Health-Related Quality of Life in Oral Cancer Patients: Scoping Review and Critical Appraisal of Investigated Determinants
Abstract
:Simple Summary
Abstract
1. Introduction
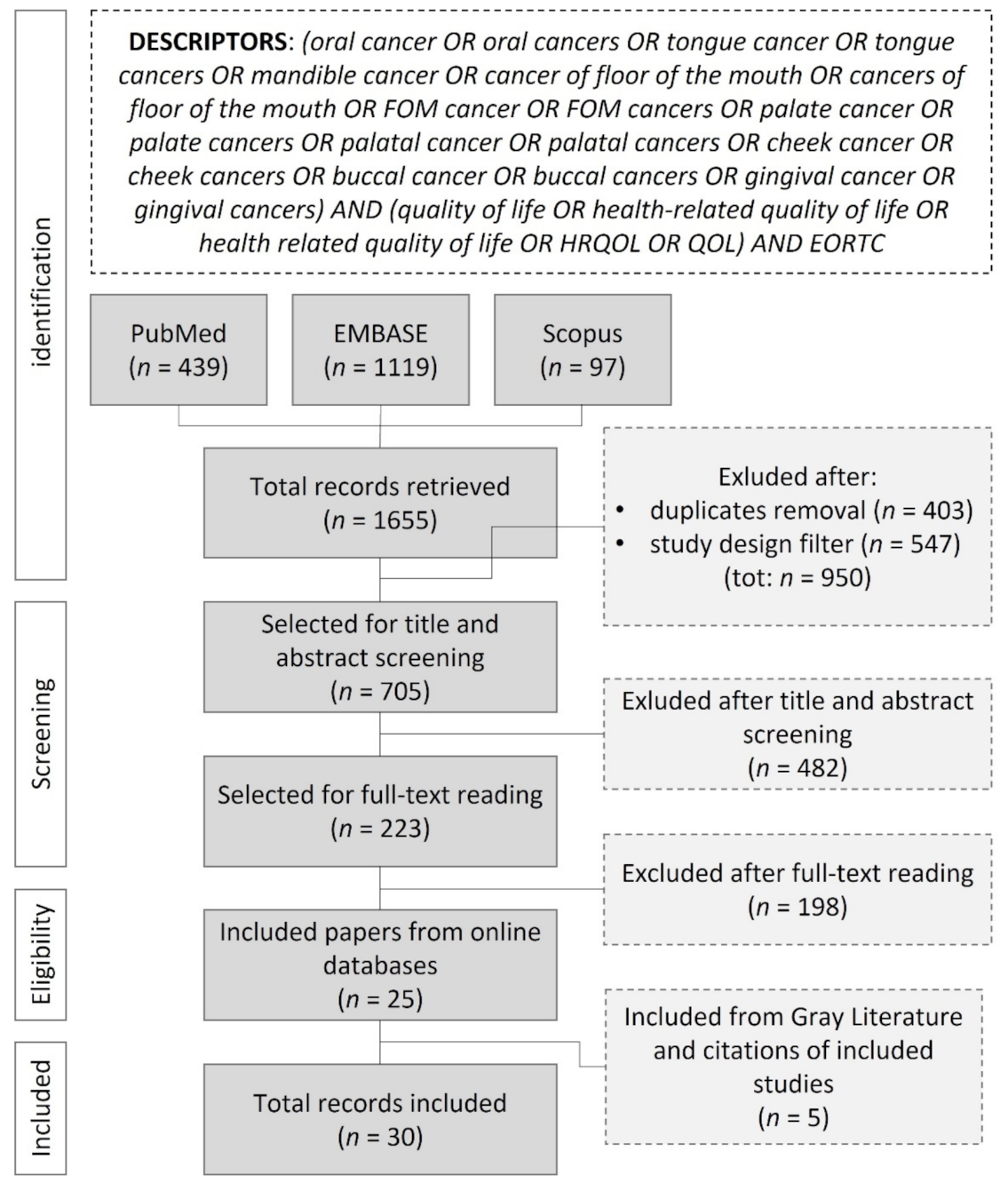
2. Materials and Methods
2.1. Data Extraction
2.2. Critical Appraisal
- “Stratified” for each independent variable related to EORTC QLQ-C30 and/or EORTC QLQ-H&N35/43 *.
- “Homogeneous” for each independent variable when all the included cases were equal concerning that specific feature.
- “Excluded” or “not present in the sample” for each independent variable if the cases reporting that specific feature were excluded during cohort selection, or if that specific feature was not observed in the screened population.
- “Incomplete stratification” for each independent variable related to EORTC QLQ-C30 and/or EORTC QLQ-H&N35/43, in case of uneven or incomplete sample grouping rules.
- “Not stratified” for each independent variable that was reported but not related to EORTC QLQ-C30 and/or EORTC QLQ-H&N35/43.
- “Not available” for each independent variable that did not clearly describe or was not described in the sample features.
- GREEN: stratified, stratified by oral subsites, homogeneous, excluded, not present in the sample.
- YELLOW: incomplete stratification, incomplete stratification by oral subsites.
- LIGHT RED: not stratified, not stratified by oral subsites.
- RED: not available, not clear.
3. Results
3.1. Study Design
3.2. Sociodemographic Variables (SDG)
3.3. Disease- and Treatment-Specific Variables (DT)
| DISEASE AND TREATMENT VARIABLES (DT) | SOCIODEMOGRAPHIC VARIABLES (SDG) | TOTAL VARIABLES CONSIDERED FOR DATA STRATIFICATION (DT+SDG) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Article | OC Sample | (1) S/SOS/H/EX/NP | (2) IS/ISOS | (3) NS/NSOS | (4) na | Considered Var (Tot 1 + 2) | Ignored Var (Tot 3 + 4) | (1) S/SOS/H/EX/NP | (2) IS/ISOS | (3) NS/NSOS | (4) na | Considered Var (Tot 1 + 2) | Ignored Var (Tot 3 + 4) | (1) S/SOS/H/EX/NP | (2) IS/ISOS | (3) NS/NSOS | (4) na | Considered Var (Tot 1 + 2) | Ignored Var (Tot 3 + 4) |
| Airoldi 2011 [22] | 50 | 2 | 0 | 3 | 10 | 2 | 13 | 0 | 0 | 7 | 1 | 0 | 8 | 2 | 0 | 10 | 11 | 2 | 21 |
| Beck 2017 [24] | 45 | 2 | 3 | 1 | 9 | 5 | 10 | 0 | 0 | 2 | 6 | 0 | 8 | 2 | 3 | 3 | 15 | 5 | 18 |
| Becker 2012 [23] | 50 | 1 | 4 | 4 | 6 | 5 | 10 | 0 | 0 | 4 | 4 | 0 | 8 | 1 | 4 | 8 | 10 | 5 | 18 |
| Borggreven 2007 [25] | 38 | 1 | 1 | 8 | 5 | 2 | 13 | 3 | 0 | 1 | 4 | 3 | 5 | 4 | 1 | 9 | 9 | 5 | 18 |
| Bozec 2009 [26] | 21 | 3 | 2 | 3 | 7 | 5 | 10 | 3 | 0 | 0 | 5 | 3 | 5 | 6 | 2 | 3 | 12 | 8 | 15 |
| Bozec 2020 [27] | 48 | 1 | 1 | 2 | 11 | 2 | 13 | 7 | 0 | 0 | 1 | 7 | 1 | 8 | 1 | 2 | 12 | 9 | 14 |
| Canis 2016 [28] | 40 | 10 | 1 | 1 | 3 | 11 | 4 | 0 | 0 | 4 | 4 | 0 | 8 | 10 | 1 | 5 | 7 | 11 | 12 |
| Crombie 2014 [10] | 16 | 0 | 1 | 9 | 5 | 1 | 14 | 0 | 0 | 2 | 6 | 0 | 8 | 0 | 1 | 11 | 11 | 1 | 22 |
| Davudov 2019 [29] | 120 | 2 | 0 | 3 | 10 | 2 | 13 | 0 | 0 | 3 | 5 | 0 | 8 | 2 | 0 | 6 | 15 | 2 | 21 |
| Dzioba 2017 [30] | 117 | 2 | 1 | 6 | 6 | 3 | 12 | 0 | 0 | 2 | 6 | 0 | 8 | 2 | 1 | 8 | 12 | 3 | 20 |
| Ferri 2020 [31] | 55 | 12 | 1 | 1 | 1 | 13 | 2 | 0 | 0 | 2 | 6 | 0 | 8 | 12 | 1 | 3 | 7 | 13 | 10 |
| Girod 2009 [32] | 34 | 2 | 2 | 1 | 10 | 4 | 11 | 0 | 0 | 3 | 5 | 0 | 8 | 2 | 2 | 4 | 15 | 4 | 19 |
| Huang 2010 [33] | 129 | 1 | 5 | 1 | 8 | 6 | 9 | 6 | 0 | 0 | 2 | 6 | 2 | 7 | 5 | 1 | 10 | 12 | 11 |
| Infante-Cossio 2009 [34] | 70 | 5 | 1 | 1 | 8 | 6 | 9 | 0 | 0 | 2 | 6 | 0 | 8 | 5 | 1 | 3 | 14 | 6 | 17 |
| Kessler 2004 [35] | 41 | 5 | 1 | 6 | 3 | 6 | 9 | 0 | 0 | 2 | 6 | 0 | 8 | 5 | 2 | 7 | 9 | 7 | 16 |
| Khandelwal 2017 [9] | 50 | 1 | 1 | 3 | 10 | 2 | 13 | 2 | 0 | 0 | 6 | 2 | 6 | 3 | 1 | 3 | 16 | 4 | 19 |
| Klug 2002 [36] | 67 | 5 | 2 | 1 | 7 | 7 | 8 | 1 | 0 | 1 | 6 | 1 | 7 | 6 | 2 | 2 | 13 | 8 | 15 |
| Kovacs 2015 [37] | 110 | 4 | 1 | 3 | 7 | 5 | 10 | 1 | 0 | 1 | 6 | 1 | 7 | 5 | 1 | 4 | 13 | 6 | 17 |
| Lin 2020 [38] | 22 | 5 | 0 | 3 | 7 | 5 | 10 | 0 | 0 | 3 | 5 | 0 | 8 | 5 | 0 | 6 | 12 | 5 | 18 |
| Mair 2017 [39] | 225 | 10 | 1 | 0 | 4 | 11 | 4 | 0 | 0 | 2 | 6 | 0 | 8 | 10 | 1 | 2 | 10 | 11 | 12 |
| Moubayed 2014 [40] | 13 | 2 | 0 | 3 | 10 | 2 | 13 | 0 | 0 | 1 | 7 | 0 | 8 | 2 | 0 | 4 | 17 | 2 | 21 |
| Nordgren 2008 [41] | 122 | 1 | 0 | 2 | 12 | 1 | 14 | 0 | 0 | 3 | 5 | 0 | 8 | 1 | 0 | 5 | 17 | 1 | 22 |
| Oates 2008 [42] | 47 | 1 | 0 | 2 | 12 | 1 | 14 | 0 | 0 | 0 | 8 | 0 | 8 | 1 | 0 | 2 | 20 | 1 | 22 |
| Oskam 2013 [43] | 38 | 0 | 1 | 3 | 11 | 1 | 14 | 2 | 1 | 3 | 2 | 3 | 5 | 2 | 2 | 6 | 13 | 4 | 19 |
| Peisker 2016 [44] | 100 | 0 | 0 | 3 | 12 | 0 | 15 | 0 | 0 | 2 | 6 | 0 | 8 | 0 | 0 | 5 | 18 | 0 | 23 |
| Petruson 2005 * [45] | 30 | 11 | 0 | 1 | 3 | 11 | 4 | 0 | 0 | 2 | 6 | 0 | 8 | 11 | 0 | 3 | 9 | 11 | 12 |
| Pierre 2014 [46] | 37 | 4 | 2 | 3 | 6 | 6 | 9 | 3 | 0 | 0 | 5 | 3 | 5 | 7 | 2 | 3 | 11 | 9 | 14 |
| Schoen 2008 [47] | 41 | 1 | 0 | 3 | 11 | 1 | 14 | 0 | 0 | 2 | 6 | 0 | 8 | 1 | 0 | 5 | 17 | 1 | 22 |
| Van Gemert 2015 [48] | 37 | 5 | 3 | 1 | 6 | 8 | 7 | 1 | 1 | 0 | 6 | 2 | 6 | 6 | 4 | 1 | 12 | 10 | 13 |
| Yoshimura 2009 * [49] | 20 | 13 | 2 | 0 | 0 | 15 | 0 | 2 | 0 | 0 | 6 | 2 | 6 | 15 | 2 | 0 | 6 | 17 | 6 |
| AVG (SD) | 3.7 (3.8) | 1.2 (1.3) | 2.7 (2.2) | 7.3 (3.3) | 5.0 (4.0) | 10.0 (4.0) | 1.0 (1.8) | 0.1 (0.3) | 1.8 (1.6) | 5.1 (1.7) | 1.1 (1.8) | 6.9 (1.8) | 4.8 (3.9) | 1.3 (1.3) | 4.5 (2.8) | 12.4 (3.5) | 6.1 (4.3) | 16.9 (4.3) | |
| WEIGHTED AVG BY OC SAMPLE (SD) | 3.8 (3.7) | 1.3 (1.4) | 2.4 (1.9) | 7.5 (3.2) | 5.1 (3.8) | 9.9 (3.8) | 1.0 (1.9) | 0.0 (0.2) | 1.9 (1.4) | 5.1 (1.6) | 1.0 (1.9) | 7.0 (1.9) | 4.8 (3.7) | 1.3 (1.4) | 4.2 (2.5) | 12.6 (3.2) | 6.1 (4.2) | 16.9 (4.2) | |
3.4. Descriptive Analysis
4. Discussion
4.1. Sociodemographic Variables
4.1.1. Gender and Age
4.1.2. Marital Status and Family
4.1.3. Comorbidity
4.1.4. Alcohol, Smoke, and Educational Level
4.2. Disease- and Treatment-Specific Variables
4.2.1. Cancer Site
4.2.2. Cancer Stage
4.2.3. Mandibular Resection
4.2.4. Extent of Resection
4.2.5. Surgical Approach
4.2.6. Neck Dissection
4.2.7. Reconstruction
4.2.8. Radiotherapy and Chemotherapy
4.2.9. Synchronous Lesions, Recurrences, and Metachronous Lesions
4.2.10. Major Surgical Complications and Secondary Surgery
4.2.11. Other Variables
5. Conclusions and Recommendations for Future Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Medicines Agency. Pre-Authorisation Evaluation of Medicines for Human Use. Doc. Ref. EMEA/CHMP/EWP/139391/2004. London. 2005. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-regulatory-guidance-use-healthrelated-quality-life-hrql-measures-evaluation_en.pdf (accessed on 28 August 2021).
- Apolone, G.; De Carli, G.; Brunetti, M.; Garattini, S. Health-related quality of life (HR-QOL) and regulatory issues. An assessment of the European Agency for the Evaluation of Medicinal Products (EMEA) recommendations on the use of HR-QOL measures in drug approval. PharmacoEconomics 2001, 19, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Marquis, P.; Caron, M.; Emery, M.-P.; Scott, J.A.; Arnould, B.; Acquadro, C. The Role of Health-Related Quality of Life Data in the Drug Approval Processes in the US and Europe. Pharm. Med. 2011, 25, 147–160. [Google Scholar] [CrossRef]
- Ferrans, C.E.P. Definitions and Conceptual Models of Quality of Life. Outcomes Assessment in Cancer: Measures, Methods, and Applications; Cambridge University Press (CUP): New York, NY, USA, 2005; pp. 14–30. [Google Scholar]
- Heckscher, A.L. The quality of American culture. In Goals for Americans: (The Report of the Presidential Commission on National Goals and Chapters Submitted for the Consideration of the Commission of the American Assembly); Prentice-Hall Washington: Washington, DC, USA, 1960; pp. 127–146. [Google Scholar]
- Morton, R.P. Evolution of quality of life assessment in head and neck cancer. J. Laryngol. Otol. 1995, 109, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Dietz, A.; Meyer, A.; Singer, S. Lebensqualitätsmessungen bei Patienten mit Kopf-Hals-Malignomen. HNO 2009, 57, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Shumaker, S.A.; Ellis, S.; Naughton, M. Assessing health-related quality of life in HIV disease: Key measurement issues. Qual. Life Res. 1997, 6, 475–480. [Google Scholar] [CrossRef]
- Khandelwal, A.; Neeli, A.; Gadiyar, A.; Khandelwal, A. Assessment of quality of life of patients 1–5 years after treatment for oral cancer. Indian J. Dent. Res. 2017, 28, 538. [Google Scholar] [CrossRef]
- Crombie, A.K.; Farah, C.S.; Batstone, M.D. Health-related quality of life of patients treated with primary chemoradiotherapy for oral cavity squamous cell carcinoma: A comparison with surgery. Br. J. Oral Maxillofac. Surg. 2014, 52, 111–117. [Google Scholar] [CrossRef]
- Smith, S.C.; Lamping, D.L.; Banerjee, S.; Harwood, R.H.; Foley, B.; Smith, P.; Cook, J.C.; Murray, J.; Prince, M.; Levin, E.; et al. Development of a new measure of health-related quality of life for people with dementia: DEMQOL. Psychol. Med. 2006, 37, 737–746. [Google Scholar] [CrossRef]
- Torres-Carranza, E.; Infante-Cossío, P.; Hernández-Guisado, J.M.; Hens-Aumente, E.; Gutierrez-Pérez, J.L. Assessment of quality of life in oral cancer. Med. Oral Patol. Oral Cir. Bucal 2008, 13, E735–E741. [Google Scholar]
- Bjordal, K.; de Graeff, A.; Fayers, P.; Hammerlid, E.; van Pottelsberghe, C.; Curran, D.; Ahlner-Elmqvist, M.; Maher, E.; Meyza, J.; Brédart, A.; et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. Eur. J. Cancer 2000, 36, 1796–1807. [Google Scholar] [CrossRef]
- Singer, S.; Amdal, C.D.; Hammerlid, E.; Tomaszewska, I.M.; Silva, J.C.; Mehanna, H.; Santos, M.; Inhestern, J.; Brannan, C.; Yarom, N.; et al. International validation of the revised European Organisation for Research and Treatment of Cancer Head and Neck Cancer Module, the EORTC QLQ-HN43: Phase IV. Head Neck 2019, 41, 1725–1737. [Google Scholar] [CrossRef]
- Quinten, C.; Coens, C.; Ghislain, I.; Zikos, E.; Sprangers, M.A.; Ringash, J.; Martinelli, F.; Ediebah, D.E.; Maringwa, J.; Reeve, B.B.; et al. The effects of age on health-related quality of life in cancer populations: A pooled analysis of randomized controlled trials using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 involving 6024 cancer patients. Eur. J. Cancer 2015, 51, 2808–2819. [Google Scholar] [CrossRef]
- Chandu, A.; Smith, A.C.; Rogers, S.N. Health-Related Quality of Life in Oral Cancer: A Review. J. Oral Maxillofac. Surg. 2006, 64, 495–502. [Google Scholar] [CrossRef]
- Murphy, B.A.; Ridner, S.; Wells, N.; Dietrich, M. Quality of life research in head and neck cancer: A review of the current state of the science. Crit. Rev. Oncol. 2007, 62, 251–267. [Google Scholar] [CrossRef]
- Samuel, S.R.; Maiya, A.G.; Fernandes, D.J.; Guddattu, V.; Saxena, P.P.; Kurian, J.R.; Lin, P.-J.; Mustian, K.M. Effectiveness of exercise-based rehabilitation on functional capacity and quality of life in head and neck cancer patients receiving chemo-radiotherapy. Support. Care Cancer 2019, 27, 3913–3920. [Google Scholar] [CrossRef] [PubMed]
- So, W.; Chan, R.; Chan, D.; Hughes, B.; Chair, S.; Choi, K.; Chan, C. Quality-of-life among head and neck cancer survivors at one year after treatment—A systematic review. Eur. J. Cancer 2012, 48, 2391–2408. [Google Scholar] [CrossRef] [PubMed]
- Talmi, Y.P. Quality of life issues in cancer of the oral cavity. J. Laryngol. Otol. 2002, 116, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis. 2020. Available online: https://synthesismanual.jbi.global (accessed on 13 November 2020).
- Airoldi, M.; Garzaro, M.; Raimondo, L.; Pecorari, G.; Giordano, C.; Varetto, A.; Caldera, P.; Torta, R. Functional and psychological evaluation after flap reconstruction plus radiotherapy in oral cancer. Head Neck 2011, 33, 458–468. [Google Scholar] [CrossRef]
- Becker, S.T.; Menzebach, M.; Küchler, T.; Hertrampf, K.; Wenz, H.-J.; Wiltfang, J. Quality of life in oral cancer patients—Effects of mandible resection and socio-cultural aspects. J. Cranio-Maxillofac. Surg. 2012, 40, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Beck-Broichsitter, B.E.; Huck, J.; Küchler, T.; Hauke, D.; Hedderich, J.; Wiltfang, J.; Becker, S.T. Self-perception versus professional assessment of functional outcome after ablative surgery in patients with oral cancer. J. Cancer Res. Clin. Oncol. 2016, 143, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Borggreven, P.A.; Aaronson, N.K.; Leeuw, I.M.V.-D.; Muller, M.J.; Heiligers, M.L.; de Bree, R.; Langendijk, J.A.; Leemans, C.R. Quality of life after surgical treatment for oral and oropharyngeal cancer: A prospective longitudinal assessment of patients reconstructed by a microvascular flap. Oral Oncol. 2007, 43, 1034–1042. [Google Scholar] [CrossRef]
- Bozec, A.; Poissonnet, G.; Chamorey, E.; Casanova, C.; Vallicioni, J.; Demard, F.; Peyrade, F.; Follana, P.; Bensadoun, R.J.; Benezery, K.; et al. Quality of life after oral and oropharyngeal recon-struction with a radial forearm free flap: Prospective study. J. Otolaryngol. Head Neck Surg. = Le J. D’oto-Rhino-Laryngol. Chir. Cervico-Faciale 2009, 38, 401–408. [Google Scholar]
- Bozec, A.; Majoufre, C.; De Boutray, M.; Gal, J.; Chamorey, E.; Roussel, L.-M.; Philouze, P.; Testelin, S.; Coninckx, M.; Bach, C.; et al. Oral and oropharyngeal cancer surgery with free-flap reconstruction in the elderly: Factors associated with long-term quality of life, patient needs and concerns. A GETTEC cross-sectional study. Surg. Oncol. 2020, 35, 81–88. [Google Scholar] [CrossRef]
- Canis, M.; Weiss, B.G.; Ihler, F.; Hummers-Pradier, E.; Matthias, C.; Wolff, H.A. Quality of life in patients after resection of pT3 lateral tongue carcinoma: Microvascular reconstruction versus primary closure. Head Neck 2014, 38, 89–94. [Google Scholar] [CrossRef]
- Davudov, M.M.; Harirchi, I.; Arabkheradmand, A.; Garajei, A.; Mahmudzadeh, H.; Shirkhoda, M.; Motiee-Langroudi, M.; Mirzajani, Z.; Zebardast, J.; Montazeri, A. Evaluation of quality of life in patients with oral cancer after mandibular resection. Medicine 2019, 98, e17431. [Google Scholar] [CrossRef]
- Dzioba, A.; Head and Neck Research Network; Aalto, D.; Papadopoulos-Nydam, G.; Seikaly, H.; Rieger, J.; Wolfaardt, J.; Osswald, M.; Harris, J.R.; O’Connell, D.A.; et al. Functional and quality of life outcomes after partial glossectomy: A multi-institutional longitudinal study of the head and neck research network. J. Otolaryngol. Head Neck Surg. 2017, 46, 56. [Google Scholar] [CrossRef] [Green Version]
- Ferri, A.; Perlangeli, G.; Montalto, N.; Lizarazo, J.L.C.; Bianchi, B.; Ferrari, S.; Nicolai, P.; Sesenna, E.; Grammatica, A. Transoral resection with buccinator flap reconstruction vs. pull-through resection and free flap reconstruction for the management of T1/T2 cancer of the tongue and floor of the mouth. J. Cranio-Maxillofac. Surg. 2020, 48, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Girod, D.A.; Sykes, K.; Jorgensen, J.; Tawfik, O.; Tsue, T. Acellular dermis compared to skin grafts in oral cavity reconstruction. Laryngoscope 2009, 119, 2141–2149. [Google Scholar] [CrossRef]
- Huang, T.-L.; Tsai, W.-L.; Chien, C.-Y.; Lee, T.-F.; Fang, F.-M. Quality of life for head and neck cancer patients treated by combined modality therapy: The therapeutic benefit of technological advances in radiotherapy. Qual. Life Res. 2010, 19, 1243–1254. [Google Scholar] [CrossRef]
- Infante-Cossio, P.; Torres-Carranza, E.; Cayuela, A.; Hens-Aumente, E.; Pastor-Gaitan, P.; Gutiérrez-Pérez, J. Impact of treatment on quality of life for oral and oropharyngeal carcinoma. Int. J. Oral Maxillofac. Surg. 2009, 38, 1052–1058. [Google Scholar] [CrossRef]
- Kessler, P.A.; Bloch-Birkholz, A.; Leher, A.; Neukam, F.W.; Wiltfang, J. Evaluation of quality of life of patients with oral squamous cell carcinoma. Comparison of two treatment protocols in a prospective study. Radiother. Oncol. 2004, 70, 275–282. [Google Scholar] [CrossRef]
- Klug, C.; Neuburg, J.; Gläser, C.; Schwarz, B.; Kermer, C.; Millesi, W. Quality of life 2–10 years after combined treatment for advanced oral and oropharyngeal cancer. Int. J. Oral Maxillofac. Surg. 2002, 31, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Stefenelli, U.; Thorn, G. Long-term quality of life after intensified multi-modality treatment of oral cancer including intra-arterial induction chemotherapy and adjuvant chemoradiation. Ann. Maxillofac. Surg. 2015, 5, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Lin, N.-C.; Lin, S.-L.; Tsai, K.-Y. Barrel-shaped design of the forearm free flap for lower lip reconstruction: A pilot case-control study. BMC Surg. 2020, 20, 132. [Google Scholar] [CrossRef]
- Mair, M.D.; Nair, S.; Nikam, S.; Nair, D.; Agarwal, J.P.; Chaturvedi, P. Longitudinal and cross-sectional assessment of quality of life in surgically treated advanced (T4) cancer of the buccal mucosa. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 529–536. [Google Scholar] [CrossRef]
- Moubayed, S.P.; L’Heureux-Lebeau, B.; Christopoulos, A.; Sampalis, J.S.; Letourneau-Guillon, L.; Bissada, E.; Guertin, L.; Harris, P.G.; Danino, A.M.; Ayad, T. Osteocutaneous free flaps for mandibular reconstruction: Systematic review of their frequency of use and a preliminary quality of life comparison. J. Laryngol. Otol. 2014, 128, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, M.; Hammerlid, E.; Bjordal, K.; Boysen, M.; Jannert, M.; Ahlner-Elmqvist, M. Quality of life in oral carcinoma: A 5-year prospective study. Head Neck 2008, 30, 461–470. [Google Scholar] [CrossRef]
- Oates, J.; Clark, J.R.; Read, J.; Reeves, N.; Gao, K.; O’Brien, C.J. Integration of prospective quality of life and nutritional assessment as routine components of multidisciplinary care of patients with head and neck cancer. ANZ J. Surg. 2008, 78, 34–41. [Google Scholar] [CrossRef]
- Oskam, I.M.; Leeuw, I.M.V.-D.; Aaronson, N.K.; Witte, B.I.; De Bree, R.; Doornaert, P.; Langendijk, J.A.; Leemans, C.R. Prospective evaluation of health-related quality of life in long-term oral and oropharyngeal cancer survivors and the perceived need for supportive care. Oral Oncol. 2013, 49, 443–448. [Google Scholar] [CrossRef]
- Peisker, A.; Raschke, G.F.; Guentsch, A.; Roshanghias, K.; Eichmann, F.; Schultze-Mosgau, S. Longterm quality of life after oncologic surgery and microvascular free flap reconstruction in patients with oral squamous cell carcinoma. Medicina Oral Patologia Oral y Cirugia Bucal 2016, 21, e420–e424. [Google Scholar] [CrossRef] [PubMed]
- Petruson, K.; Mercke, C.; Lundberg, L.-M.; Silander, E.; Hammerlid, E. Longitudinal evaluation of patients with cancer in the oral tongue, tonsils, or base of tongue—Does interstitial radiation dose affect quality of life? Brachytherapy 2005, 4, 271–277. [Google Scholar] [CrossRef]
- Pierre, C.S.; Dassonville, O.; Chamorey, E.; Poissonnet, G.; Ettaiche, M.; Santini, J.; Peyrade, F.; Benezery, K.; Sudaka, A.; Bozec, A. Long-term quality of life and its predictive factors after oncologic surgery and microvascular reconstruction in patients with oral or oropharyngeal cancer. Eur. Arch. Oto-Rhino-Laryngol. 2013, 271, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Schoen, P.; Raghoebar, G.; Bouma, J.; Reintsema, H.; Burlage, F.; Roodenburg, J.; Vissink, A. Prosthodontic rehabilitation of oral function in head–neck cancer patients with dental implants placed simultaneously during ablative tumour surgery: An assessment of treatment outcomes and quality of life. Int. J. Oral Maxillofac. Surg. 2008, 37, 8–16. [Google Scholar] [CrossRef]
- van Gemert, J.; Holtslag, I.; van der Bilt, A.; Merkx, M.A.; Koole, R.; Van Cann, E. Health-related quality of life after segmental resection of the lateral mandible: Free fibula flap versus plate reconstruction. J. Cranio-Maxillofac. Surg. 2015, 43, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, R.-I.; Shibuya, H.; Miura, M.; Watanabe, H.; Ayukawa, F.; Hayashi, K.; Toda, K. Quality of Life of Oral Cancer Patients After Low-Dose-Rate Interstitial Brachytherapy. Int. J. Radiat. Oncol. 2009, 73, 772–778. [Google Scholar] [CrossRef]
- Bozec, A.; Poissonnet, G.; Chamorey, E.; Casanova, C.; Vallicioni, J.; Demard, F.; Mahdyoun, P.; Peyrade, F.; Follana, P.; Bensadoun, R.-J.; et al. Free-Flap Head and Neck Reconstruction and Quality of Life: A 2-Year Prospective Study. Laryngoscope 2008, 118, 874–880. [Google Scholar] [CrossRef]
- Oskam, I.M.; Leeuw, I.V.-D.; Aaronson, N.K.; Kuik, D.J.; de Bree, R.; Doornaert, P.; Langendijk, J.A.; Leemans, R.C. Quality of life as predictor of survival: A prospective study on patients treated with combined surgery and radiotherapy for advanced oral and oropharyngeal cancer. Radiother. Oncol. 2010, 97, 258–262. [Google Scholar] [CrossRef]
- Robbins, K.T.; Clayman, G.; Levine, P.A.; Medina, J.; Sessions, R.; Shaha, A.; Som, P.; Wolf, G.T. Neck Dissection Classification Update. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 751–758. [Google Scholar] [CrossRef] [Green Version]
- Van Cann, E.M.; Dom, M.; Koole, R.; Merkx, M.A.; Stoelinga, P.J. Health related quality of life after mandibular resection for oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2005, 41, 687–693. [Google Scholar] [CrossRef]
- Extermann, M.; Aapro, M.; Bernabei, R.; Cohen, H.J.; Droz, J.-P.; Lichtman, S.; Mor, V.; Monfardini, S.; Repetto, L.; Sørbye, L.; et al. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit. Rev. Oncol. 2005, 55, 241–252. [Google Scholar] [CrossRef]
- Pallis, A.; Fortpied, C.; Wedding, U.; Van Nes, M.; Penninckx, B.; Ring, A.; Lacombe, D.; Monfardini, S.; Scalliet, P.; Wildiers, H. EORTC elderly task force position paper: Approach to the older cancer patient. Eur. J. Cancer 2010, 46, 1502–1513. [Google Scholar] [CrossRef]
- Pottel, L.; Lycke, M.; Boterberg, T.; Pottel, H.; Goethals, L.; Duprez, F.; Noortgate, N.V.D.; De Neve, W.; Rottey, S.; Geldhof, K.; et al. Serial comprehensive geriatric assessment in elderly head and neck cancer patients undergoing curative radiotherapy identifies evolution of multidimensional health problems and is indicative of quality of life. Eur. J. Cancer Care 2014, 23, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.H.; Feinstein, A.R. The importance of classifying initial co-morbidity in evaluating the outcome of diabetes mellitus. J. Chronic Dis. 1974, 27, 387–404. [Google Scholar] [CrossRef]
- Piccirillo, J.F. Importance of comborbidity in head and neck cancer. Laryngoscope 2015, 125, 2242. [Google Scholar] [CrossRef] [Green Version]
- Bang, D.; Piccirillo, J.F.; Littenberg, B.; Johnston, A. The adult comorbidity evaluation-27 (ACE-27) test: A new comorbidity index for patients with cancer. Presented at the 36th Annual meeting of the American Society of Clinical Oncology, New Orleans, LA, USA, 20–23 May 2000. [Google Scholar]
- Demiral, A.N.; Sen, M.; Demiral, Y.; Kinay, M.; Kınay, M. The Effect of Socioeconomic Factors on Quality of Life After Treatment in Patients with Head and Neck Cancer. Int. J. Radiat. Oncol. 2008, 70, 23–27. [Google Scholar] [CrossRef]
- Ronis, D.L.; Duffy, S.A.; Fowler, K.E.; Khan, M.J.; Terrell, J.E. Changes in Quality of Life Over 1 Year in Patients with Head and Neck Cancer. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 241–248. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, A.K.; Chan, J.K.C.; Rubin Grandis, J.; Takata, T.; Slootweg, P.J. WHO Classification of Head and Neck Tumours, 7th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2017; p. 109. [Google Scholar]
- Leeuw, I.M.V.-D.; Buffart, L.M.; Heymans, M.; Rietveld, D.H.; Doornaert, P.; de Bree, R.; Buter, J.; Aaronson, N.K.; Slotman, B.J.; Leemans, C.R.; et al. The course of health-related quality of life in head and neck cancer patients treated with chemoradiation: A prospective cohort study. Radiother. Oncol. 2014, 110, 422–428. [Google Scholar] [CrossRef]
- Schliephake, H.; Neukam, F.W.; Schmelzeisen, R.; Varoga, B.; Schneller, H. Long-term quality of life after ablative intraoral tumour surgery. J. Cranio-Maxillofac. Surg. 1995, 23, 243–249. [Google Scholar] [CrossRef]
- Hartl, D.M.; Dauchy, S.; Escande, C.; Bretagne, E.; Janot, F.; Kolb, F. Quality of life after free-flap tongue reconstruction. J. Laryngol. Otol. 2008, 123, 550–554. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.-P.; Meng, L.; Shen, J.; Liu, H.; Zhang, J.; Xiang, X.; Yan, Y.-B. Free radial forearm flap and anterolateral thigh flap for reconstruction of hemiglossectomy defects: A comparison of quality of life. J. Cranio-Maxillofac. Surg. 2018, 46, 2157–2163. [Google Scholar] [CrossRef]
- Goh, H.K.C.; Ng, Y.H.; Teo, D.T.W. Minimally invasive surgery for head and neck cancer. Lancet Oncol. 2010, 11, 281–286. [Google Scholar] [CrossRef]
- Hartl, D.M.; Ferlito, A.; Silver, C.E.; Takes, R.P.; Stoeckli, S.J.; Suárez, C.; Rodrigo, J.P.; Sesterhenn, A.M.; Snyderman, C.H.; Terris, D.J.; et al. Minimally invasive techniques for head and neck malignancies: Current indications, outcomes and future directions. Eur. Arch. Oto-Rhino-Laryngol. 2011, 268, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Rauso, R.; Colella, G.; Franco, R.; Ronchi, A.; Chirico, F. Ossified Carcinoma Ex Pleomorphic Adenoma in accessory lobe of parotid gland: Complexity in clinical, imaging and histologic diagnosis and minimally invasive surgery. Oral Oncol. 2019, 92, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.; Sayan, A.; Ramchandani, P.; Ilankovan, V. Minimally-invasive neck dissection and free flap reconstruction in patients with cancer of the head and neck. Br. J. Oral Maxillofac. Surg. 2016, 55, 46–49. [Google Scholar] [CrossRef]
- Madhura, M.G.; Rao, R.; Patil, S.; Alhazmi, Y.A.; Jafer, M.; Habib, S.R.; Awan, K.H. Minimally invasive procedures for the recognition and diagnosis of oral precancer and cancer. Disease-a-Month 2020, 66, 101033. [Google Scholar] [CrossRef]
- Gilbert, R.W. Reconstruction of the oral cavity; past, present and future. Oral Oncol. 2020, 108, 104683. [Google Scholar] [CrossRef]
- Lam, L.; Samman, N. Speech and swallowing following tongue cancer surgery and free flap reconstruction—A systematic review. Oral Oncol. 2013, 49, 507–524. [Google Scholar] [CrossRef]
- Saint-Cyr, M.; Wong, C.; Schaverien, M.; Mojallal, A.; Rohrich, R.J. The Perforasome Theory: Vascular Anatomy and Clinical Implications. Plast. Reconstr. Surg. 2009, 124, 1529–1544. [Google Scholar] [CrossRef] [PubMed]
- Colella, G.; Rauso, R.; De Cicco, D.; Boschetti, C.E.; Iorio, B.; Spuntarelli, C.; Franco, R.; Tartaro, G. Clinical management of squamous cell carcinoma of the tongue: Patients not eligible for free flaps, a systematic review of the literature. Expert Rev. Anticancer. Ther. 2021, 21, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Rauso, R.; Chirico, F.; Federico, F.; Nicoletti, G.F.; Colella, G.; Fragola, R.; Pafundi, P.C.; Tartaro, G. Maxillo-facial reconstruction following cancer ablation during COVID-19 pandemic in southern Italy. Oral Oncol. 2021, 115, 105114. [Google Scholar] [CrossRef]
- Joseph, S.; Naveen, B.S.; Mohan, M.T.; Tharayil, J. Comparison of islanded facial artery myomucosal flap with fasciocutaneous free flaps in the reconstruction of lateral oral tongue defects. Int. J. Oral Maxillofac. Surg. 2020, 49, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.W.; Nuyen, B.A.; Divi, V.; Sirjani, D.; Rosenthal, E.L. Outcomes in Head and Neck Resections That Require Multiple-Flap Reconstructions. JAMA Otolaryngol. Neck Surg. 2018, 144, 746–752. [Google Scholar] [CrossRef]
- Sukato, D.C.; Timashpolsky, A.; Ferzli, G.; Rosenfeld, R.M.; Gordin, E.A. Systematic Review of Supraclavicular Artery Island Flap vs Free Flap in Head and Neck Reconstruction. Otolaryngol. Neck Surg. 2018, 160, 215–222. [Google Scholar] [CrossRef]
- Rauso, R.; Tartaro, G.; Califano, L.; Rugge, L.; Chirico, F.; Colella, G. Pedicled palatal flap for surgical repair of oro-nasal fistula. J. Biol. Regul. Homeost. Agents 2018, 32, 1565–1567. [Google Scholar]
- Halama, D.; Dreilich, R.; Lethaus, B.; Bartella, A.; Pausch, N.C. Donor-site morbidity after harvesting of radial forearm free flaps—Comparison of vacuum-assisted closure with conventional wound care: A randomized controlled trial. J. Cranio-Maxillofac. Surg. 2019, 47, 1980–1985. [Google Scholar] [CrossRef]
- Hu, S.; Fan, C.; Bs, B.P.; Rosenberg, J.D. Submental island flap vs free tissue transfer in oral cavity reconstruction: Systematic review and meta-analysis. Head Neck 2020, 42, 2155–2164. [Google Scholar] [CrossRef]
- Ling, X.F.; Peng, X. What Is the Price to Pay for a Free Fibula Flap? A Systematic Review of Donor-Site Morbidity following Free Fibula Flap Surgery. Plast. Reconstr. Surg. 2012, 129, 657–674. [Google Scholar] [CrossRef]
- Lakhiani, C.; DeFazio, M.V.; Han, K.; Falola, R.; Evans, K. Donor-Site Morbidity Following Free Tissue Harvest from the Thigh: A Systematic Review and Pooled Analysis of Complications. J. Reconstr. Microsurg. 2016, 32, 342–357. [Google Scholar] [CrossRef]
- Ettyreddy, A.R.; Chen, C.L.; Zenga, J.; Simon, L.; Pipkorn, P. Complications and Outcomes of Chimeric Free Flaps: A Systematic Review. Otolaryngol. Neck Surg. 2019, 161, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Rauso, R.; Nicoletti, G.F.; Sesenna, E.; Faro, C.L.; Chirico, F.; Fragola, R.; Giudice, G.L.; Tartaro, G. Superficial Temporal Artery Perforator Flap: Indications, Surgical Outcomes, and Donor Site Morbidity. Dent. J. 2020, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.S.; Garden, A.S.; Kies, M.S.; Martin, J.W. Radiation-induced Xerostomia in patients with head and neck cancer: Pathogenesis, impact on quality of life, and management. Head Neck 2004, 26, 796–807. [Google Scholar] [CrossRef]
- De Felice, F.; Pranno, N.; Papi, P.; Brugnoletti, O.; Tombolini, V.; Polimeni, A. Xerostomia and Clinical Outcomes in Definitive Intensity Modulated Radiotherapy (IMRT) Versus Three-dimensional Conformal Radiotherapy (3D-CRT) for Head and Neck Squamous Cell Carcinoma: A Meta-analysis. In Vivo 2020, 34, 623–629. [Google Scholar] [CrossRef] [Green Version]
- Jensen, S.B.; Pedersen, A.M.L.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.F.; et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Management strategies and economic impact. Support. Care Cancer 2010, 18, 1061–1079. [Google Scholar] [CrossRef]
- Marta, G.N.; Silva, V.; De Andrade Carvalho, H.; de Arruda, F.F.; Hanna, S.A.; Gadia, R.; da Silva, J.L.F.; Correa, S.F.M.; Vita Abreu, C.E.C.; Riera, R. Intensity-modulated radiation therapy for head and neck cancer: Systematic review and meta-analysis. Radiother. Oncol. 2014, 110, 9–15. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Rumble, R.; Warde, P. Intensity-modulated Radiotherapy in the Treatment of Head and Neck Cancer. Clin. Oncol. 2012, 24, 474–487. [Google Scholar] [CrossRef]
- Rademaker, A.W.; Vonesh, E.F.; Logemann, J.A.; Pauloski, B.R.; Liu, D.; Lazarus, C.L.; Newman, L.A.; Ma, A.H.M.; Ms, E.M.; Gaziano, J.; et al. Eating ability in head and neck cancer patients after treatment with chemoradiation: A 12-month follow-up study accounting for dropout. Head Neck 2003, 25, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Kotz, T.; Costello, R.; Li, Y.; Posner, M.R. Swallowing dysfunction after chemoradiation for advanced squamous cell carcinoma of the head and neck. Head Neck 2004, 26, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Livergant, J.; Ringash, J. Health related quality of life in head and neck cancer treated with radiation therapy with or without chemotherapy: A systematic review. Oral Oncol. 2014, 50, 254–262. [Google Scholar] [CrossRef]
- Bian, X.; Song, T.; Wu, S. Outcomes of xerostomia-related quality of life for nasopharyngeal carcinoma treated by IMRT: Based on the EORTC QLQ-C30 and H&N35 questionnaires. Expert Rev. Anticancer. Ther. 2014, 15, 109–119. [Google Scholar] [CrossRef]
- Arpino, L.; Iavarone, A.; Parlato, C.; Moraci, A. Prognostic role of depression after lumbar disc surgery. Neurol. Sci. 2004, 25, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Mathews, A.; Ridgeway, V. Personality and surgical recovery: A review. Br. J. Clin. Psychol. 1981, 20, 243–260. [Google Scholar] [CrossRef]
- Powell, R.; Scott, N.; Manyande, A.; Bruce, J.; Vögele, C.; Byrne-Davis, L.M.T.; Unsworth, M.; Osmer, C.; Johnston, M. Psychological preparation and postoperative outcomes for adults undergoing surgery under general anaesthesia. Cochrane Database Syst. Rev. 2016, 5, CD008646. [Google Scholar] [CrossRef]
- Morris, N.; Moghaddam, N.; Tickle, A.; Biswas, S. The relationship between coping style and psychological distress in people with head and neck cancer: A systematic review. Psycho-Oncology 2017, 27, 734–747. [Google Scholar] [CrossRef]
- Montazeri, A. Quality of life data as prognostic indicators of survival in cancer patients: An overview of the literature from 1982 to 2008. Heal. Qual. Life Outcomes 2009, 7, 102. [Google Scholar] [CrossRef] [Green Version]
- Dunne, S.; Mooney, O.; Coffey, L.; Sharp, L.; Desmond, D.; Timon, C.; O’Sullivan, E.; Gallagher, P. Psychological variables associated with quality of life following primary treatment for head and neck cancer: A systematic review of the literature from 2004 to 2015. Psycho-Oncology 2016, 26, 149–160. [Google Scholar] [CrossRef] [Green Version]
- De Cicco, V.; Barresi, M.; Fantozzi, M.P.T.; Cataldo, E.; Parisi, V.; Manzoni, D. Oral Implant-Prostheses: New Teeth for a Brighter Brain. PLoS ONE 2016, 11, e0148715. [Google Scholar] [CrossRef] [Green Version]
- Fantozzi, M.P.T.; Banfi, T.; De Cicco, V.; Barresi, M.; Cataldo, E.; De Cicco, D.; Bruschini, L.; D’Ascanio, P.; Ciuti, G.; Faraguna, U.; et al. Assessing Pupil-linked Changes in Locus Coeruleus-mediated Arousal Elicited by Trigeminal Stimulation. J. Vis. Exp. 2019, 2019, e59970. [Google Scholar] [CrossRef] [PubMed]
- Fantozzi, M.P.T.; De Cicco, V.; Argento, S.; De Cicco, D.; Barresi, M.; Cataldo, E.; Bruschini, L.; D’Ascanio, P.; Faraguna, U.; Manzoni, D. Trigeminal input, pupil size and cognitive performance: From oral to brain matter. Brain Res. 2021, 1751, 147194. [Google Scholar] [CrossRef]
- Fantozzi, M.P.T.; De Cicco, V.; Barresi, M.; Cataldo, E.; Faraguna, U.; Bruschini, L.; Manzoni, D. Short-Term Effects of Chewing on Task Performance and Task-Induced Mydriasis: Trigeminal Influence on the Arousal Systems. Front. Neuroanat. 2017, 11, 68. [Google Scholar] [CrossRef] [Green Version]
- Fantozzi, M.P.T.; Diciotti, S.; Tessa, C.; Castagna, B.; Chiesa, D.; Barresi, M.; Ravenna, G.; Faraguna, U.; Vignali, C.; De Cicco, V.; et al. Unbalanced Occlusion Modifies the Pattern of Brain Activity During Execution of a Finger to Thumb Motor Task. Front. Neurosci. 2019, 13, 499. [Google Scholar] [CrossRef] [Green Version]
- Fantozzi, M.P.T.; Lazzarini, G.; De Cicco, V.; Briganti, A.; Argento, S.; De Cicco, D.; Barresi, M.; Cataldo, E.; Bruschini, L.; D’Ascanio, P.; et al. The path from trigeminal asymmetry to cognitive impairment: A behavioral and molecular study. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Barbier, L.; Pottel, L.; De Ceulaer, J.; Lamoral, P.; Duyck, J.; Jacobs, R.; Abeloos, J. Evaluation of Quality of Life After Mandibular Reconstruction Using a Novel Fixed Implant-Supported Dental Prosthesis Concept: A Pilot Study. Int. J. Prosthodont. 2019, 32, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Hundepool, A.; Dumans, A.; Hofer, S.; Fokkens, N.; Rayat, S.; van der Meij, E.; Schepman, K. Rehabilitation after mandibular reconstruction with fibula free-flap: Clinical outcome and quality of life assessment. Int. J. Oral Maxillofac. Surg. 2008, 37, 1009–1013. [Google Scholar] [CrossRef]
- Jacobsen, H.-C.; Wahnschaff, F.; Trenkle, T.; Sieg, P.; Hakim, S.G. Oral rehabilitation with dental implants and quality of life following mandibular reconstruction with free fibular flap. Clin. Oral Investig. 2015, 20, 187–192. [Google Scholar] [CrossRef]
- Kumar, V.V.; Jacob, P.; Ebenezer, S.; Kuriakose, M.A.; Kekatpure, V.; Baliarsing, A.S.; Al-Nawas, B.; Wagner, W. Implant supported dental rehabilitation following segmental mandibular reconstruction- quality of life outcomes of a prospective randomized trial. J. Cranio-Maxillofac. Surg. 2016, 44, 800–810. [Google Scholar] [CrossRef]
- Petrovic, I.; Baser, R.; Blackwell, T.; McCarthy, C.; Ganly, I.; Patel, S.; Cordeiro, P.; Shah, J. Long-term functional and esthetic outcomes after fibula free flap reconstruction of the mandible. Head Neck 2019, 41, 2123–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tribius, S.; Meyer, M.S.; Pflug, C.; Hanken, H.; Busch, C.-J.; Krüll, A.; Petersen, C.; Bergelt, C. Socioeconomic status and quality of life in patients with locally advanced head and neck cancer. Strahlenther. Onkol. 2018, 194, 737–749. [Google Scholar] [CrossRef]
- Winkler, J.; Stolzenberg, H. Social class index in the Federal Health Survey. Gesundh. (Bundesverb. Arzte Offentlichen Gesundh. (Ger.)) 1999, 61, S178–S183. [Google Scholar]





| INCLUSION CRITERIA | |
|---|---|
| Type of study | Randomized/non-randomized trials, cohort studies, cross-sectional, case control, prospective, retrospective studies |
| Cohort | Patients treated for oral cancer |
| Sample | ≥10 |
| Data | Quality of life assessed by using both EORTC QLQ-C30 and EORTC QLQ-H&N35/43 |
| Timing | Evaluation of HRQOL performed after at least 12 months since treatment |
| EXCLUSION CRITERIA | |
| Type of study | Case series, case reports, reviews, letters, technical notes, conference documents, books, book chapters, editorials, surveys |
| Cohort | Studies on patients treated for non-oral/oropharyngeal cancers without stratification. Studies on non-treated patients |
| Sample | <10 |
| Data | Studies using other HRQOL evaluation tools |
| Timing | Last HRQOL assessment performed before 12-months post-treatment |
| Article | Study Design | Country | Sample | Cohorts Definition | Independent Variables Considered (EORTC Questionnaires as Dependent Variable) | Findings |
|---|---|---|---|---|---|---|
| Airoldi, 2011 [22] | Cross-sectional study | Italy | 38 | OSCC undergoing RFFF and adjuvant RT | Other: dysphagia severity (grouping algorithm not clearly stated); psychological status (HADS) | Dysphagia severity: severe dysphagia group showed significantly worse global health status/QoL, fatigue, physical and social functioning, sexuality, social eating, and contacts Psychological status: depression showed positive correlation with poor head- and neck-specific functional domains (data not available) |
| Beck-Broichsitter, 2017 [24] | Cross-sectional study | Germany | 50 | OC undergoing surgery as primary treatment | Disease/treatment: T stage (Tis-2 vs. T3/4); mandibular involvement (no resection vs. marginal/segmental resection); reconstruction (local flaps NOS vs. distant flaps, including together PMMC, FFF, RFFF) | Reconstruction: local flaps group showed significantly better swallowing No statistical significance of other independent variables |
| Becker, 2012 [23] | Cross-sectional study | Germany | 50 | OC | Disease/treatment: site; T stage (Tis-2 vs. T3/4); mandibular involvement (no resection vs. marginal vs. segmental resection); reconstruction (not clearly reported) | Mandibular involvement: no resection showed significantly better results for all scales with the exception of cognitive functioning; marginal resection (compared to segmental resection) showed significantly better results for role functioning and financial difficulties T stage: early-stage group showed significantly better results in all scales; Reconstruction: “more invasive techniques” and combined reconstructions showed significantly worse results for role, emotional and social functioning, financial difficulties, pain, swallowing, speech problems, trouble with social eating, trouble with social contact No statistical significance of other independent variables |
| Borggreven, 2007 [25] | Prospective cohort study | The Netherlands | 45 | OOPC undergoing RFFF | Time (baseline vs. 6 months vs. 1 year) Sociodemographic: age; gender; marital status; comorbidity Disease/treatment: site (oral cavity vs. oropharynx); stage (T2 vs. T3-4); metachronous lesions/recurrence | Time:
|
| Bozec, 2009 [26] | Prospective Cohort study | France | 50 | OOPC undergoing RFFF without flap failure | Time (baseline vs. 6 months vs. 1 year) Sociodemographic: age; gender; comorbidity (KFI < 2 vs. ≥ 2) Disease/treatment: site (oral cavity vs. oropharynx); stage (AJCC2002 II vs. III/IV); RT | Time: significant progressive worsening of mouth opening from baseline to 6 and 1 year after treatment The statistical analysis on all sociodemographic and disease- and treatment-specific variables was performed on 6-month follow-up questionnaires and not considered for critical appraisal. |
| Bozec, 2020 [27] | Multicenter cross-sectional study | France | 21 | OOPC undergoing free flaps in elderly patients | Sociodemographic: age (<80 years vs. >80years); gender; educational level (< vs. ≥high school diploma); marital status/family (living at home alone vs. not); alcohol consumption (yes vs. no); tobacco consumption (yes vs. no) Disease/treatment: site (oral cavity vs. oropharynx); T stage (4 vs. <4); N stage (0 vs. >0); adjuvant RT Other: HADS (<15 vs. >15); Geriatric 8 health status scores (G8 < 15 vs. >15); number of patients concerns inventory (PCI) | HADS > 15 and G8 <15: significantly associated with poorer scores in global QoL score, functioning scales, general symptoms, H&N symptoms. The authors also administered the EORTC QLQ-ELD14 questionnaire, reporting significantly poorer results in patients older than 80 years, living alone, and with HADS > 15 in motility, as well as significantly poorer results in patients with HADS > 15 in joint stiffness, worries about the future, worries about others, burden of illness, maintaining purpose. Oropharyngeal cancers, G8 < 15 and HADS ≥ 15 were significantly associated with lower scores in the Dysphagia Outcome and Severity Scale (DOSS). HADS ≥ 15 has been significantly associated with a higher number of PCI. No statistical significance of other independent variables |
| Canis, 2016 [28] | Retrospective cohort study | Germany | 48 | Lateral tongue pT3 SCC primarily treated by surgical excision, neck dissection followed by CRT | Disease/treatment: reconstruction (RFFF vs. primary closure) | Reconstruction: RFFF group showed significant better speech, swallowing, and social eating |
| Crombie, 2014 [10] | Cross-sectional study | Australia | 40 | OC | Treatment by CRT alone vs. surgery alone/surgery with adjuvant RT/surgery with adjuvant CT | No statistically significant differences between compared groups |
| Davudov, 2019 [29] | Cross-sectional study | Iran | 16 | OCC undergoing mandible segmental resection | Disease/treatment: reconstruction (no reconstruction vs. free flap vs. plate) | Reconstruction: no reconstruction showed significantly worse outcomes in speech problems, dry mouth, and feeling ill |
| Dzioba, 2017 [30] | Prospective cohort study | Canada | 120 | Cancer of the anterior two-thirds of the tongue, treated by surgical excision and reconstruction alone or by a combination of surgery + RT or surgery + CRT | Time (baseline vs. 1 month vs. 6 months vs. 1 year) substratified by treatment protocol (surgery only vs. surgery + RT vs. surgery + CRT) only for some EORTC items | Surgery + RT group:
|
| Ferri, 2020 [31] | Multicenter retrospective cohort study | Italy | 70 | OSCC (T1-2, N0) involving the tongue and FOM undergoing transoral partial pelviglossectomy/BAMM flap or pull-through partial pelviglossectomy/free flap | Other: treatment protocol (transoral partial pelviglossectomy followed by BAMM flap vs. pull-through partial pelviglossectomy followed by free flap) | Significantly better results in transoral/BAMM flap group for average H&N35 questionnaire. The authors did not provide item-specific data, except for swallowing, which had significantly better result in the transoral/BAMM group |
| Girod, 2009 [32] | Prospective cohort study | USA | 122 | OC | Disease/treatment: reconstruction (ADM vs. STSG) substratified by RT (not specified if pre- or post-treatment); major complications (graft failure vs. regular healing) | Reconstruction: ADM group showed significantly better social eating Reconstruction stratified by RT: ADM/RT scored significantly better results in swallowing scale compared to STSG/RT No statistical significance of other independent variables |
| Huang, 2010 [33] | Cross-sectional study | Taiwan | 41 | HNC free from disease at least 2 y after combined treatment with curative intent | Sociodemographic: gender; age (32–48 years vs. 49–56 years vs. 57–83 years); marital status; educational level (≤6 years vs. 6–12 years vs. >12 years); family income (annual: <0.6 million NTD vs. 0.6–1.2 million NTD vs. ≥1.2 million NTD); comorbidity (Charlson Comorbidity Index [CCI]: 0 vs. ≥1) Disease/treatment: site (oral cavity vs. oropharynx vs. hypopharynx/larynx); stage (AJCC: II vs. III vs. IV); Other: treatment protocol (surgery + RT vs. surgery + RT + CT vs. RT + CT); RT dose (<63 Gy vs. ≥63 Gy); RT technique (2DRT vs. 3DCRT vs. IMRT); length of follow-up (2.2–3.5 years vs. 3.5–4.7 years vs. 4.7–13.2 years) | The study applied an interesting statistical model to compare several independent variables simultaneously in a double-step general linear model multivariate analysis of variance (GML-MANOVA). Annual family income: patients with ≥1.2 million NTD annual income showed significantly better results for physical functioning, role functioning, social functioning, financial problems, swallowing, speech, social eating, and social contact Site:
No statistically significant differences were found analyzing other independent variables (age, gender, educational level, marital status, comorbidity, cancer stage, RT dose, treatment protocol, length of follow-up) |
| Infante-Cossio, 2009 [34] | Prospective cohort study | Spain | 67 | OOPC | Time (baseline, 1 year, 3 years) Disease/treatment: site (oral cavity vs. oropharynx); adjuvant CRT Other: AJCC stage (I/II vs. III/IV) | Time: the study demonstrated three different evolution patterns among questionnaires items: (I) Improvement at the first and third year for emotional functioning, general pain, and specific H&N pain; (II) Worsening at the first year and improvement at the third year for global QoL, physical, role and social functioning, financial problems, sensory problems, social eating, social relationships, sexuality, mouth opening, and use of painkillers; (III) Worsening at the first and third year: cognitive functioning, fatigue, constipation, diarrhea, swallowing, speech, dry mouth, sticky saliva, cough, feeling ill, and weight loss. Site: oropharyngeal cancer showed worse results in overall QoL, functioning role, tiredness, nausea/emesis, appetite loss, pain, use of painkillers, dyspnea, social relationships Stage: III/IV stage cancers showed significantly worse state of health and QoL, pain, tiredness, loss of appetite, swallowing function, speech, social contacts, eating in public, mouth opening, cough, weight loss, use of pain killers Adjuvant CRT: patients undergoing adjuvant CRT showed significantly worse overall QoL, swallowing function, pain, dry mouth, sticky saliva, mouth opening, sensory disorders, speech, social eating |
| Kessler, 2004 [35] | Prospective cohort study | Germany | 55 | Primary OC undergoing nCRT + surgical excision or primary surgical excision + adjuvant RT | Time (baseline vs. 3 month vs. 1 year) substratified by treatment protocols (nCRT + surgery vs. surgery+RT) | nCRT + surgery group:
|
| Khandelwal, 2017 [9] | Cross-sectional study | India | 34 | OC undergoing free flaps | Time (1–2 years vs. 3–5 years) Sociodemographic: age (<45 years vs. >45 years); gender Disease/treatment: site (anterior floor of the mouth/sublingual sulcus vs. retromolar region/tonsillar fossa/tongue); T stage (T2 vs. T3 vs. T4) Other: use of feeding tubes | T stage: progressively better results have been found for smaller tumors for global health status/QoL, functional scales, symptom scale, H and NSS (NOS). Feeding tubes: significantly worse results in patients using feeding tubes for functional status and H and N scales (NOS) No statistical significance of other independent variables |
| Klug, 2002 [36] | Retrospective cohort study | Austria | 110 | OC undergoing multimodal treatment (preoperative CRT followed by surgery and free flaps) | Disease/treatment: site (anterior vs. posterior); T stage (T2 vs. T4), mandibular involvement (segmental vs. marginal resection); neck dissection (SND vs. MRND (NOS)/bilateral ND) | No statistically significant differences between compared groups |
| Kovács, 2015 [37] | Cross-sectional study | Germany | 100 | OOPC undergoing various combinations of multimodality treatment | Sociodemographic: gender Disease/treatment: site (FOM vs. tongue vs. oropharynx vs. retromolar trigone vs. oral cheek vs. mandibular crest vs. lip vs. maxilla); neck dissection laterality (no vs. unilateral vs. bilateral) and type (super selective I-IIa vs. MRND-III); reconstruction (no vs. local flaps NOS vs. distant flaps NOS vs. free flaps NOS); adjuvant RT; adjuvant CRT; adjuvant CT. Other: time since treatment; comparison with EORTC group | Time since treatment: patients evaluated at the 4-years follow-up demonstrated statistically significant worse results for social eating and nutritional support compared to the 1-year follow-up evaluation. Gender: men showed significantly worse results for financial difficulties and cognitive and social functioning Site: cancers of the FOM showed significantly worse social contact compared to tongue; oropharyngeal cancers showed significantly worse results for feeding tubes and sticky saliva compared to tongue and retromolar trigone Reconstruction:
|
| Lin, 2020 [38] | Case control study | Taiwan | 13 | Cancer of the lower lip undergoing surgical resection and reconstruction with RFFF or barrel-shaped RFFF | Disease/treatment: reconstruction (RFFF vs. barrel-shaped RFFF) | Reconstruction: patients undergone barrel-shaped RFFF reconstruction scored better results for swallowing, speech, social eating, social contact and dry mouth |
| Mair, 2017 [39] | Prospective cohort study | India | 38 | T4 cancers of the buccal mucosa undergoing surgery (ablation, neck dissection and reconstruction with PMMC) as first-line treatment | Time (baseline vs. 3 months vs. 6 months vs. 9 months vs. 1 year) on the disease-free sub cohort and sub stratified by adjuvant therapy Disease/treatment: adjuvant therapy (RT vs. CRT) | Baseline differences between disease-free patients and those who developed a relapse: significantly worse results in the latter group for global QOL, dyspnea, appetite loss and weight loss Adjuvant therapy: no differences at 1-year evaluation between groups |
| Moubayed, 2014 [40] | Cross-sectional study and systematic review of literature | Canada | 37 | OSCC undergoing segmental resection of the mandible and free flaps | Disease/treatment: reconstruction (FFF vs. ORFFF vs. Scapular flap) | No statistically significant differences between compared groups |
| Nordgren, 2008 [41] | Multicenter prospective cohort study | Sweden/Norway | 37 | OC | Time (baseline vs. 3 months vs. 6 months vs. 1 year vs. 5 years) in entire cohort and substratified by treatment protocol and survival Other: treatment protocol (surgery alone vs. RT alone vs. combined); survival (5-year survivors vs. 5-year non-survivors and 5-year survivors vs. died after the first year) | Time (baseline vs. 5 years) entire cohort: significant improvement in emotional functioning, significant deterioration in physical and role functioning, dyspnea, problems with senses, teeth, mouth opening, dry mouth, and sticky saliva Time (1 year vs. 5 years) entire cohort: significant deterioration in role functioning, sticky saliva, and mouth opening Time (baseline vs. 5 years) surgery alone: stability of all items Time (baseline vs. 5 years) RT alone: significant improvement of sleep disturbance, H&N pain, social eating and mouth opening; deterioration in physical and role functioning, dyspnea, senses, and dry mouth. Time (baseline vs. 5 years) combined group: significant improvement for emotional functioning and sleep problems; deterioration for role functioning, senses, mouth opening, dry mouth, and sticky saliva. 5-year survivors vs. 5-year non-survivors (compared at baseline): survivors showed significantly better results at baseline for physical, cognitive, and social functioning; fatigue; pain; dyspnea; sleep disturbance; appetite loss; H&N pain; senses; speech; social eating and contacts; dental status; mouth opening; sticky saliva; and dry mouth 5-year survivors vs. died after the first year (compared at baseline): survivors showed significantly better results for physical, cognitive, and social functioning; fatigue; pain; dyspnea; sleep disturbance; appetite loss; H&N pain; senses; speech; social eating; dental status; mouth opening; dry mouth; sticky saliva 5-year survivors vs. died after the first year (compared at 1 year): survivors showed significantly better results for physical and role functioning, fatigue, nausea/vomiting, appetite loss, constipation, diarrhea, swallowing, social eating, sexuality, mouth opening. |
| Oates, 2008 [42] | Prospective cohort study | Australia | 47 | HNC | Time (baseline vs. 3 months vs. 6 months vs. 1 year) substratified by site and treatment protocol Disease/treatment: site (oral cavity vs. oropharynx vs. larynx vs. nasopharynx vs. parotid vs. occult primary vs. paranasal sinus) Other: treatment protocol (surgery vs. RT only) substratified by site | Patients undergoing RT only over time:
|
| Oskam, 2013 [43] | Prospective cohort study | The Netherlands | 129 | OOPC | Time (baseline vs. 6 months vs. 1 year vs. ≥8 years) Sociodemographic: age (NOS); gender; marital status Disease/treatment: tumor site (OC vs. OP); stage (NOS) Other: long-term survival | Time: the mixed-effects model showed significant deterioration from baseline to long-term evaluation for dry mouth, sticky saliva, speech, coughing, senses, swallowing, and social functioning. Long-term survival: non-survivors showed significantly worse baseline global health status/QoL, general pain, appetite loss, swallowing, dental status, and feeling ill No statistical significance of other independent variables |
| Peisker, 2016 [44] | Cross-sectional study | Germany | 22 | OSCC undergoing free flaps | None | Authors performed a bivariate intraquestionnaire analysis to correlate impact of symptom scales on global health status/QoL scale |
| Petruson, 2005 [45] | Prospective cohort study | Sweden | 225 | Primary OOPC (mobile tongue vs. OPC) undergoing brachytherapy | Time (baseline vs. 3 months vs. 1 year vs. 3 years) substratified by site (mobile tongue vs. OPC), brachytherapy quality indices dose, dose rate, and tumor target volume | Mobile tongue group:
|
| Pierre, 2014 [46] | Prospective cohort study | France | 117 | OOPC undergoing free flaps without flap failure and disease free | Sociodemographic: age (>70 years vs. <70 years); gender; comorbidity (KFI ≥2 vs. <2); Disease/treatment: site (oral cavity vs. oropharynx) and OOP subsites (mobile tongue vs. FOM vs. cheek vs. hard palate vs. BOT vs. pharyngeal wall vs. soft palate vs. posterior pharyngeal wall); T stage (T2 vs. T3 vs. T4); mandibular involvement (no vs. segmental resection); reconstruction (FFF/scapular vs. RFFF/ALT); adjuvant RT; neoadjuvant RT; N stage (N ≥ 1 vs. N0) | T stage: T3–4 stage group showed significantly worse results in mean QoL global score, mean C30 symptom domains score and mean H&N35 module score Subsite: BOT showed a significantly worse result in mean H&N35 module score Adjuvant RT: significantly worse results in mean H&N35 module score Neoadjuvant RT: significantly worse results in mean H&N35 module score No statistical significance of other independent variables |
| Schoen, 2008 [47] | Prospective cohort study | The Netherlands | 41 | OOPC in edentulous undergoing surgical excision and implant retained prosthesis rehabilitation | Time (baseline vs. 6 weeks vs. 1 year) substratified by adjuvant RT Disease/treatment: adjuvant RT | Adjuvant RT: patients undergoing adjuvant radiotherapy showed significantly worse results for H&N pain, swallowing, speech, social eating, sexuality, mouth opening, dry mouth, and sticky saliva. Significantly better result was shown in nausea/vomiting. |
| Van Gemert, 2015 [48] | Cross-sectional study | The Netherlands | 20 | OC undergoing lateral segmental resection of the mandible | Sociodemographic: age (NOS); gender Disease/treatment: site (retromolar area vs. FOM vs. gingiva vs. cheek); neck dissection (no vs. unilateral NOS vs. bilateral NOS); reconstruction (of the bony defect [FFF vs. plate] and of soft tissue defect among plate group [primary closure vs. RFFF vs. PMMC]); adjuvant RT Other: cN stage (0 vs. +); horizontal defect size; occlusion (achieved vs. not achieved); accessory nerve sacrifice | Age: significant inverse relation with mouth opening (OVB or selection bias) Gender: relation NOS with feeding tube (OVB or selection bias) Reconstruction of the bony defect: significant relation NOS with functional scales and feeling ill Reconstruction of soft tissue defect: significant relation NOS with mouth opening and feeling ill Bilateral neck dissection NOS: significant relation NOS with social eating and contact, dental status, and feeding tube Horizontal defect size: significant relation NOS with feeding tube Accessory nerve sacrifice: significant relation with swallowing and speech troubles No statistical significance of other independent variables |
| Yoshimura, 2009 [49] | Prospective cohort study | Japan | 30 | OC undergoing primary low-dose-rate brachytherapy with no cervical lymph node or distant metastases, no other active malignancies | Time (baseline vs. 3 months vs. 6 months vs. 1 year) Sociodemographic: gender; age (<65 years or >65 years) Disease/treatment: site (tongue vs. others); T stage (T1 vs. T2–3) Other: brachytherapy source (iridium vs. cesium vs. gold) | Site: patients affected by cancer of the tongue scored worse results at baseline for swallowing, senses and sticky saliva. The latter two remained worse during the follow-up period (1 y), while swallowing item improved toward results comparable with those of the other group at 1 y assessment T stage: T1 stage patients demonstrated higher scores for global health status at baseline and at the 1-year evaluation No statistical significance of other independent variables |
| Tot | 1833 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Cicco, D.; Tartaro, G.; Ciardiello, F.; Fasano, M.; Rauso, R.; Fiore, F.; Spuntarelli, C.; Troiano, A.; Lo Giudice, G.; Colella, G. Health-Related Quality of Life in Oral Cancer Patients: Scoping Review and Critical Appraisal of Investigated Determinants. Cancers 2021, 13, 4398. https://doi.org/10.3390/cancers13174398
De Cicco D, Tartaro G, Ciardiello F, Fasano M, Rauso R, Fiore F, Spuntarelli C, Troiano A, Lo Giudice G, Colella G. Health-Related Quality of Life in Oral Cancer Patients: Scoping Review and Critical Appraisal of Investigated Determinants. Cancers. 2021; 13(17):4398. https://doi.org/10.3390/cancers13174398
Chicago/Turabian StyleDe Cicco, Davide, Gianpaolo Tartaro, Fortunato Ciardiello, Morena Fasano, Raffaele Rauso, Francesca Fiore, Chiara Spuntarelli, Antonio Troiano, Giorgio Lo Giudice, and Giuseppe Colella. 2021. "Health-Related Quality of Life in Oral Cancer Patients: Scoping Review and Critical Appraisal of Investigated Determinants" Cancers 13, no. 17: 4398. https://doi.org/10.3390/cancers13174398
APA StyleDe Cicco, D., Tartaro, G., Ciardiello, F., Fasano, M., Rauso, R., Fiore, F., Spuntarelli, C., Troiano, A., Lo Giudice, G., & Colella, G. (2021). Health-Related Quality of Life in Oral Cancer Patients: Scoping Review and Critical Appraisal of Investigated Determinants. Cancers, 13(17), 4398. https://doi.org/10.3390/cancers13174398









