Glutamine Availability Controls BCR/Abl Protein Expression and Functional Phenotype of Chronic Myeloid Leukemia Cells Endowed with Stem/Progenitor Cell Potential
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Culture Conditions
2.2. Reagents
2.3. Cell Lysis and Western Blotting
2.4. RNA Isolation and Quantitative Real Time PCR
2.5. Culture Repopulation Ability (CRA) Assay
2.6. Statistical Analysis
3. Results
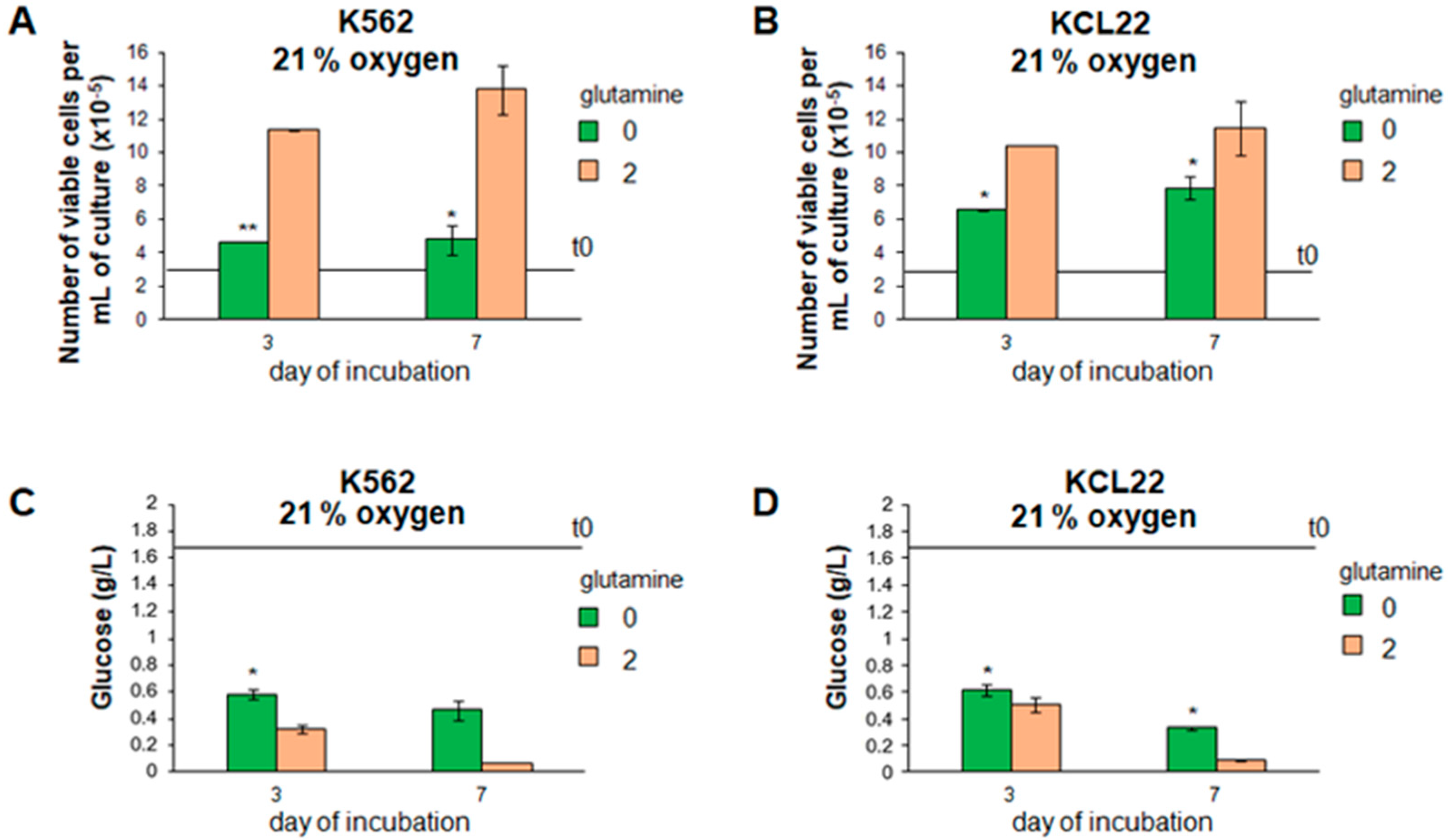
3.1. Cell Proliferation and Glucose Consumption Were Reduced in Glutamine-Free CML Cell Cultures
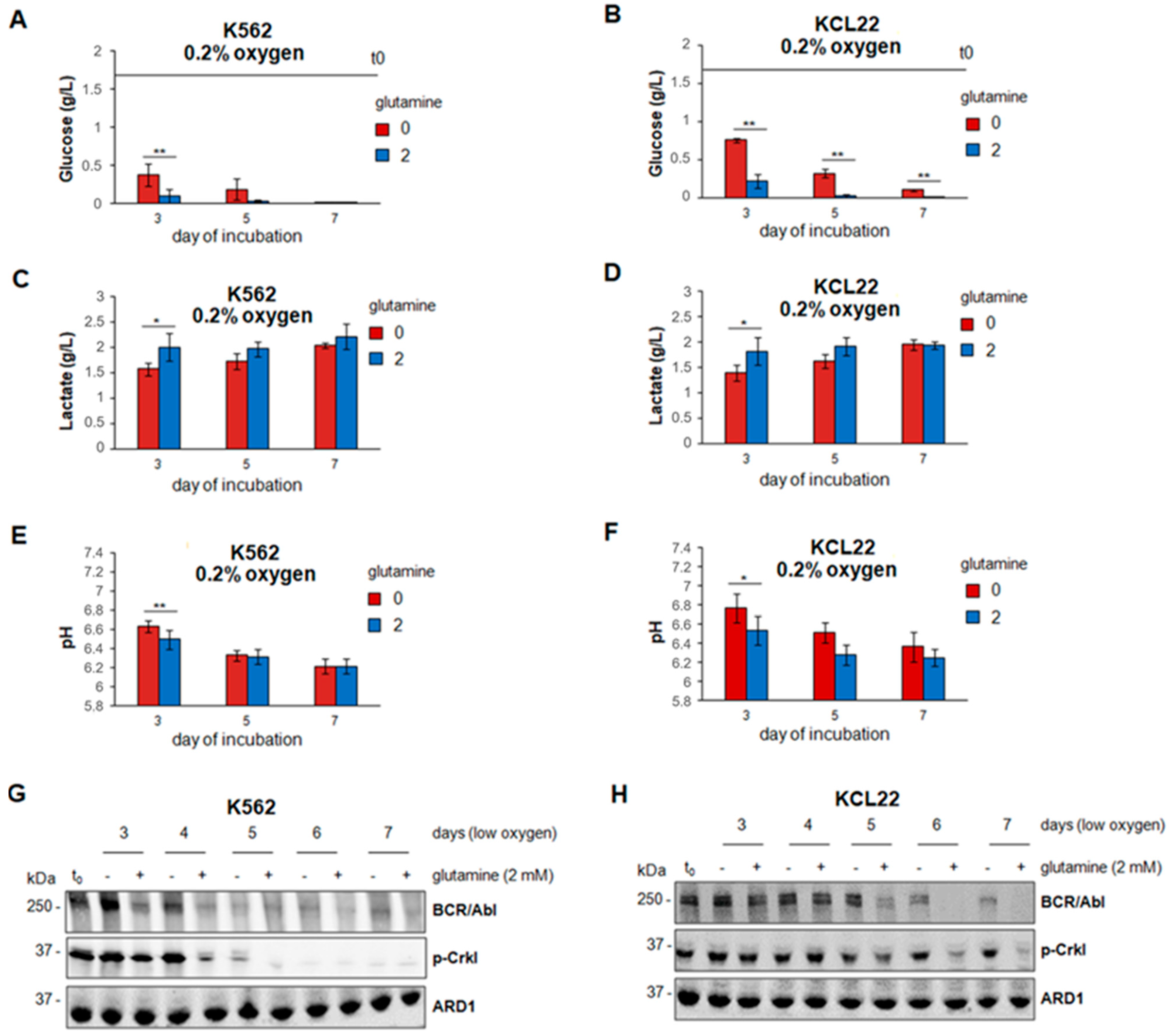
3.2. Glucose Consumption and BCR/Abl Protein Suppression in Low Oxygen Were Delayed in the Absence of Glutamine
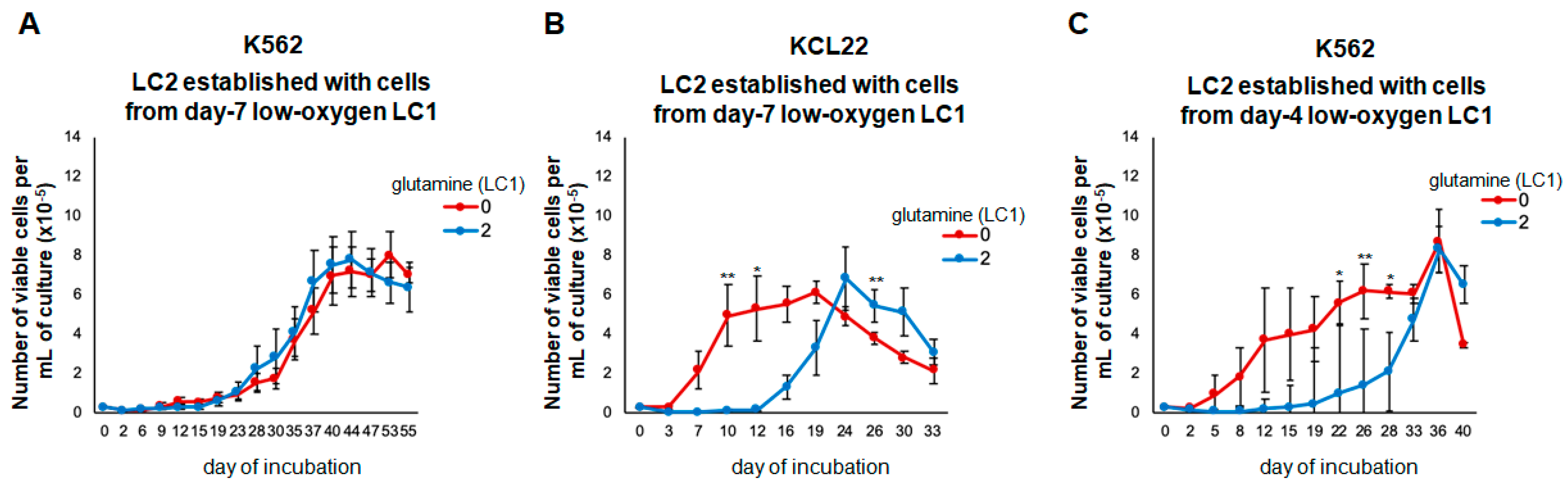
3.3. Glutamine Controls the Maintenance of Stem/Progenitor Cell Potential in Low Oxygen
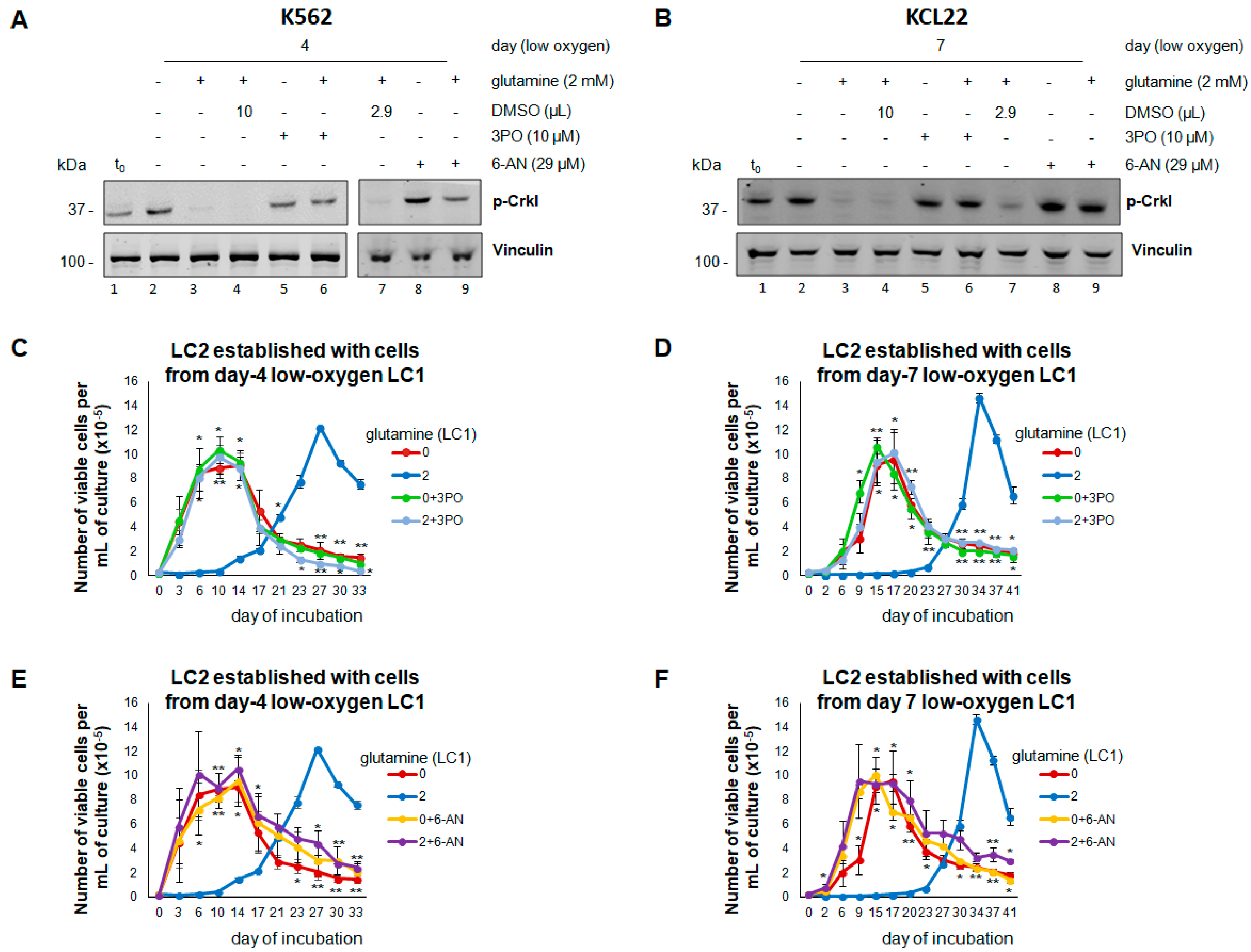
3.4. Inhibition of Glucose Catabolism in the Presence of Glutamine Mimics the Effects of Its Absence
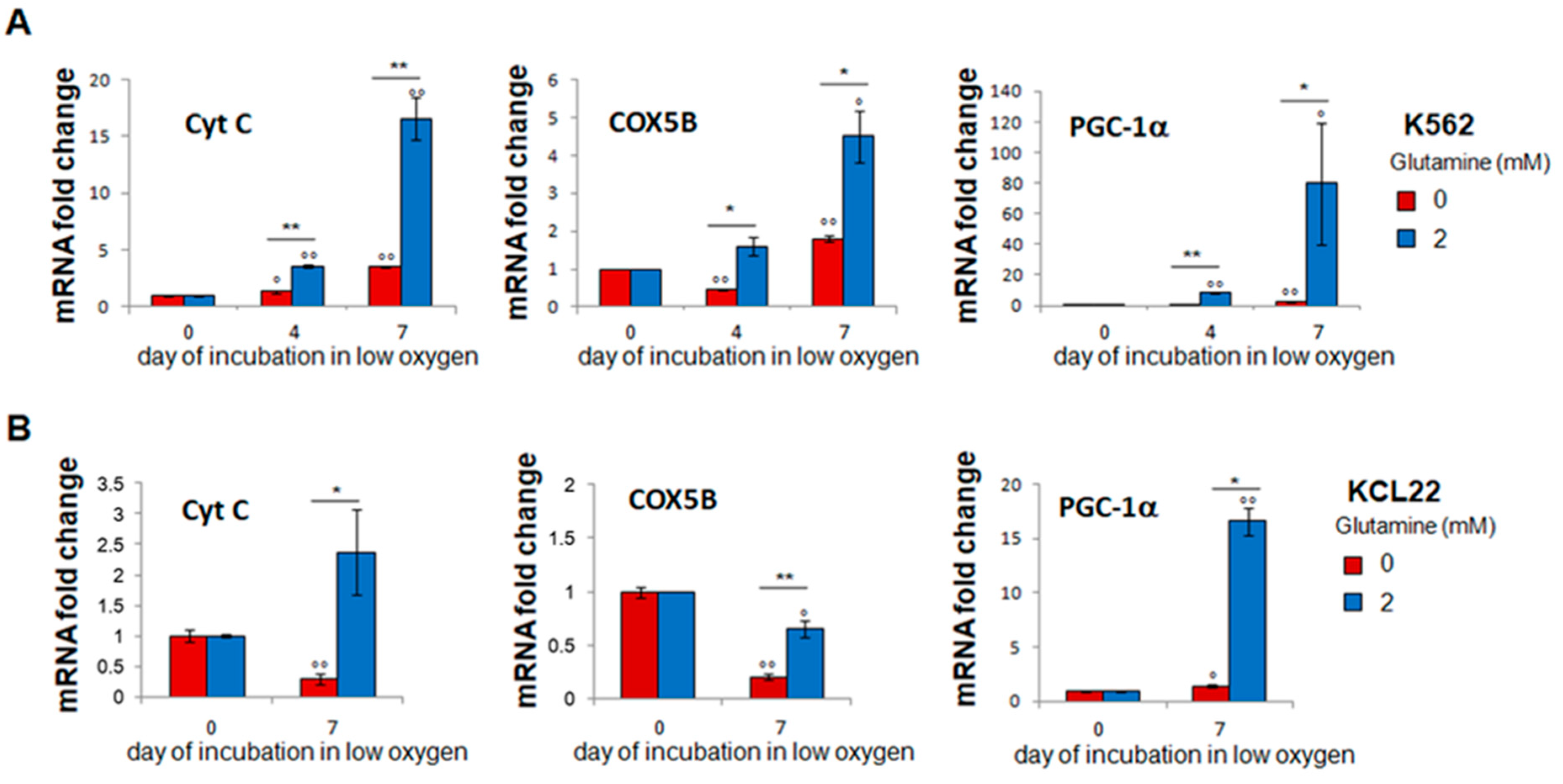
3.5. Glutamine-Dependent Glucose Exhaustion Is Counterbalanced by Respiration Induction
3.6. Inhibition of Glutamine Metabolism by BPTES Mimics the Effects of the Absence of Glutamine
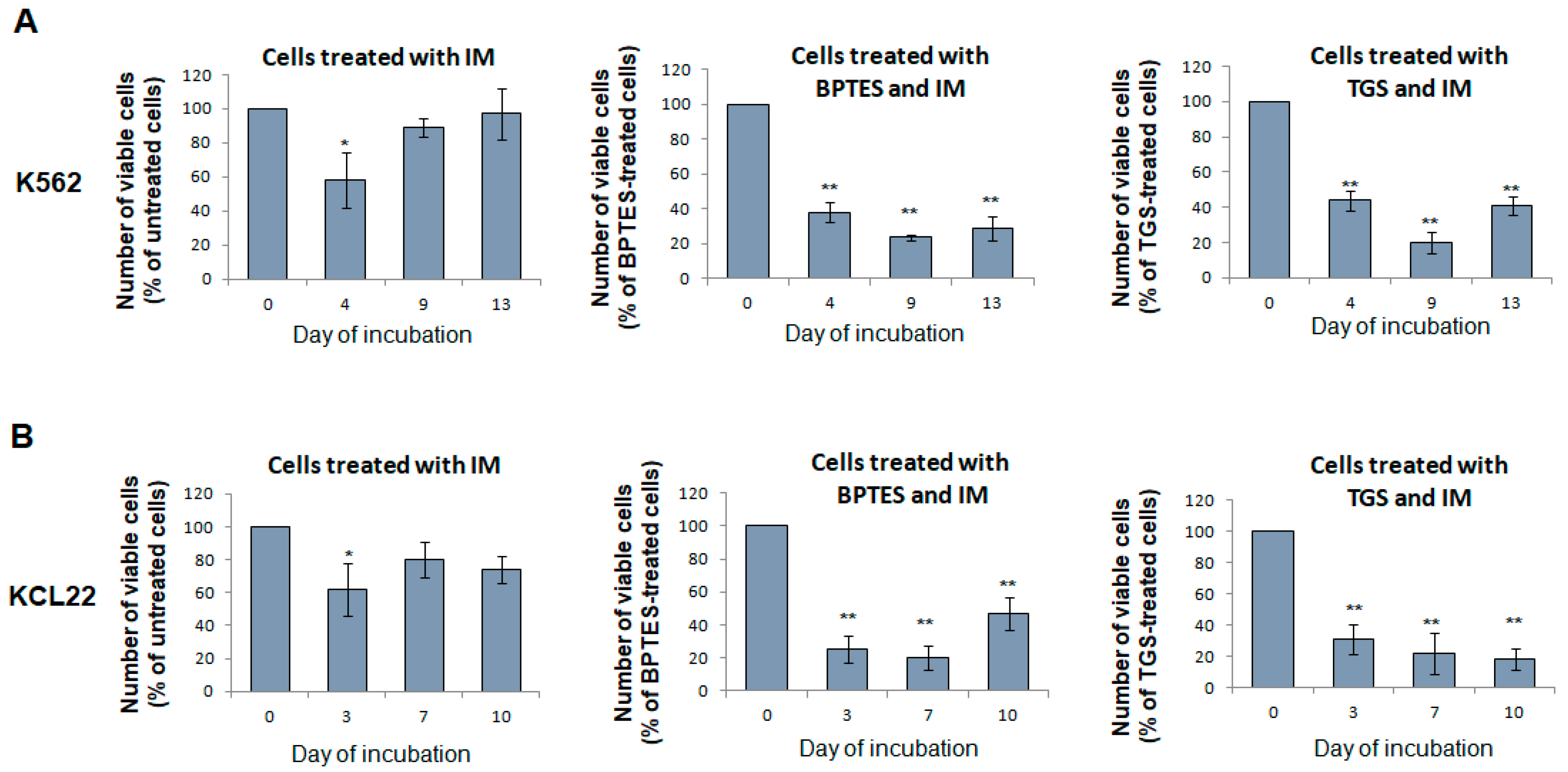
3.7. Glutaminase Inhibitors Sensitize CML Cells to TKi Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowley, J.D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973, 243, 290–293. [Google Scholar] [CrossRef]
- Chopade, P.; Akard, L.P. Improving outcomes in Chronic Myeloid Leukemia over time in the era of tyrosine kinase inhibitors. Clin. Lymph. Myel. Leuk. 2018, 18, 710–723. [Google Scholar] [CrossRef] [Green Version]
- Graham, S.M.; Jørgensen, H.G.; Allan, E.; Pearson, C.; Alcorn, M.J.; Richmond, L.; Holyoake, T.L. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 2002, 99, 319–325. [Google Scholar] [CrossRef]
- Cipolleschi, M.G.; Dello Sbarba, P.; Olivotto, M. The role of hypoxia in the maintenance of hemopoietic stem cells. Blood 1993, 82, 2031–2037. [Google Scholar] [CrossRef] [Green Version]
- Parmar, K.; Mauch, P.; Vergilio, J.A.; Sackstein, R.; Down, J.D. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl. Acad. Sci. USA 2007, 104, 5431–5436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohyeldin, A.; Garzon-Muvdi, T.; Quinones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simsek, T.; Kocabas, F.; Zheng, J.; Deberardinis, R.J.; Mahmoud, A.I.; Olson, E.N.; Schneider, J.W.; Zhang, C.C.; Sadek, H.A. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 2010, 7, 380–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, T.; Takubo, K.; Semenza, G.L. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 2011, 9, 298–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipolleschi, M.-G.; Rovida, E.; Ivanovic, Z.; Praloran, V.; Olivotto, M.; Dello Sbarba, P. The expansion of murine bone marrow cells preincubated in hypoxia as an in vitro indicator of their marrow-repopulating ability. Leukemia 2000, 14, 735–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuntoli, S.; Rovida, E.; Gozzini, A.; Barbetti, V.; Cipolleschi, M.-G.; Olivotto, M.; Dello Sbarba, P. Severe hypoxia defines heterogeneity and selects highly immature progenitors within clonal erythroleukemia cells. Stem Cells 2007, 25, 1119–1125. [Google Scholar] [CrossRef]
- Giuntoli, S.; Rovida, E.; Barbetti, V.; Cipolleschi, M.-G.; Olivotto, M.; Dello Sbarba, P. Hypoxia suppresses BCR/Abl and selects imatinib-insensitive progenitors within clonal CML populations. Leukemia 2006, 20, 1291–1293. [Google Scholar] [CrossRef]
- Giuntoli, S.; Tanturli, M.; Di Gesualdo, F.; Barbetti, V.; Rovida, E.; Dello Sbarba, P. Glucose availability in hypoxia regulates the selection of chronic myeloid leukemia progenitor subsets with different resistance to imatinib-mesylate. Haematologica 2011, 96, 204–212. [Google Scholar] [CrossRef]
- Rovida, E.; Peppicelli, S.; Bono, S.; Bianchini, F.; Tusa, I.; Cheloni, G.; Marzi, I.; Cipolleschi, M.G.; Calorini, L.; Sbarba, P.D. The metabolically-modulated stem cell niche: A dynamic scenario regulating cancer cell phenotype and resistance to therapy. Cell Cycle 2014, 13, 3169–3175. [Google Scholar] [CrossRef] [PubMed]
- Hosios, A.M.; Hecht, V.C.; Danai, L.V.; Johnson, M.O.; Rathmell, J.C.; Steinhauser, M.L.; Manalis, S.R.; Heiden, M.G.V. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev. Cell 2016, 36, 540–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellen, K.E.; Lu, C.; Mancuso, A.; Lemons, J.M.; Ryczko, M.; Dennis, J.W.; Rabinowitz, J.D.; Coller, H.A.; Thompson, C.B. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Devel. 2010, 24, 2784–2799. [Google Scholar] [CrossRef] [Green Version]
- Qie, S.; Liang, D.; Yin, C.; Gu, W.; Meng, M.; Wang, C.; Sang, N. Glutamine depletion and glucose depletion trigger growth inhibition via distinctive gene expression reprogramming. Cell Cycle 2012, 11, 3679–3690. [Google Scholar] [CrossRef] [Green Version]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef] [PubMed]
- Wappler, J.; Arts, M.; Röth, A.; Heeren, R.M.A.; Neumann, U.P.; Olde Damink, S.W.; Soons, Z.; Cramer, T. Glutamine deprivation counteracts hypoxia-induced chemoresistance. Neoplasia 2020, 22, 22–32. [Google Scholar] [CrossRef]
- Pantel, A.R.; Ackerman, D.; Lee, S.C.; Mankoff, D.A.; Gade, T.P. Imaging cancer metabolism: Underlying biology and emerging strategies. J. Nucl. Med. 2018, 59, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Lozzio, C.B.; Lozzio, B.B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood 1975, 45, 321–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubonishi, I.; Miyoshi, I. Establishment of a Ph1 chromosome-positive cell line from chronic myelogenous leukemia in blast crisis. Int. J. Cell Cloning 1983, 1, 105–117. [Google Scholar] [CrossRef]
- Sottnik, J.L.; Lori, J.C.; Rose, B.J.; Thamm, D.H. Glycolysis inhibition by 2-deoxy-d-glucose reverts the metastatic phenotype in vitro and in vivo. Clin. Exp. Metast. 2011, 28, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Ashton, T.M.; McKenna, W.G.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative phosphorylation as an emerging target in cancer therapy. Clin. Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoors, S.; De Bock, K.; Cantelmo, A.R.; Georgiadou, M.; Ghesquière, B.; Cauwenberghs, S.; Kuchnio, A.; Wong, B.W.; Quaegebeur, A.; Goveia, J.; et al. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 2014, 19, 37–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Xu, Q.; Ji, D.; Wei, Y.; Chen, H.; Li, T.; Wan, B.; Yuan, L.; Huang, R.; Chen, G. Inhibition of pentose phosphate pathway suppresses acute myelogenous leukemia. Tumor Biol. 2016, 37, 6027–6034. [Google Scholar] [CrossRef] [PubMed]
- Lukey, M.J.; Wilson, K.F.; Cerione, R.A. Therapeutic strategies impacting cancer cell glutamine metabolism. Future Med. Chem. 2013, 5, 1685–1700. [Google Scholar] [CrossRef] [Green Version]
- Cipolleschi, M.G.; Rovida, E.; Dello Sbarba, P. The Culture-Repopulating Ability assays and incubation in low oxygen: A simple way to test drugs on leukaemia stem or progenitor cells. Curr. Pharm. Des. 2013, 19, 5374–5383. [Google Scholar] [CrossRef] [Green Version]
- Cheloni, G.; Tanturli, M. The Culture Repopulation Ability (CRA) assay and incubation in low oxygen to test antileukemic drugs on imatinib-resistant CML stem-like cells. Methods Mol. Biol. 2016, 1465, 73–85. [Google Scholar]
- Cheloni, G.; Tanturli, M.; Tusa, I.; DeSouza, N.H.; Shan, Y.; Gozzini, A.; Mazurier, F.; Rovida, E.; Li, S.; Sbarba, P.D. Targeting chronic myeloid leukemia stem cells with the hypoxia-inducible factor inhibitor acriflavine. Blood 2017, 130, 655–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tusa, I.; Cheloni, G.; Poteti, M.; Gozzini, A.; DeSouza, N.H.; Shan, Y.; Deng, X.; Gray, N.S.; Li, S.; Rovida, E.; et al. Targeting the extracellular signal-regulated kinase 5 pathway to suppress human chronic myeloid leukemia stem cells. Stem Cell Rep. 2018, 11, 929–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cellai, C.; Laurenzana, A.; Vannucchi, A.M.; Della Malva, N.; Bianchi, L.; Paoletti, F. Specific PAF antagonist WEB-2086 induces terminal differentiation of murine and human leukemia cells. FASEB J. 2002, 16, 733–735. [Google Scholar] [CrossRef]
- Barnes, K.; McIntosh, E.; Whetton, A.D.; Daley, G.Q.; Bentley, J.; Baldwin, S.A. Chronic myeloid leukaemia: An investigation into the role of Bcr-Abl-induced abnormalities in glucose transport regulation. Oncogene 2005, 24, 3257–3267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wierenga, A.T.J.; Cunningham, A.; Erdem, A.; Vilaplana Lopera, N.; Brouwers-Vos, A.Z.; Pruis, M.; Mulder, A.B.; Günther, U.L.; Martens, J.H.A.; Vellenga, E.; et al. HIF1/2-exerted control over glycolytic gene expression is not functionally relevant for glycolysis in human leukemic stem/progenitor cells. Cancer Metab. 2019, 7, 11–27. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiani, C.; Colombo, R.; Gaglio, D.; Mastroianni, F.; Pescini, D.; Westerhoff, H.V.; Mauri, G.; Vanoni, M.; Alberghina, L. A metabolic core model elucidates how enhanced utilization of glucose and glutamine, with enhanced glutamine-dependent lactate production, promotes cancer cell growth: The WarburQ effect. PLoS Comput. Biol. 2017, 13, e1005758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fendt, S.-M.; Bell, E.L.; Keibler, M.A.; Davidson, S.M.; Wirth, G.J.; Fiske, B.; Mayers, J.; Schwab, M.; Bellinger, G.; Csibi, A.; et al. Metformin decreases glucose oxidation and increases the dependency of prostate cancer cells on reductive glutamine metabolism. Cancer Res. 2013, 73, 4429–4438. [Google Scholar] [CrossRef] [Green Version]
- Kaadige, M.R.; Looper, R.E.; Kamalanaadhan, S.; Ayer, D.E. Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proc. Natl. Acad. Sci. USA 2009, 106, 14878–14883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bono, S.; Lulli, M.; D’Agostino, V.G.; Di Gesualdo, F.; Loffredo, R.; Cipolleschi, M.-G.; Provenzani, A.; Rovida, E.; Sbarba, P.D. Different BCR/Abl protein suppression patterns as a converging trait of chronic myeloid leukemia cell adaptation to energy restriction. Oncotarget 2016, 7, 84810–84825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munksgaard Persson, M.; Johansson, M.E.; Monsef, N.; Planck, M.; Beckman, S.; Seckl, M.J.; Ronnstrand, L.; Påhlman, S.; Pettersson, H.M. HIF-2alpha expression is suppressed in SCLC cells, which survive in moderate and severe hypoxia when HIF-1alpha is repressed. Am. J. Pathol. 2012, 180, 494–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovida, E.; Marzi, I.; Cipolleschi, M.G.; Dello Sbarba, P. One more stem cell niche: How the sensitivity of chronic myeloid leukemia cells to imatinib-mesylate is modulated within a “hypoxic” environment. Hypoxia 2014, 2, 1–10. [Google Scholar] [PubMed] [Green Version]
- Cheloni, G.; Poteti, M.; Bono, S.; Masala, E.; Mazure, N.M.; Rovida, E.; Lulli, M.; Dello Sbarba, P. The leukemic stem cell niche: Adaptation to “hypoxia” versus oncogene addiction. Stem Cells Intern. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Kamphorst, J.J.; Mathew, R.; Chung, M.K.; White, E.; Shlomi, T.; Rabinowitz, J.D. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol. Syst. Biol. 2013, 9, 712–722. [Google Scholar] [CrossRef]
- Eales, K.L.; Hollinshead, K.E.R.; Tennant, D.A. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 2016, 5, e190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonveaux, P.; Végran, F.; Schroeder, T.; Wergin, M.; Verrax, J.; Rabbani, Z.N.; de Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar] [CrossRef] [Green Version]
- Lagadinou, E.D.; Sach, A.; Callahan, K.; Rossi, R.M.; Neering, S.J.; Minhajuddin, M.; Ashton, J.M.; Pei, S.; Grose, V.; O’Dwyer, K.M.; et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 2013, 12, 329–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuntz, E.M.; Baquero, P.; Michie, A.M.; Dunn, K.; Tardito, S.; Holyoake, T.L.; Helgason, G.V.; Gottlieb, E. Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells. Nat. Med. 2017, 23, 1234–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Meng, Y.; Li, L.; Xu, P.F.; Wang, J.; Li, Z.; Bian, J. Overview of the Development of Glutaminase Inhibitors: Achievements and Future Directions. J. Med. Chem. 2019, 62, 1096–1115. [Google Scholar] [CrossRef]


| Gene | Forward | Reverse |
|---|---|---|
| b-actin | 5′-TCGAGCCATAAAAGGCAACT-3′ | 5′-CTTCCTCAATCTCGCTCTCG-3′ |
| Cyt-C | 5′-TTGCACTTACACCGGTACTTAAGC-3′ | 5′-ACGTCCCCACTCTCTAAGTCCAA-3′ |
| COX5B | 5′-TGCGCTCCATGGCATCT-3′ | 5′-CCCAGTCGCCTGCTCTTC-3′ |
| PGC-1 | 5′-GGGAAAGTGAGCGATTAGTTGAG-3′ | 5′-CATGTAGAATTGGCAGGTGGAA-3′ |
| 18S | 5′-CGCCGCTAGAGGTGAAATTCT-3′ | 5′-CGAACCTCCGA CTTTCGTTCT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poteti, M.; Menegazzi, G.; Peppicelli, S.; Tusa, I.; Cheloni, G.; Silvano, A.; Mancini, C.; Biagioni, A.; Tubita, A.; Mazure, N.M.; et al. Glutamine Availability Controls BCR/Abl Protein Expression and Functional Phenotype of Chronic Myeloid Leukemia Cells Endowed with Stem/Progenitor Cell Potential. Cancers 2021, 13, 4372. https://doi.org/10.3390/cancers13174372
Poteti M, Menegazzi G, Peppicelli S, Tusa I, Cheloni G, Silvano A, Mancini C, Biagioni A, Tubita A, Mazure NM, et al. Glutamine Availability Controls BCR/Abl Protein Expression and Functional Phenotype of Chronic Myeloid Leukemia Cells Endowed with Stem/Progenitor Cell Potential. Cancers. 2021; 13(17):4372. https://doi.org/10.3390/cancers13174372
Chicago/Turabian StylePoteti, Martina, Giulio Menegazzi, Silvia Peppicelli, Ignazia Tusa, Giulia Cheloni, Angela Silvano, Caterina Mancini, Alessio Biagioni, Alessandro Tubita, Nathalie M. Mazure, and et al. 2021. "Glutamine Availability Controls BCR/Abl Protein Expression and Functional Phenotype of Chronic Myeloid Leukemia Cells Endowed with Stem/Progenitor Cell Potential" Cancers 13, no. 17: 4372. https://doi.org/10.3390/cancers13174372








