macrophage Colony-Stimulating Factor. J. Clin. Oncol. 2006, 24, 2885–2890. [Google Scholar] [CrossRef]
- Matlung, H.L.; Babes, L.; Zhao, X.W.; Van Houdt, M.; Treffers, L.W.; Van Rees, D.J.; Franke, K.; Schornagel, K.; Verkuijlen, P.; Janssen, H.; et al. Neutrophils Kill Antibody-Opsonized Cancer Cells by Trogoptosis. Cell Rep. 2018, 23, 3946–3959.e6. [Google Scholar] [CrossRef] [PubMed]
- Treffers, L.W.; Zhao, X.W.; van der Heijden, J.; Nagelkerke, S.Q.; van Rees, D.J.; Gonzalez, P.; Geissler, J.; Verkuijlen, P.; van Houdt, M.; de Boer, M.; et al. Genetic variation of human neutrophil Fcgamma receptors and SIRPalpha in antibody-dependent cellular cytotoxicity towards cancer cells. Eur. J. Immunol. 2018, 48, 344–354. [Google Scholar] [CrossRef]
- Derer, S.; Glorius, P.; Schlaeth, M.; Lohse, S.; Klausz, K.; Muchhal, U.; Desjarlais, J.R.; Humpe, A.; Valerius, T.; Peipp, M. Increasing FcgammaRIIa affinity of an FcgammaRIII-optimized anti-EGFR antibody restores neutrophil-mediated cytotoxicity. MAbs 2014, 6, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Barker, E.; Mueller, B.M.; Handgretinger, R.; Herter, M.; Yu, A.L.; Reisfeld, R.A. Effect of a chimeric anti-ganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer Res. 1991, 51, 144–149. [Google Scholar]
- Kushner, B.H.; Cheung, N.K. Clinically effective monoclonal antibody 3F8 mediates nonoxidative lysis of human neuroectodermal tumor cells by polymorphonuclear leukocytes. Cancer Res. 1991, 51, 4865–4870. [Google Scholar]
- Michon, J.; Moutel, S.; Barbet, J.; Romet-Lemonne, J.L.; Deo, Y.M.; Fridman, W.H.; Teillaud, J.L. In vitro killing of neuroblastoma cells by neutrophils derived from granulocyte colony-stimulating factor-treated cancer patients using an anti-disialoganglioside/anti-Fc gamma RI bispecific antibody. Blood 1995, 86, 1124–1130. [Google Scholar] [CrossRef]
- Chen, R.-L.; Reynolds, C.P.; Seeger, R.C. Neutrophils are cytotoxic and growth-inhibiting for neuroblastoma cells with an anti-GD2 antibody but, without cytotoxicity, can be growth-stimulating. Cancer Immunol. Immunother. 2000, 48, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Treffers, L.W.; van Houdt, M.; Bruggeman, C.W.; Heineke, M.H.; Zhao, X.W.; van der Heijden, J.; Nagelkerke, S.Q.; Verkuijlen, P.; Geissler, J.; Lissenberg-Thunnissen, S.; et al. FcgammaRIIIb Restricts Antibody-Dependent Destruction of Cancer Cells by Human Neutrophils. Front. Immunol. 2018, 9, 3124. [Google Scholar] [CrossRef]
- Kushner, B.H.; Cheung, N.K. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood 1989, 73, 1936–1941. [Google Scholar] [CrossRef] [PubMed]
- Van Der Kolk, L.E.; De Haas, M.; Grillo-López, A.J.; Baars, J.W.; Van Oers, M.H.J. Analysis of CD20-dependent cellular cytotoxicity by G-CSF-stimulated neutrophils. Leukemia 2002, 16, 693–699. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ragnhammar, P.F.J.; Trotta, P.P.; Mellstedt, H. Cytotoxicity of white blood cells activated by granulocyte-colony-stimulating factor, granulocyte/macrophage-colony-stimulating factor and macrophage-colony-stimulating factor against tumor cells in the presence of various monoclonal antibodies. Cancer Immunol. Immunother. 1994, 39, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Sanz, P.M.; van Rees, D.J.; van Zogchel, L.M.J.; Klein, B.; Bouti, P.; Olsman, H.; Schornagel, K.; Kok, I.; Sunak, A.; Leeuwenburg, K.; et al. G-CSF as a suitable alternative to GM-CSF to boost dinutuximab-mediated neutrophil cytotoxicity in neuroblastoma treatment. J. Immunother. Cancer 2021, 9, e002259. [Google Scholar] [CrossRef]
- Favier, B. Regulation of neutrophil functions through inhibitory receptors: An emerging paradigm in health and disease. Immunol. Rev. 2016, 273, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Van Rees, D.J.; Szilagyi, K.; Kuijpers, T.W.; Matlung, H.L.; Berg, T.K.V.D. Immunoreceptors on neutrophils. Semin. Immunol. 2016, 28, 94–108. [Google Scholar] [CrossRef]
- Willingham, S.B.; Volkmer, J.-P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef]
- Zhao, X.W.; van Beek, E.M.; Schornagel, K.; van der Maaden, H.; van Houdt, M.; Otten, M.A.; Finetti, P.; van Egmond, M.; Matozaki, T.; Kraal, G.; et al. CD47-signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction. Proc. Natl. Acad. Sci. USA 2011, 108, 18342–18347. [Google Scholar] [CrossRef]
- Ring, N.G.; Herndler-Brandstetter, D.; Weiskopf, K.; Shan, L.; Volkmer, J.-P.; George, B.M.; Lietzenmayer, M.; McKenna, K.; Naik, T.J.; McCarty, A.; et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc. Natl. Acad. Sci. USA 2017, 114, e10578–e10585. [Google Scholar] [CrossRef] [PubMed]
- Horrigan, S.K.; Iorns, E. Reproducibility Project: Cancer, Replication Study: The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. eLife 2017, 6, e18173. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, K. Cancer immunotherapy targeting the CD47/SIRPα axis. Eur. J. Cancer 2017, 76, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.; et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Sikic, B.I.; Lakhani, N.; Patnaik, A.; Shah, S.A.; Chandana, S.R.; Rasco, D.; Colevas, A.D.; O’Rourke, T.; Narayanan, S.; Papadopoulos, K.; et al. First-in-Human, First-in-Class Phase I Trial of the Anti-CD47 Antibody Hu5F9-G4 in Patients with Advanced Cancers. J. Clin. Oncol. 2019, 37, 946–953. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, Y.; Hertwig, F.; Thierry-Mieg, J.; Zhang, W.; Thierry-Mieg, D.; Wang, J.; Furlanello, C.; Devanarayan, V.; Cheng, J.; et al. Comparison of RNA-seq and microarray-based models for clinical endpoint prediction. Genome Biol. 2015, 16, 1–12. [Google Scholar] [CrossRef]
- Grassi, L.; Pourfarzad, F.; Ullrich, S.; Merkel, A.; Were, F.; Pau, E.C.D.S.; Yi, G.; Hiemstra, I.H.; Tool, A.T.; Mul, E.; et al. Dynamics of Transcription Regulation in Human Bone Marrow Myeloid Differentiation to Mature Blood Neutrophils. Cell Rep. 2018, 24, 2784–2794. [Google Scholar] [CrossRef]
- Kuijpers, T.W.; Tool, A.T.; Van Der Schoot, C.E.; Ginsel, L.A.; Onderwater, J.J.; Roos, D.; Verhoeven, A.J. Membrane surface antigen expression on neutrophils: A reappraisal of the use of surface markers for neutrophil activation. Blood 1991, 78, 1105–1111. [Google Scholar] [CrossRef]
- Bate-Eya, L.T.; Ebus, M.E.; Koster, J.; Hartog, I.J.D.; Zwijnenburg, D.A.; Schild, L.; van der Ploeg, I.; Dolman, M.E.M.; Caron, H.N.; Versteeg, R.; et al. Newly-derived neuroblastoma cell lines propagated in serum-free media recapitulate the genotype and phenotype of primary neuroblastoma tumours. Eur. J. Cancer 2014, 50, 628–637. [Google Scholar] [CrossRef]
- Neuroblastoma Stages and Prognostic Markers. Am. Cancer Soc. 2021. Available online: https://www.cancer.org/cancer/neuroblastoma/detection-diagnosis-staging/staging.html (accessed on 10 January 2021).
- Brisse, H.J.; McCarville, M.B.; Granata, C.; Krug, K.B.; Wootton-Gorges, S.L.; Kanegawa, K.; Giammarile, F.; Schmidt, M.; Shulkin, B.; Matthay, K.K.; et al. Guidelines for Imaging and Staging of Neuroblastic Tumors: Consensus Report from the International Neuroblastoma Risk Group Project. Radiology 2011, 261, 243–257. [Google Scholar] [CrossRef]
- Maris, J.M. Recent Advances in Neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Van Groningen, T.; Koster, J.; Valentijn, L.J.; Zwijnenburg, D.A.; Akogul, N.; Hasselt, N.E.; Broekmans, M.; Haneveld, F.; Nowakowska, N.E.; Bras, J.; et al. Neuroblastoma is composed of two super-enhancer-associated differentiation states. Nat. Genet. 2017, 49, 1261–1266. [Google Scholar] [CrossRef]
- Boeva, V.; Louis-Brennetot, C.; Peltier, A.; Durand, S.; Pierre-Eugène, C.; Raynal, V.; Etchevers, H.C.; Thomas, S.; Lermine, A.; Daudigeos-Dubus, E.; et al. Heterogeneity of neuroblastoma cell identity defined by transcriptional circuitries. Nat. Genet. 2017, 49, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Van Wezel, E.M.; van Zogchel, L.M.; van Wijk, J.; Timmerman, I.; Vo, N.-K.; Zappeij-Kannegieter, L.; Decarolis, B.; Simon, T.; van Noesel, M.M.; Molenaar, J.J.; et al. Mesenchymal Neuroblastoma Cells Are Undetected by Current mRNA Marker Panels: The Development of a Specific Neuroblastoma Mesenchymal Minimal Residual Disease Panel. JCO Precis. Oncol. 2019, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Foster, J. High-Risk Neuroblastoma Treatment Review. Children 2018, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Vakkila, J.; Jaffe, R.; Michelow, M.; Lotze, M.T. Pediatric Cancers Are Infiltrated Predominantly by Macrophages and Contain a Paucity of Dendritic Cells: A Major Nosologic Difference with Adult Tumors. Clin. Cancer Res. 2006, 12, 2049–2054. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.; Gerald, W.L.; Kushner, B.H.; Larson, S.M.; Hameed, M.; Cheung, N.K. Disaloganglioside GD2 loss following monoclonal antibody therapy is rare in neuroblastoma. Med. Pediatr. Oncol. 2001, 36, 194–196. [Google Scholar] [CrossRef]
- Schumacher-Kuckelkorn, R.; Volland, R.; Gradehandt, A.; Hero, B.; Simon, T.; Berthold, F. Lack of immunocytological GD2 expression on neuroblastoma cells in bone marrow at diagnosis, during treatment, and at recurrence*. Pediatr. Blood Cancer 2016, 64, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Terzic, T.; Cordeau, M.; Herblot, S.; Teira, P.; Cournoyer, S.; Beaunoyer, M.; Peuchmaur, M.; Duval, M.; Sartelet, H. Expression of Disialoganglioside (GD2) in Neuroblastic Tumors: A Prognostic Value for Patients Treated with Anti-GD2 Immunotherapy. Pediatr. Dev. Pathol. 2018, 21, 355–362. [Google Scholar] [CrossRef]
- Seeger, R.C.; Rosenblatt, H.M.; Imai, K.; Ferrone, S. Common antigenic determinants on human melanoma, glioma, neuroblastoma, and sarcoma cells defined with monoclonal antibodies. Cancer Res. 1981, 41, 2714–2717. [Google Scholar]
- Loo, D.; Alderson, R.F.; Chen, F.Z.; Huang, L.; Zhang, W.; Gorlatov, S.; Burke, S.; Ciccarone, V.; Li, H.; Yang, Y.; et al. Development of an Fc-Enhanced Anti–B7-H3 Monoclonal Antibody with Potent Antitumor Activity. Clin. Cancer Res. 2012, 18, 3834–3845. [Google Scholar] [CrossRef] [PubMed]
- Dondero, A.; Morini, M.; Cangelosi, D.; Mazzocco, K.; Serra, M.; Spaggiari, G.M.; Rotta, G.; Tondo, A.; Locatelli, F.; Castellano, A.; et al. Multiparametric flow cytometry highlights B7-H3 as a novel diagnostic/therapeutic target in GD2neg/low neuroblastoma variants. J. Immunother. Cancer 2021, 9, e002293. [Google Scholar] [CrossRef] [PubMed]
- Castriconi, R.; Dondero, A.; Augugliaro, R.; Cantoni, C.; Carnemolla, B.; Sementa, A.R.; Negri, F.; Conte, R.; Corrias, M.V.; Moretta, L.; et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc. Natl. Acad. Sci. USA 2004, 101, 12640–12645. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Sanz, P.; Hoogendijk, A.J.; Verkuijlen, P.J.J.H.; Schornagel, K.; van Bruggen, R.; van den Berg, T.K.; Tytgat, G.A.M.; Franke, K.; Kuijpers, T.W.; Matlung, H.L. CD47-SIRPα Checkpoint Inhibition Enhances Neutrophil-Mediated Killing of Dinutuximab-Opsonized Neuroblastoma Cells. Cancers 2021, 13, 4261. https://doi.org/10.3390/cancers13174261
Martínez-Sanz P, Hoogendijk AJ, Verkuijlen PJJH, Schornagel K, van Bruggen R, van den Berg TK, Tytgat GAM, Franke K, Kuijpers TW, Matlung HL. CD47-SIRPα Checkpoint Inhibition Enhances Neutrophil-Mediated Killing of Dinutuximab-Opsonized Neuroblastoma Cells. Cancers. 2021; 13(17):4261. https://doi.org/10.3390/cancers13174261
Chicago/Turabian StyleMartínez-Sanz, Paula, Arjan J. Hoogendijk, Paul J. J. H. Verkuijlen, Karin Schornagel, Robin van Bruggen, Timo K. van den Berg, Godelieve A. M. Tytgat, Katka Franke, Taco W. Kuijpers, and Hanke L. Matlung. 2021. "CD47-SIRPα Checkpoint Inhibition Enhances Neutrophil-Mediated Killing of Dinutuximab-Opsonized Neuroblastoma Cells" Cancers 13, no. 17: 4261. https://doi.org/10.3390/cancers13174261
APA StyleMartínez-Sanz, P., Hoogendijk, A. J., Verkuijlen, P. J. J. H., Schornagel, K., van Bruggen, R., van den Berg, T. K., Tytgat, G. A. M., Franke, K., Kuijpers, T. W., & Matlung, H. L. (2021). CD47-SIRPα Checkpoint Inhibition Enhances Neutrophil-Mediated Killing of Dinutuximab-Opsonized Neuroblastoma Cells. Cancers, 13(17), 4261. https://doi.org/10.3390/cancers13174261






