The T/Tn-Specific Helix pomatia Lectin Induces Cell Death in Lymphoma Cells Negative for T/Tn Antigens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
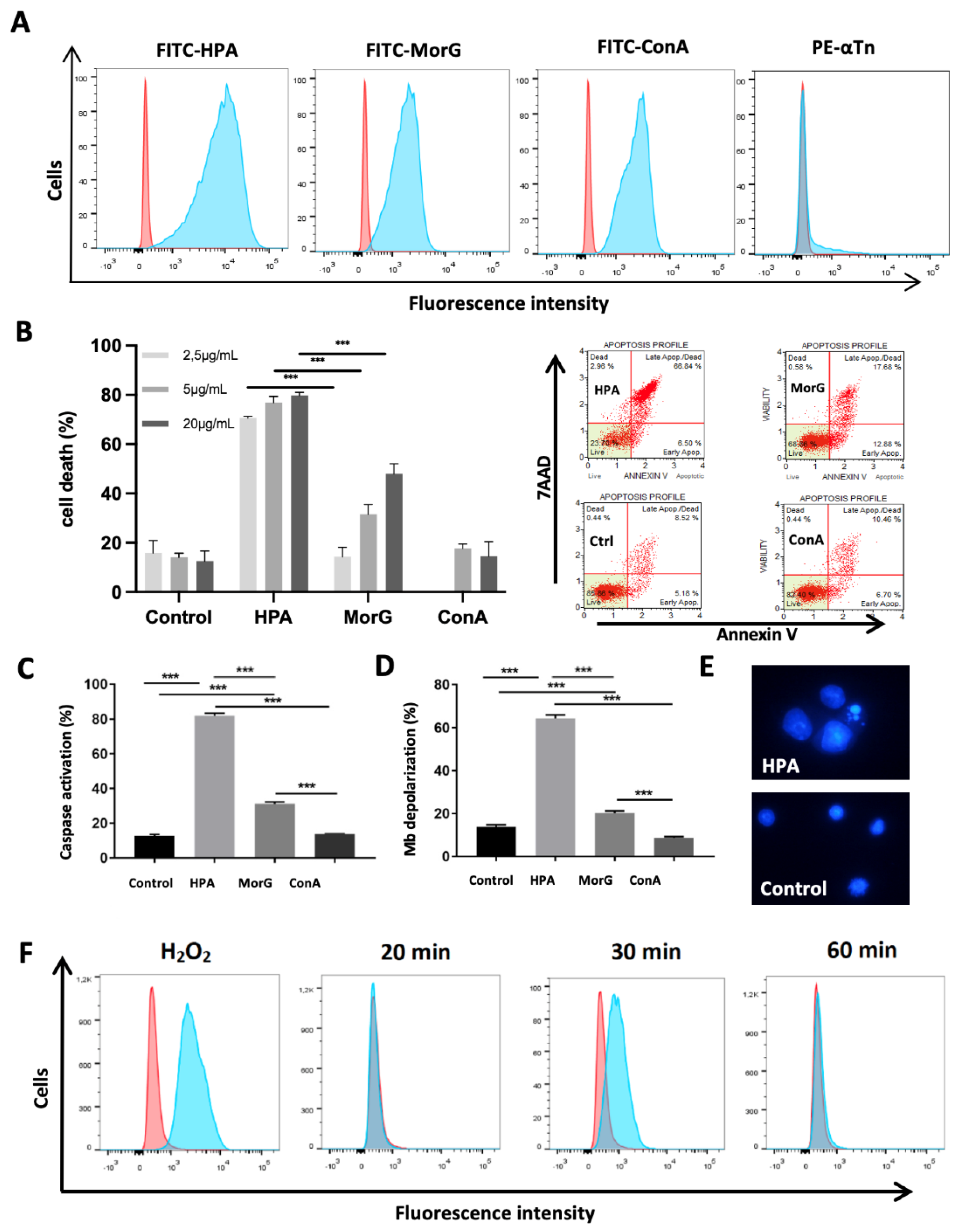
2.1. HPA Kills In Vitro the Tn-Positive Jurkat T-Cell Leukemia
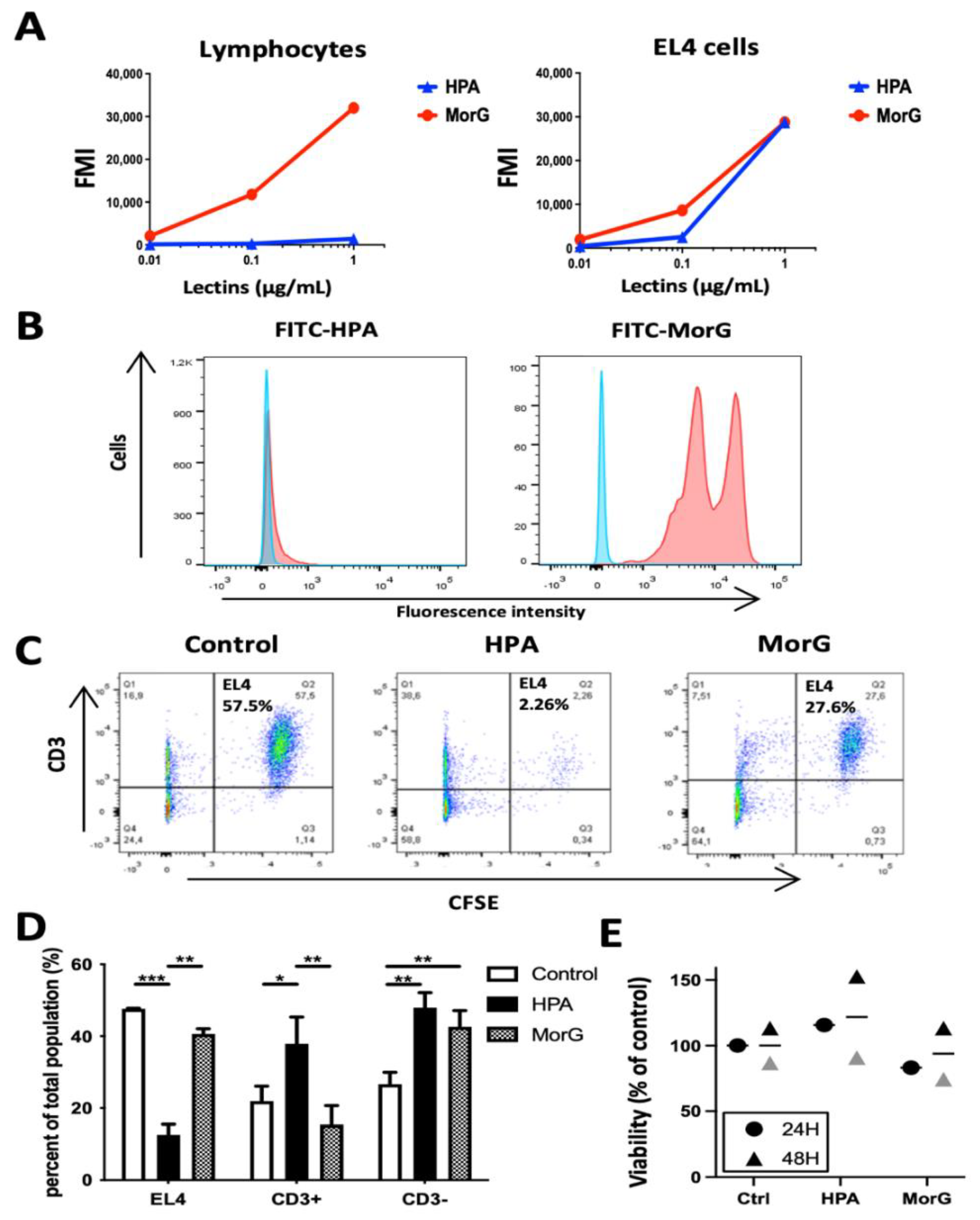
2.2. HPA Kills T/Tn-Negative EL4 T-Lymphoma Cells
2.3. HPA Induces Death in EL4 T-Cells but Not in Healthy T-Cells
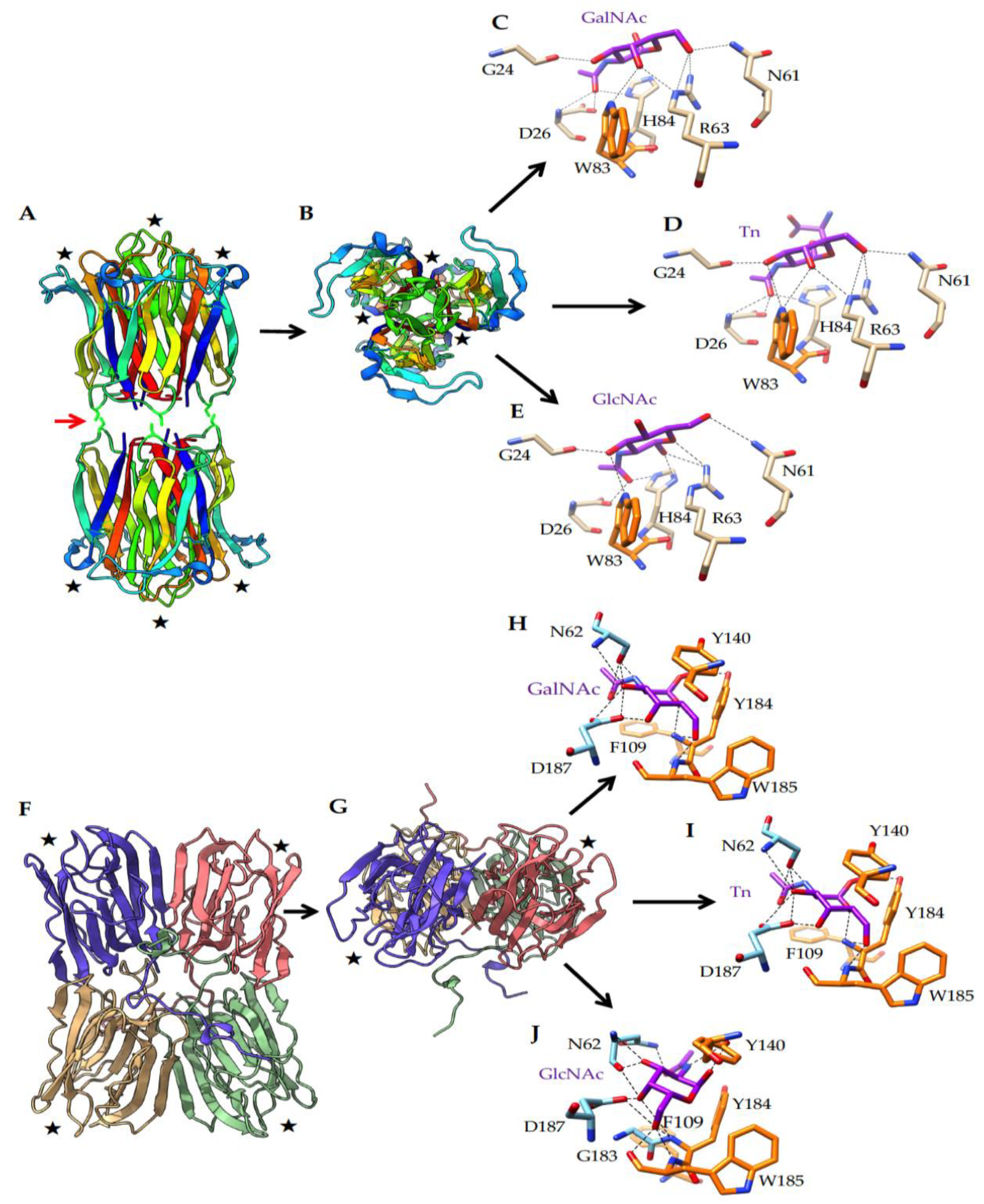
2.4. What Could Be the Targets of HPA on EL4 T-Lymphoma Cells?
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Lectin-Mediated Cytotoxicity Assay and Cell Death Evaluation
4.3. Preparation of Healthy Lymphocytes and EL4 Mixtures
4.4. Cell Surface-Binding Experiments
4.5. Neuraminidase Treatment of EL4 Cells
4.6. ROS Detection
4.7. Statistical Analyses
4.8. In Silico Molecular Modeling and Docking Experiments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Divya, T.; Ashok, K.R.; Prakash, R. Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188464. [Google Scholar]
- Dall’Olio, F.; Trinchera, M. Epigenetic bases of aberrant glycosylation in cancer. Int. J. Mol. Sci. 2017, 18, 998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihara, S.; Miyoshi, E.; Ko, J.H.; Murata, K.; Nakahara, S.; Honke, K.; Dickson, R.B.; Lin, C.Y.; Taniguchi, N. Prometastatic effect of N-acetylglucosaminyltransferase V is due to modification and stabilization of active matriptase by adding beta 1-6 GlcNAc branching. J. Biol. Chem. 2002, 277, 16960–16967. [Google Scholar] [CrossRef] [Green Version]
- Ju, T.; Lanneau, G.S.; Gautam, T.; Wang, Y.; Xia, B.; Stowell, S.R.; Willard, M.T.; Wang, W.; Xia, J.Y.; Zuna, R.E.; et al. Human tumor anti- gens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Res. 2008, 68, 1636–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrascal, M.A.; Severino, P.F.; Guadalupe Cabral, M.; Silva, M.; Ferreira, J.A.; Calais, F.; Quinto, H.; Pen, C.; Ligeiro, D.; Santos, L.L.; et al. Sialyl Tn-expressing bladder cancer cells induce a tolerogenic phenotype in innate and adaptive immune cells. Mol. Oncol. 2014, 8, 753–765. [Google Scholar] [CrossRef]
- Marcos, N.T.; Pinho, S.; Grandela, C.; Cruz, A.; Samyn-Petit, B.; Harduin-Lepers, A.; Almeida, R.; Silva, F.; Morais, V.; Costa, J.; et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004, 64, 7050–7057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, E.; Macauley, M.S. Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities. Cancers 2018, 10, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, J.A.; Videira, P.A.; Lima, L.; Pereira, S.; Silva, M.; Carrascal, M.; Severino, P.F.; Fernandes, E.; Almeida, A.; Costa, C.; et al. Overexpression of tumour-associated carbohydrate antigen sialyl-Tn in advanced bladder tumours. Mol. Oncol. 2013, 7, 719–731. [Google Scholar] [CrossRef] [Green Version]
- Sinha, D.; Mandal, C.; Bhattacharya, D.K. Identification of 9-O acetyl sialoglycoconjugates (9-OAcSGs) as biomarkers in childhood acute lymphoblastic leukemia using a lectin, AchatininH, as a probe. Leukemia 1999, 13, 119–125. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, H.; Song, X.; Shi, S.; Zhang, J.; Jia, L. Modification of sialylation is associated with multidrug resistance in human acute myeloid leukemia. Oncogene 2015, 34, 726–740. [Google Scholar] [CrossRef] [Green Version]
- Laack, E.; Nikbakht, H.; Peters, A.; Kugler, C.; Jasiewicz, Y.; Edler, L.; Hossfeld, D.K.; Schumacher, U. Lectin histochemistry of resected adenocarcinoma of the lung: Helix pomatia agglutinin binding is an independent prognostic factor. Am. J. Pathol. 2002, 160, 1001–1008. [Google Scholar] [CrossRef]
- Poiroux, G.; Barre, A.; van Damme, E.J.M.; Benoist, H.; Rougé, P. Plant lectins targeting O-glycans at the cell surface as tools for cancer diagnostic, pronostic and therapy. Int. J. Mol. Sci. 2017, 18, 1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, D.J.; Tham, K.M.; Chia, J.; Wang, S.C.; Steentoft, C.; Clausen, H.; Bard-Chapeau, E.A.; Bard, F.A. Initiation of GalNAc-type O-glycosylation in the endoplasmic reticulum promotes cancer cell invasiveness. Proc. Natl. Acad. Sci. USA 2013, 110, 3152–3161. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Wen, T.; Yan, R.; Kim, S.R.; Stowell, S.R.; Wang, W.; Wang, Y.; An, G.; Cummings, R.D.; Ju, T. O-glycans on death receptors in cells modulate their sensitivity to TRAIL-induced apoptosis through affecting on their stability and oligomerization. FASEB J. 2020, 34, 11786–11801. [Google Scholar] [CrossRef]
- Poiroux, G.; Barre, A.; Simplicien, M.; Pelofy, S.; Segui, B.; Van Damme, E.J.M.; Rougé, P.; Benoist, H. Morniga-G, a T/Tn-Specific Lectin, Induces Leukemic Cell Death via Caspase and DR5 Receptor-Dependent Pathways. Int. J. Mol. Sci. 2019, 20, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, K.W.; Punnoose, E.A.; Januario, T.; Lawrence, D.A.; Pitti, R.M.; Lancaster, K.; Lee, D.; von Goetz, M.; Yee, S.F.; Totpal, K.; et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat. Med. 2007, 13, 1070–1077. [Google Scholar] [CrossRef]
- Dufour, F.; Rattier, T.; Shirley, S.; Picarda, G.; Constantinescu, A.A.; Morle, A.; Zakaria, A.B.; Marcion, G.; Causse, S.; Szegezdi, E.; et al. N-glycosylation of mouse TRAIL-R and human TRAIL-R1 enhances TRAIL-induced death. Cell Death Differ. 2017, 24, 500–510. [Google Scholar] [CrossRef] [Green Version]
- Rabinovich, G.A.; van Kooyk, Y.; Cobb, B.A. Glycobiology of immune responses. Ann. N.Y. Acad. Sci. 2012, 1253, 1–15. [Google Scholar] [CrossRef]
- Seyrek, K.; Richter, M.; Lavrik, I.N. Decoding the sweet regulation of apoptosis: The role of glycosylation and galectins in apoptotic signaling pathways. Cell Death Differ. 2019, 26, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, S.K.; Panda, P.K.; Sinha, N.; Praharaj, P.P.; Bhol, C.S.; Panigrahi, D.P.; Mahapatra, K.K.; Saha, S.; Patra, S.; Mishra, S.R.; et al. Plant lectins in cancer therapeutics: Targeting apoptosis and autophagy-dependent cell death. Pharmacol. Res. 2019, 44, 8–18. [Google Scholar] [CrossRef]
- Takayama, H.; Ohta, M.; Iwashita, Y.; Uchida, H.; Shitomi, Y.; Yada, K.; Inomata, M. Altered glycosylation associated with dedifferentiation of hepatocellular carcinoma: A lectin microarray-based study. BMC Cancer 2020, 20, 192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhan, T.; Deng, Z.; Li, Q.; Liu, Y.; Yang, S.; Ji, D.; Li, Y. Glycan analysis of colorectal cancer samples reveals stage-dependent changes in CEA glycosylation patterns. Clin. Proteom. 2018, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, Y.; Zhu, T.S.; Xie, X.; Costello, M.A.; Talsma, C.E.; Flack, C.G.; Crowley, J.G.; Dimeco, F.; Vescovi, A.L.; et al. Glycoproteomic analysis of glioblastoma stem cell differentiation. J. Proteome Res. 2011, 10, 330–338. [Google Scholar] [CrossRef]
- Benoist, H.; Culerrier, R.; Poiroux, G.; Ségui, B.; Jauneau, A.; Van Damme, E.J.; Peumans, W.J.; Barre, A.; Rougé, P. Two structurally identical mannose-specific jacalin-related lectins display different effects on human T lymphocyte activation and cell death. J. Leukoc. Biol. 2009, 86, 103–114. [Google Scholar] [CrossRef]
- Tejero, J.; Jiménez, P.; Quinto, E.J.; Cordoba-Diaz, D.; Garrosa, M.; Cordoba-Diaz, M.; Gayoso, J.; Girbés, T. Elderberries: A source of ribosome-inactivating proteins with lectin activity. Molecules 2015, 20, 2364–2387. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-H.; Liu, J.-F.; Chiang, Y.-C.; Hu, S.C.-S.; Hsu, L.-F.; Lin, Y.-C.; Lin, Z.-C.; Lee, H.-C.; Chen, M.-C.; Huang, C.-L.; et al. Artocarpin, an isoprenyl flavonoid, induces p53-dependent or independent apoptosis via ROS-mediated MAPKs and Akt activation in non-small cell lung cancer cells. Oncotarget 2017, 8, 28342–28358. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.; Wu, J.H.; Peumans, W.J.; Rougé, P.; Van Damme, E.J.; Wu, A.M. Recognition profile of Morus nigra agglutinin (Morniga G) expressed by monomeric ligands, simple clusters and mammalian polyvalent glycotopes. Mol. Immunol. 2007, 44, 451–462. [Google Scholar] [CrossRef]
- Pietrzyk-Brzezinska, A.J.; Bujacz, A. H-type lectins-Structural characteristics and their applications in diagnostics, analytics and drug delivery. Int. J. Biol. Macromol. 2020, 152, 735–747. [Google Scholar] [CrossRef]
- Peiris, D.; Ossondo, M.; Fry, S.; Loizidou, M.; Smith-Ravin, J.; Dwek, M.V. Identification of O-Linked Glycoproteins Binding to the Lectin Helix pomatia Agglutinin as Markers of Metastatic Colorectal Cancer. PLoS ONE. 2015, 10, e0138345. [Google Scholar] [CrossRef] [PubMed]
- Milde-Langosch, K.; Schütze, D.; Oliveira-Ferrer, L.; Wikman, H.; Müller, V.; Lebok, P.; Pantel, K.; Schröder, C.; Witzel, I.; Schumacher, U. Relevance of βGal-βGalNAc-containing glycans and the enzymes involved in their synthesis for invasion and survival in breast cancer patients. Breast Cancer Res. Treat. 2015, 151, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Thöm, I.; Schult-Kronefeld, O.; Burkholder, I.; Goern, M.; Andritzky, B.; Blonski, K.; Kugler, C.; Edler, L.; Bokemeyer, C.; Schumacher, U.; et al. Lectin histochemistry of metastatic adenocarcinomas of the lung. Lung Cancer 2007, 56, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Thies, A.; Moll, I.; Berger, J.; Schumacher, U. Lectin binding to cutaneous malignant melanoma: HPA is associated with metastasis formation. Br. J. Cancer 2001, 84, 819–823. [Google Scholar] [CrossRef] [Green Version]
- Alaeddine, M.; Prat, M.; Poinsot, V.; Gouazé-Andersson, V.; Authier, H.; Meunier, E.; Lefèvre, L.; Alric, C.; Dardenne, C.; Bernad, J.; et al. IL13-Mediated Dectin-1 and Mannose Receptor Overexpression Promotes Macrophage Antitumor Activities through Recognition of Sialylated Tumor Cells. Cancer Immunol. Res. 2019, 7, 321–334. [Google Scholar] [CrossRef]
- Rambaruth, N.D.S.; Greenwell, P.; Dwek, M.V. The lectin Helix pomatia agglutinin recognizes O-GlcNAc containing glycoproteins in human breast cancer. Glycobiology 2012, 22, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, J.-F.; Lescar, J.; Chazalet, V.; Audfray, A.; Gagnon, J.; Alvarez, R.; Breton, C.; Imberty, A.; Mitchell, E.P. Biochemical and structural analysis of Helix pomatia agglutinin. A hexameric lectin with a novel fold. J. Biol. Chem. 2006, 281, 20171–20180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rougé, P.; Peumans, W.J.; Van Damme, E.J.M.; Barre, A.; Singh, T.; Wu, J.H.; Wu, A.M. Glycotope structures and intramolecular affinity factors of plant lectins for T/Tn antigens. Adv. Exp. Med. Biol. 2011, 705, 143–154. [Google Scholar]
- Cutler, C.E.; Jones, M.B.; Cutler, A.A.; Mener, A.; Arthur, C.M.; Stowell, S.R.; Cummings, R.D. Cosmc is required for T cell persistence in the periphery. Glycobiology 2019, 29, 776–788. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Xu, X.; Yu, X.; Du, Z.; Luan, X.; Liu, D.; Hu, T. CD3/CD28 dynabeads induce expression of Tn antigen in human T cells accompanied by hypermethylation of the cosmc promoter. Mol. Immunol. 2017, 90, 98–105. [Google Scholar] [CrossRef]
- Brooks, S.A.; Carter, T.M. N-acetylgalactosamine, N-acetylglucosamine and sialic acid expression in primary breast cancers. Acta Histochem. 2001, 103, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Gays, F.; Unnikrishnan, M.; Shrestha, S.; Fraser, K.P.; Brown, A.R.; Tristram, C.M.G.; Chrzanowska-Lightowlers, Z.M.A.; Brooks, C.G. The mouse tumor cell lines EL4 and RMA display mosaic expression of NK-related and certain other surface molecules and appear to have a common origin. J. Immunol. 2000, 164, 5094–5102. [Google Scholar] [CrossRef] [PubMed]
- Kovar, L.; Etrych, T.; Kabešová, M.; Subr, V.; Vetvicka, D.; Hovorka, O.; Strohalm, J.; Sklenar, J.; Chytil, P.; Ulbrich, K.; et al. Doxorubicin attached to HPMA copolymer via amide bond modifies the glycosylation pattern of EL4 cells. Tumor Biol. 2010, 31, 233–242. [Google Scholar] [CrossRef]
- Rajasagi, M.; von Au, A.; Singh, R.; Hartmann, N.; Zöller, M.; Marhaba, R. Anti-CD44 induces apoptosis in T lymphoma via mitochondrial depolarization. J. Cell. Mol. Med. 2010, 14, 1453–1467. [Google Scholar] [CrossRef]
- Clark, M.C.; Baum, L.G. T cells modulate glycans on CD43 and CD45 during development and activation, signal regulation, and survival. Ann. N.Y. Acad. Sci. 2012, 1253, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Earl, L.A.; Bi, S.; Baum, L.G. N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death. J. Biol. Chem. 2010, 285, 2232–2244. [Google Scholar] [CrossRef] [Green Version]
- Poiroux, G.; Pitié, M.; Culerrier, R.; Lafont, E.; Ségui, B.; Van Damme, E.J.M.; Peumans, W.J.; Bernadou, J.; Levade, T.; Rougé, P.; et al. Targeting of T/Tn antigens with a plant lectin to kill human leukemia cells by photochemotherapy. PLoS ONE 2011, 6, 23315. [Google Scholar] [CrossRef] [Green Version]
- Madani, S.Y.; Tan, A.; Dwek, M.; Seifalian, A.M. Functionalization of single-walled carbon nanotubes and their binding to cancer cells. Int. J. Nanomed. 2012, 7, 905–914. [Google Scholar]
- Krieger, E.; Koraimann, G.; Vriend, G. Increasing the precision of comparative models with YASARA NOVA—A self-parametrizing force field. Proteins 2002, 47, 393–402. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Z.; Wang, D.; Ogata, C.M.; Palczewski, K.; Lee, X.; Young, N.M. Characterization of the secondary binding sites of Maclura pomifera agglutinin by glycan array and crystallographic analyses. Glycobiology 2010, 20, 1643–1653. [Google Scholar] [CrossRef] [Green Version]
- Jeyaprakash, A.A.; Katyar, S.; Swaminathan, C.P.; Sekar, K.; Surolia, A.; Vijayan, M. Structural basis of the carbohydrate specificities of jacalin: An X-ray and modeling study. J. Mol. Biol. 2003, 332, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Gabrielsen, M.; Abdul-Rahman, P.S.; Othman, S.; Hashim, O.H.; Cogdell, R.J. Structures and binding specificity of galactose- and mannose-binding lectins from champedak: Differences from jackfruit lectins. Acta Crystallogr. F Struct. Biol. Commun. 2014, 70, 709–716. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemistry of protein structures. J. Appl. Crystallogr. 1993, 126, 283–291. [Google Scholar] [CrossRef]
- Melo, F.; Feytmans, E. Assessing protein structures with a non-local atomic interaction energy. J. Mol. Biol. 1998, 277, 1141–1152. [Google Scholar] [CrossRef]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2011, 27, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lescar, J.; Sanchez, J.-F.; Audfray, A.; Coll, J.-L.; Breton, C.; Mitchell, E.P.; Imberty, A. Structural basis for recognition of breast and colon cancer epitopes Tn antigen and Forssman disaccharide by Helix pomatia lectin. Glycobiology 2007, 17, 1077–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef] [Green Version]
- Grosdidier, A.; Zoete, V.; Michielin, O. Fast docking using the CHARMM force field with EADock DSS. J. Comput. Chem. 2011, 32, 2149–2159. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Goddard, T.D.; Huang, C.C.; Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 2018, 27, 14–25. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simplicien, M.; Barre, A.; Benkerrou, Y.; Van Damme, E.J.M.; Rougé, P.; Benoist, H. The T/Tn-Specific Helix pomatia Lectin Induces Cell Death in Lymphoma Cells Negative for T/Tn Antigens. Cancers 2021, 13, 4356. https://doi.org/10.3390/cancers13174356
Simplicien M, Barre A, Benkerrou Y, Van Damme EJM, Rougé P, Benoist H. The T/Tn-Specific Helix pomatia Lectin Induces Cell Death in Lymphoma Cells Negative for T/Tn Antigens. Cancers. 2021; 13(17):4356. https://doi.org/10.3390/cancers13174356
Chicago/Turabian StyleSimplicien, Mathias, Annick Barre, Yamina Benkerrou, Els J. M. Van Damme, Pierre Rougé, and Hervé Benoist. 2021. "The T/Tn-Specific Helix pomatia Lectin Induces Cell Death in Lymphoma Cells Negative for T/Tn Antigens" Cancers 13, no. 17: 4356. https://doi.org/10.3390/cancers13174356
APA StyleSimplicien, M., Barre, A., Benkerrou, Y., Van Damme, E. J. M., Rougé, P., & Benoist, H. (2021). The T/Tn-Specific Helix pomatia Lectin Induces Cell Death in Lymphoma Cells Negative for T/Tn Antigens. Cancers, 13(17), 4356. https://doi.org/10.3390/cancers13174356







