Magnetic-Guided Axillary UltraSound (MagUS) Sentinel Lymph Node Biopsy and Mapping in Patients with Early Breast Cancer. A Phase 2, Single-Arm Prospective Clinical Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. MRI-LG
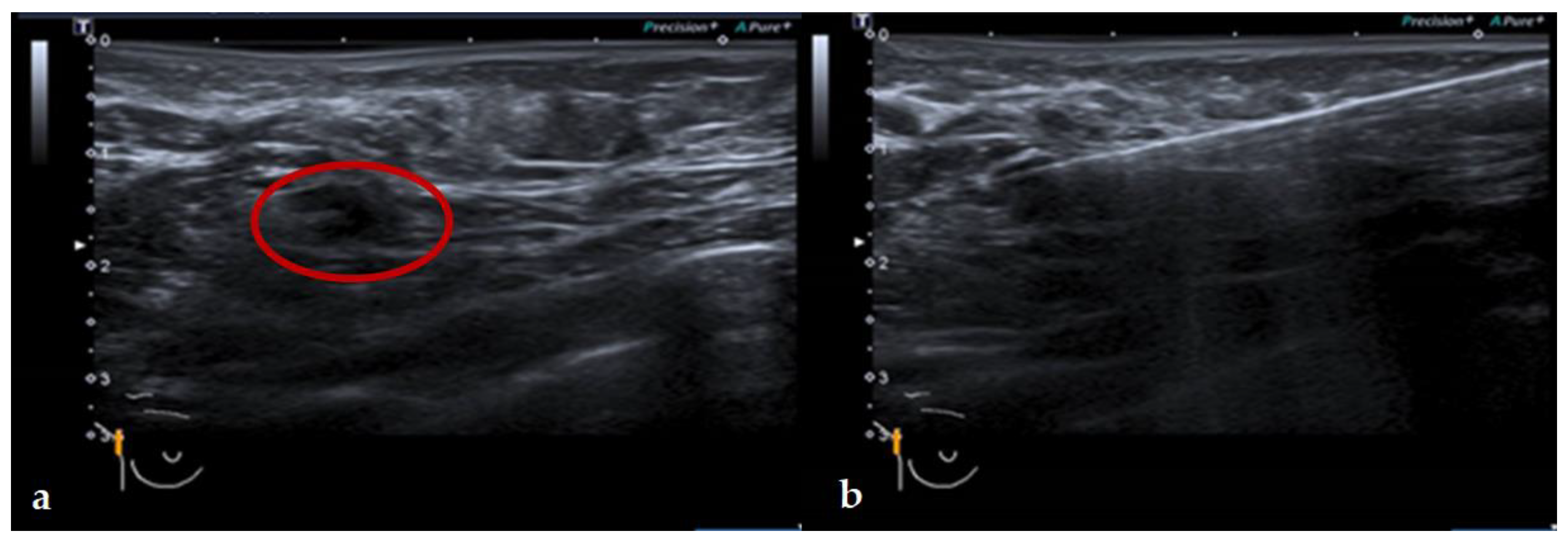
2.3. Magnetic Guided Axillary UltraSound (MagUS) and Core Needle Biopsy (CNB)
2.4. Surgery and Specimen Pathology
2.5. Trial Design and Study Endpoints
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, M.K.; Park, H.S.; Kim, J.Y.; Kim, S.; Nam, S.; Park, S.; Kim, S.I. The clinical implication of the number of lymph nodes harvested during sentinel lymph node biopsy and its effects on survival outcome in patients with node-negative breast cancer. Am. J. Surg. 2016, 214, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Stenmark Tullberg, A.; Lundstedt, D.; Olofsson Bagge, R.; Karlsson, P. Positive sentinel node in luminal A-like breast cancer patients-implications for adjuvant chemotherapy? Acta Oncol. 2019, 58, 162–167. [Google Scholar] [CrossRef]
- Veronesi, U.; Paganelli, G.; Viale, G.; Luini, A.; Zurrida, S.; Galimberti, V.; Intra, M.; Veronesi, P.; Robertson, C.; Maisonneuve, P.; et al. A Randomized Comparison of Sentinel-Node Biopsy with Routine Axillary Dissection in Breast Cancer. N. Engl. J. Med. 2003, 349, 546–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krag, D.N.; Anderson, S.; Julian, T.B.; Brown, A.M.; Harlow, S.P.; Costantino, J.P.; Ashikaga, T.; Weaver, D.L.; Mamounas, E.P.; Jalovec, L.M.; et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: Overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010, 11, 927–933. [Google Scholar] [CrossRef] [Green Version]
- Morrow, M.; Jagsi, R.; McLeod, M.C.; Shumway, D.; Katz, S.J. Surgeon Attitudes Toward the Omission of Axillary Dissection in Early Breast Cancer. JAMA Oncol. 2018, 4, 1511–1516. [Google Scholar] [CrossRef] [Green Version]
- Donker, M.; van Tienhoven, G.; Straver, M.E.; Meijnen, P.; van de Velde, C.J.H.; Mansel, R.E.; Cataliotti, L.; Westenberg, A.H.; Klinkenbijl, J.H.G.; Orzalesi, L.; et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Kuerer, H.M.; Bowling, M.; Flippo-Morton, T.S.; et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013, 310, 1455–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stearns, V.; Ewing, C.A.; Slack, R.; Penannen, M.F.; Hayes, D.F.; Tsangaris, T.N. Sentinel lymphadenectomy after neoadjuvant chemotherapy for breast cancer may reliably represent the axilla except for inflammatory breast cancer. Ann. Surg Oncol. 2002, 9, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs. No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef]
- Ahmed, M.; Jozsa, F.; Baker, R.; Rubio, I.T.; Benson, J.; Douek, M. Meta-analysis of tumour burden in pre-operative axillary ultrasound positive and negative breast cancer patients. Breast Cancer Res. Treat. 2017, 166, 329–336, Erratum in 2017, 166, 337. [Google Scholar] [CrossRef] [Green Version]
- Caudle, A.S.; Yang, W.T.; Krishnamurthy, S.; Mittendorf, E.A.; Black, D.M.; Gilcrease, M.Z.; Bedrosian, I.; Hobbs, B.P.; DeSnyder, S.M.; Hwang, R.F.; et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J. Clin. Oncol. 2016, 34, 1072–1078. [Google Scholar] [CrossRef] [Green Version]
- Dubsky, P.; Pinker, K.; Cardoso, F.; Montagna, G.; Ritter, M.; Denkert, C.; Rubio, I.T.; de Azambuja, E.; Curigliano, G.; Gentilini, O.; et al. Breast conservation and axillary management after primary systemic therapy in patients with early-stage breast cancer: The Lucerne toolbox. Lancet Oncol. 2021, 22, e18–e28. [Google Scholar] [CrossRef]
- Lucci, A.; McCall, L.M.; Beitsch, P.D.; Whitworth, P.W.; Reintgen, D.S.; Blumencranz, P.W.; Leitch, A.M.; Saha, S.; Hunt, K.K.; Giuliano, A.E. Surgical Complications Associated With Sentinel Lymph Node Dissection (SLND) Plus Axillary Lymph Node Dissection Compared With SLND Alone in the American College of Surgeons Oncology Group Trial Z0011. J. Clin. Oncol. 2007, 25, 3657–3663. [Google Scholar] [CrossRef] [PubMed]
- Mansel, R.E.; Fallowfield, L.; Kissin, M.; Goyal, A.; Newcombe, R.G.; Dixon, J.M.; Yiangou, C.; Horgan, K.; Bundred, N.; Monypenny, I.; et al. Randomized Multicenter Trial of Sentinel Node Biopsy Versus Standard Axillary Treatment in Operable Breast Cancer: The ALMANAC Trial. J. Natl. Cancer Inst. 2006, 98, 599–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbelen, H.; Tjalma, W.; Meirte, J.; Gebruers, N. Long-term morbidity after a negative sentinel node in breast cancer patients. Eur. J. Cancer Care 2019, 28, e13077. [Google Scholar] [CrossRef] [PubMed]
- Verbelen, H.; Gebruers, N.; Eeckhout, F.-M.; Verlinden, K.; Tjalma, W. Shoulder and arm morbidity in sentinel node-negative breast cancer patients: A systematic review. Breast Cancer Res. Treat. 2014, 144, 21–31. [Google Scholar] [CrossRef]
- Navalkissoor, S.; Wagner, T.; Gnanasegaran, G.; Buscombe, J. SPECT/CT in imaging sentinel nodes. Clin. Transl. Imaging 2015, 3, 203–215. [Google Scholar] [CrossRef]
- Nakagawa, M.; Morimoto, M.; Takechi, H.; Tadokoro, Y.; Tangoku, A. Preoperative diagnosis of sentinel lymph node (SLN) metastasis using 3D CT lymphography (CTLG). Breast Cancer 2015, 23, 519–524. [Google Scholar] [CrossRef]
- Cox, K.; Taylor-Phillips, S.; Sharma, N.; Weeks, J.; Mills, P.; Sever, A.; Lim, A.; Haigh, I.; Hashem, M.; De Silva, T.; et al. Enhanced pre-operative axillary staging using intradermal microbubbles and contrast-enhanced ultrasound to detect and biopsy sentinel lymph nodes in breast cancer: A potential replacement for axillary surgery. Br. J. Radiol. 2017, 91, 20170626. [Google Scholar] [CrossRef] [PubMed]
- Teshome, M.; Wei, C.; Hunt, K.K.; Thompson, A.; Rodriguez, K.; Mittendorf, E.A. Use of a Magnetic Tracer for Sentinel Lymph Node Detection in Early-Stage Breast Cancer Patients: A Meta-analysis. Ann. Surg. Oncol. 2016, 23, 1508–1514. [Google Scholar] [CrossRef]
- Karakatsanis, A.; Christiansen, P.M.; Fischer, L.; Hedin, C.; Pistioli, L.; Sund, M.; Rasmussen, N.R.; Jørnsgård, H.; Tegnelius, D.; Eriksson, S.; et al. The Nordic SentiMag trial: A comparison of super paramagnetic iron oxide (SPIO) nanoparticles versus Tc(99) and patent blue in the detection of sentinel node (SN) in patients with breast cancer and a meta-analysis of earlier studies. Breast Cancer Res. Treat. 2016, 157, 281–294. [Google Scholar] [CrossRef] [Green Version]
- Motomura, K.; Izumi, T.; Tateishi, S.; Tamaki, Y.; Ito, Y.; Horinouchi, T.; Nakanishi, K. Superparamagnetic iron oxide-enhanced MRI at 3 T for accurate axillary staging in breast cancer. Br. J. Surg. 2016, 103, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, A.; Hersi, A.; Pistiolis, L.; Bagge, R.O.; Lykoudis, P.M.; Eriksson, S.; Wärnberg, F.; Nagy, G.; Mohammed, I.; Sundqvist, M.; et al. Effect of preoperative injection of superparamagnetic iron oxide particles on rates of sentinel lymph node dissection in women undergoing surgery for ductal carcinoma in situ (SentiNot study). J. Br. Surg. 2019, 106, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Purushotham, A.D.; Douek, M. Novel techniques for sentinel lymph node biopsy in breast cancer: A systematic review. Lancet Oncol. 2014, 15, e351–e362. [Google Scholar] [CrossRef]
- Karakatsanis, A.; Daskalakis, K.; Stålberg, P.; Olofsson, H.; Andersson, Y.; Eriksson, S.; Bergkvist, L.; Wärnberg, F. Superparamagnetic iron oxide nanoparticles as the sole method for sentinel node biopsy detection in patients with breast cancer. J. Br. Surg. 2017, 104, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Baker, R.; Rubio, I.T. Meta-analysis of aberrant lymphatic drainage in recurrent breast cancer. BJS 2016, 103, 1579–1588. [Google Scholar] [CrossRef]
- Clough, K.B.; Nasr, R.; Nos, C.; Vieira, M.; Inguenault, C.; Poulet, B. New anatomical classification of the axilla with implications for sentinel node biopsy. Br. J. Surg. 2010, 97, 1659–1665. [Google Scholar] [CrossRef]
- Neven, A.; Mauer, M.; Hasan, B.; Sylvester, R.; Collette, L. Sample size computation in phase II designs combining the A’Hern design and the Sargent and Goldberg design. J. Biopharm. Stat. 2020, 30, 305–321. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.W.; et al. For the STARD Group. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilger, T.L.; Francisco, D.F.; Candido Dos Reis, F.J. Effect of sentinel lymph node biopsy on upper limb function in women with early breast cancer: A systematic review of clinical trials. Eur. J. Surg. Oncol. 2021, 30, 1497–1506. [Google Scholar] [CrossRef]
- Boughey, J.C.; Moriarty, J.P.; Degnim, A.C.; Gregg, M.S.; Egginton, J.S.; Long, K.H. Cost modeling of preoperative axillary ultrasound and fine-needle aspiration to guide surgery for invasive breast cancer. Ann. Surg. Oncol. 2010, 17, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Turaga, K.K.; Chau, A.; Eatrides, J.M.; Kiluk, J.V.; Khakpour, N.; Laronga, C.; Lee, M.C. Selective application of routine preoperative axillary ultrasonography reduces costs for invasive breast cancers. Oncologist 2011, 16, 942–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, A.; Gangi, A.; Amersi, F.; Zhang, X.; Guiliano, A. Not Performing a Sentinel Node Biopsy for Older Patients with Early-Stage Invasive Breast Cancer. JAMA Surg. 2015, 150, 683–684. [Google Scholar] [CrossRef] [Green Version]
- Ingvar, C.; Ahlgren, J.; Emdin, S.; Lofgren, L.; Nordander, M.; Nimeus, E.; Arnesson, L.-G. Long-term outcome of pT1a-b, cN0 breast cancer without axillary dissection or staging: A prospective observational study of 1543 women. Br. J. Surg. 2020, 107, 1299–1306. [Google Scholar] [CrossRef]
- O’Connell, R.L.; Rusby, J.E.; Stamp, G.F.W.; Conway, A.; Roche, N.; Barry, P.; Khabra, K.; Bonomi, R.; Rapisarda, I.F.; Della Rovere, G.Q. Long term results of treatment of breast cancer without axillary surgery–predicting a sound approach? Eur. J. Surg. Oncol. 2016, 42, 942–948. [Google Scholar] [CrossRef]
- Sentinel Node Vs Observation after Axillary Ultra-souND (SOUND). Available online: https://clinicaltrials.gov/ct2/show/NCT02167490 (accessed on 18 May 2021).
- Hughes, K.S.; Schnaper, L.A.; Bellon, J.R.; Cirrincione, C.T.; Berry, D.A.; McCormick, B.; Muss, H.B.; Smith, B.L.; Hudis, C.A.; Winer, E.P.; et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. J. Clin. Oncol. 2013, 31, 2382–2387. [Google Scholar] [CrossRef] [Green Version]
- Kunkler, I.H.; Williams, L.J.; Jack, W.J.; Cameron, D.A.; Dixon, J.M.; PRIME II Investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet Oncol. 2015, 16, 266–273. [Google Scholar] [CrossRef]
- Wickberg, Å.; Liljegren, G.; Killander, F.; Lindman, H.; Bjöhle, J.; Carlberg, M.; Blomqvist, C.; Ahlgren, J.; Villman, K. Omitting radiotherapy in women ≥ 65 years with low-risk early breast cancer after breast-conserving surgery and adjuvant endocrine therapy is safe. Eur. J. Surg. Oncol. 2018, 44, 951–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinsky, K.; Barlow, W.E.; Meric-Bernstam, F.; Gralow, J.R.; Albain, K.S.; Hayes, D.; Lin, N.; Perez, E.A.; Goldstein, L.J.; Chia, S.; et al. First results from a phase III randomized clinical trial of standard adjuvant endocrine therapy (ET) +/- chemotherapy (CT) in patients (pts) with 1-3 positive nodes, hormone receptor-positive (HR+) and HER2-negative (HER2-) breast cancer (BC) with recurrence score (RS) < 25: SWOG S1007 (RxPonder). In Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium, San Antonio, TX, USA, 8–11 December 2020. Abstract nr GS3-00. [Google Scholar]
- Van Wely, B.J.; de Wilt, J.H.; Francissen, C.; Teerenstra, S.; Strobbe, L.J. Meta-analysis of ultrasound-guided biopsy of suspicious axillary lymph nodes in the selection of patients with extensive axillary tumour burden in breast cancer. Br. J. Surg. 2015, 102, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Ciatto, S.; Turner, R.M.; Cody, H.S., 3rd; Macaskill, P. Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: Meta-analysis of its accuracy and utility in staging the axilla. Ann. Surg. 2011, 254, 243–251. [Google Scholar] [CrossRef]
- Caudle, A.; Hunt, K.K.; Kuerer, H.M.; Meric-Berstein, F.; Lucci, A.; Bedrosian, I.; Babiera, G.V.; Hwang, R.F.; Ross, M.I.; Feig, B.W.; et al. Multidisciplinary considerations in the implementation of the findings from the American College of Surgeons Oncology Group (ACOSOG) Z0011 study: A practice-changing trial. Ann. Surg. Oncol. 2011, 18, 2407–2412. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://clinicaltrials.gov/ct2/show/NCT01901094 (accessed on 18 May 2021).
- Standard or Comprehensive Radiation Therapy in Treating Patients With Early-Stage Breast Cancer Previously Treated with Chemotherapy and Surgery. Available online: https://clinicaltrials.gov/ct2/show/NCT01872975 (accessed on 18 May 2021).
- Boughey, J.C.; Ballman, K.V.; Le-Petross, H.T.; McCall, L.M.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Feliberti, E.C.; Hunt, K.K. Identification and Resection of Clipped Node Decreases the False-negative Rate of Sentinel Lymph Node Surgery in Patients Presenting With Node-positive Breast Cancer (T0–T4, N1–N2) Who Receive Neoadjuvant Chemotherapy: Results From ACOSOG Z1071 (Alliance). Ann. Surg. 2016, 263, 802–807. [Google Scholar] [CrossRef] [Green Version]
- Haffty, B.G.; McCall, L.M.; Ballman, K.V.; Buchholz, T.A.; Hunt, K.K.; Boughey, J.C. Impact of Radiation on Locoregional Control in Women with Node-Positive Breast Cancer Treated with Neoadjuvant Chemotherapy and Axillary Lymph Node Dissection: Results from ACOSOG Z1071 Clinical Trial. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Haffty, B.G.; McCall, L.M.; Ballman, K.V.; McLaughlin, S.; Jagsi, R.; Ollila, D.W.; Hunt, K.K.; Buchholz, T.A.; Boughey, J.C. Patterns of Local-Regional Management Following Neoadjuvant Chemotherapy in Breast Cancer: Results from ACOSOG Z1071 (Alliance). Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Kuemmel, S.; Heil, J.; Rueland, A.; Seiberling, C.; Harrach, H.; Schindowski, D.; Lubitz, J.; Hellerhoff, K.; Ankel, C.; Graßhoff, S.T.; et al. A Prospective, Multicenter Registry Study to Evaluate the Clinical Feasibility of Targeted Axillary Dissection (TAD) in Node-Positive Breast Cancer Patients. Ann. Surg. 2020. [Epub ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hausschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; von Minckwitz, G.; Nekljudova, V.; et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol. 2013, 14, 609–618. [Google Scholar] [CrossRef]
- Schwentner, L.; Helms, G.; Nekljudova, V.; Ataseven, B.; Bauerfeind, I.; Ditsch, N.; Fehm, T.; Fleige, B.; Hauschild, M.; Heil, J.; et al. Using ultrasound and palpation for predicting axillary lymph node status following neoadjuvant chemotherapy-Results from the multi-center SENTINA trial. Breast 2017, 31, 202–207. [Google Scholar] [CrossRef] [PubMed]
- van der Noordaa, M.E.M.; van Duijnhoven, F.H.; Cuijpers, F.N.E.; van Werkhoven, E.; Wiersma, T.G.; Elkhuizen, P.H.M.; Winter-Warnars, G.; Dezentje, V.; Sonke, G.S.; Groen, E.J.; et al. Toward omitting sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with clinically node-negative breast cancer. Br. J. Surg. 2020, 108, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Reimer, T.; Glass, A.; Botteri, E.; Loibl, S.; DGentilini, O. Avoiding Axillary Sentinel Lymph Node Biopsy after Neoadjuvant Systemic Therapy in Breast Cancer: Rationale for the Prospective, Multicentric EUBREAST-01 Trial. Cancers 2020, 12, 3698. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Gonzalez, S.J.; Lin, H.; Zhao, X.; Kiluk, J.V.; Laronga, C.; Mooney, B. Prospective trial of breast MRI versus 2D and 3D ultrasound for evaluation of response to neoadjuvant chemotherapy. Ann. Surg. Oncol. 2015, 22, 2888–2894. [Google Scholar] [CrossRef] [PubMed]
- Marinovich, M.L.; Macaskill, P.; Irwig, L.; Sardanelli, F.; von Minckwitz, G.; Mamounas, E.; Brennan, M.; Ciatto, S.; Houssami, N. Meta-analysis of agreement between MRI and pathologic breast tumour size after neoadjuvant chemotherapy. Br. J. Cancer 2013, 109, 1528–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikhbahaei, S.; Trahan, T.J.; Xiao, J.; Taghipour, M.; Mena, E.; Connolly, R.M.; Subramaniam, R.M. FDG-PET/CT and MRI for Evaluation of Pathologic Response to Neoadjuvant Chemotherapy in Patients With Breast Cancer: A Meta-Analysis of Diagnostic Accuracy Studies. Oncologist 2016, 21, 931–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Yang, Q.; Bao, J.; Liu, D.; Huang, X.; Wang, J. Direct comparison of PET/CT and MRI to predict the pathological response to neoadjuvant chemotherapy in breast cancer: A meta-analysis. Sci. Rep. 2017, 7, 8479. [Google Scholar] [CrossRef] [Green Version]
- Ugras, S.; Matsen, C.; Eaton, A.; Stempel, M.; Morrow, M.; Cody, H.S., 3rd. Reoperative Sentinel Lymph Node Biopsy is Feasible for Locally Recurrent Breast Cancer, But is it Worthwhile? Ann. Surg. Oncol. 2016, 23, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Vugts, G.; Maaskant-Braat, A.J.; Voogd, A.C.; van Riet, Y.E.; Luiten, E.J.; Rutgers, E.J.; Rutten, H.J.; Roumen, R.M.; Nieuwenhuijzen, G.A. Repeat sentinel node biopsy should be considered in patients with locally recurrent breast cancer. Breast Cancer Res. Treat. 2015, 153, 549–556. [Google Scholar] [CrossRef]
- Kuemmel, S.; Holtschmidt, J.; Gerber, B.; Von der Assen, A.; Heil, J.; Thill, M.; Krug, D.; Schem, C.; Denkert, C.; Lubitz, J.; et al. Prospective, Multicenter, Randomized Phase III Trial Evaluating the Impact of Lymphoscintigraphy as Part of Sentinel Node Biopsy in Early Breast Cancer: SenSzi (GBG80) Trial. J. Clin. Oncol. 2019, 37, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Motomura, K.; Izumi, T.; Tateishi, S.; Sumino, H.; Noguchi, A.; Horinouchi, T.; Nakanishi, K. Correlation between the area of high-signal intensity on SPIO-enhanced MR imaging and the pathologic size of sentinel node metastases in breast cancer patients with positive sentinel nodes. BMC Med. Imaging 2013, 13, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Patient Characteristics. | |
|---|---|
| Patient age at operation (median, range) | 64 (38–87) |
| Body mass index (median, range) | 24.8 (19.1–43.8) |
| Preoperative tumor extent mm (median, range) | 20 (5–120) |
| Days between injection and Surgery (median, range) | 12 (0–140) |
| Laterality, number, % | |
| Right | 41 (51.9) |
| Left | 38 (48.1) |
| Previous breast surgery | |
| Right | 21 (26.6) |
| Left | 58 (73.4) |
| Previous axillary surgery | |
| Right | 19 (24.4) |
| Left | 59 (75.6) |
| Neo adjuvant treatment | |
| Right | 12 (15.2) |
| Left | 67 (84.8) |
| Localization in the breast, number, % | |
| Upper outer | 31 (39.2) |
| Upper inner | 12 (15.2) |
| Lower outer | 9 (11.4) |
| Lower inner | 7 (8.9) |
| Central | 7 (8.9) |
| Multicentric | 11 (13.9) |
| Chest wall | 2 (2.5) |
| Histological type (n = 79) | |
| Invasive ductal (n, (%)) | 66 (83.5) |
| Invasive lobular (n, (%)) | 11 (13.9) |
| Other Histology (n, (%)) | 2 (2.5) |
| Intrinsic Subtype (n = 79) | |
| Luminal A (n, (%)) | 36 |
| Luminal B, erbb2− (n, (%)) | 20 |
| Luminal B, erbb2+ (n, (%)) | 10 |
| Non luminal erbb2+ (n, (%)) | 3 |
| Triple negative (n, (%)) | 9 |
| Type of surgery (n = 79) | |
| Wide local excision (n, (%)) | 28 (35.4) |
| Mastectomy (n, (%)) | 23 (29.1) |
| Oncoplastic breast conservation (n, (%)) | 28 (34.4) |
| Preoperative MagUS Assessment for Metastases | ||||
|---|---|---|---|---|
| No n, (%) | Yes n, (%) | Total n, (%) | ||
| Metastases at histopathology | No | 62 (98.4) | 0 (0) | 62 (84.9) |
| Yes | 1 (1.6) | 10 (100) | 11 (15.1) | |
| Total | 63 (100) | 10 (100) | 73 (100) | |
| Rate | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|
| Sensitivity | 90.9% | 58.7% | 99.8% |
| Specificity | 100% | 94.2% | 100% |
| PPV | 100% | 69.1% | 100% |
| NPV | 98.4% | 91.5% | 99.9% |
| Accuracy | 98.6% | 92.6% | 99.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jazrawi, A.; Pantiora, E.; Abdsaleh, S.; Bacovia, D.V.; Eriksson, S.; Leonhardt, H.; Wärnberg, F.; Karakatsanis, A. Magnetic-Guided Axillary UltraSound (MagUS) Sentinel Lymph Node Biopsy and Mapping in Patients with Early Breast Cancer. A Phase 2, Single-Arm Prospective Clinical Trial. Cancers 2021, 13, 4285. https://doi.org/10.3390/cancers13174285
Jazrawi A, Pantiora E, Abdsaleh S, Bacovia DV, Eriksson S, Leonhardt H, Wärnberg F, Karakatsanis A. Magnetic-Guided Axillary UltraSound (MagUS) Sentinel Lymph Node Biopsy and Mapping in Patients with Early Breast Cancer. A Phase 2, Single-Arm Prospective Clinical Trial. Cancers. 2021; 13(17):4285. https://doi.org/10.3390/cancers13174285
Chicago/Turabian StyleJazrawi, Allan, Eirini Pantiora, Shahin Abdsaleh, Daniel Vasiliu Bacovia, Staffan Eriksson, Henrik Leonhardt, Fredrik Wärnberg, and Andreas Karakatsanis. 2021. "Magnetic-Guided Axillary UltraSound (MagUS) Sentinel Lymph Node Biopsy and Mapping in Patients with Early Breast Cancer. A Phase 2, Single-Arm Prospective Clinical Trial" Cancers 13, no. 17: 4285. https://doi.org/10.3390/cancers13174285






