Characterizing Low-Risk Breast and Gynecological Cancer Patients for Transition into an Oncology/Primary Care Coordinated Care Model: Findings from a Survey of Diverse Survivors in a Rural U.S. State
Abstract
:Simple Summary
Abstract
1. Introduction
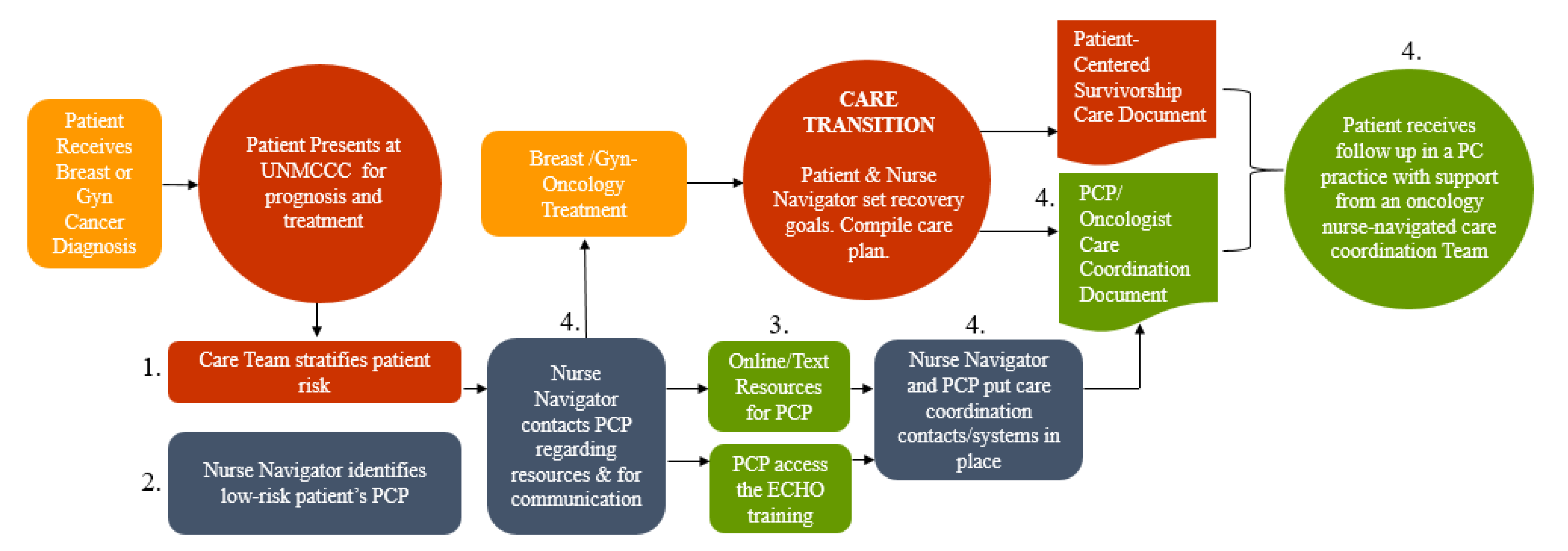
2. Materials and Methods
2.1. Cross-Sectional Survey Data and Sample
2.2. Survey Instrument
2.3. Data Collection
2.4. Analysis Plan
3. Results
3.1. Respondent Demographics
3.2. Health Status and Guideline Concordance
3.3. Primary Health Care Utilization
3.4. Cancer Care Access and Perceived Burden
3.5. Receipt of Survivorship Care Document
4. Discussion
4.1. Implications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mayer, D.K.; Nasso, S.F.; Earp, J.A. Defining cancer survivors, their needs, and perspectives on survivorship health care in the USA. Lancet Oncol. 2017, 18, e11–e18. [Google Scholar] [CrossRef]
- Burg, M.A.; Adorno, G.; Lopez, E.D.S.; Loerzel, V.; Stein, K.; Wallace, C.; Sharma, D.K.B. Current unmet needs of cancer survivors: Analysis of open-ended responses to the American Cancer Society Study of Cancer Survivors II. Cancer 2015, 121, 623–630. [Google Scholar] [CrossRef]
- Perlmutter, J. From Cancer Patient to Cancer Survivor: Lost in Translation; National Academies Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Palmer, S.; DeMichele, A.; Schapira, M.; Glanz, K.; Blauch, A.; Pucci, D.; Jacobs, L. Symptoms, unmet need, and quality of life among recent breast cancer survivors. J. Community Support. Oncol. 2016, 14, 299–306. [Google Scholar] [CrossRef]
- Melkonian, S.C.; Jim, M.A.; Haverkamp, D.; Wiggins, C.L.; McCollum, J.; White, M.C.; Kaur, J.S.; Espey, D.K. Disparities in Cancer Incidence and Trends among American Indians and Alaska Natives in the United States, 2010–2015. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1604–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavala, V.A.; Bracci, P.M.; Carethers, J.M.; Carvajal-Carmona, L.; Coggins, N.B.; Cruz-Correa, M.R.; Davis, M.; de Smith, A.J.; Dutil, J.; Figueiredo, J.C.; et al. Cancer health disparities in racial/ethnic minorities in the United States. Br. J. Cancer 2020, 124, 315–332. [Google Scholar] [CrossRef]
- Gonzales, M.; Qeadan, F.; Mishra, S.I.; Rajput, A.; Hoffman, R.M. Racial-Ethnic Disparities in Late-Stage Colorectal Cancer Among Hispanics and Non-Hispanic Whites of New Mexico. Hisp. Health Care Int. 2017, 15, 180. [Google Scholar] [CrossRef]
- Cooper, R.A. The Medical Oncology Workforce: An Economic and Demographic Assessment of the Demand for Medical Oncologists and Hematologist-Oncologists to Serve the Adult Population to the Year 2020. 2007. Available online: https://www.asco.org/sites/new-www.asco.org/files/content-files/research-and-progress/documents/medical-oncology-workforce-cooper-study.pdf (accessed on 5 May 2020).
- Pascal, J.; Johnson, N.; Dickson-Swift, V.; Kenny, A. Returning home: Psychosocial care during the re-entry phase of cancer survivorship in rural Australia. Eur. J. Cancer Care (Engl.) 2015, 24, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, M.; Greenfield, S.; Stovall, E. From Cancer Patient to Cancer Survivor; National Academies Press: Washington, DC, USA, 2005; ISBN 0309095956. [Google Scholar]
- Nekhlyudov, L.; O’malley, D.M.; Hudson, S.V. Integrating primary care providers in the care of cancer survivors: Gaps in evidence and future opportunities. Lancet Oncol. 2017, 18, e30–e38. [Google Scholar] [CrossRef] [Green Version]
- Howell, D.; Hack, T.F.; Oliver, T.K.; Chulak, T.; Mayo, S.; Aubin, M.; Chasen, M.; Earle, C.C.; Friedman, A.J.; Green, E.; et al. Models of care for post-treatment follow-up of adult cancer survivors: A systematic review and quality appraisal of the evidence. J. Cancer Surviv. 2012, 6, 359–371. [Google Scholar] [CrossRef]
- Halpern, M.T.; Viswanathan, M.; Evans, T.S.; Birken, S.A.; Basch, E.; Mayer, D.K. Models of Cancer Survivorship Care: Overview and Summary of Current Evidence. J. Oncol. Pract. 2015, 11, e19–e27. [Google Scholar] [CrossRef]
- Jacobsen, P.B.; Rowland, J.H.; Paskett, E.D.; Van Leeuwen, F.; Moskowitz, C.; Katta, S.; Wollins, D.; Robison, L.L. Identification of Key Gaps in Cancer Survivorship Research: Findings From the American Society of Clinical Oncology Survey. J. Oncol. Pract. 2016, 12, 190–193. [Google Scholar] [CrossRef]
- McCabe, M.S.; Partridge, A.H.; Grunfeld, E.; Hudson, M.M. Risk-based health care, the cancer survivor, the oncologist, and the primary care physician. Semin. Oncol. 2013, 40, 804–812. [Google Scholar] [CrossRef] [Green Version]
- Keesing, S.; Rosenwax, L.; McNamara, B. A call to action: The need for improved service coordination during early survivorship for women with breast cancer and partners. Women Health 2019, 59, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Fuchsia Howard, A.; Smillie, K.; Turnbull, K.; Zirul, C.; Munroe, D.; Ward, A.; Tobin, P.; Kazanjian, A.; Olson, R. Access to medical and supportive care for rural and remote cancer survivors in northern British Columbia. J. Rural Health 2014, 30, 311–321. [Google Scholar] [CrossRef]
- Cusimano, M.C.; Simpson, A.N.; Han, A.; Hayeems, R.; Bernardini, M.Q.; Robertson, D.; Kives, S.L.; Satkunaratnam, A.; Baxter, N.N.; Ferguson, S.E. Barriers to care for women with low-grade endometrial cancer and morbid obesity: A qualitative study. BMJ Open 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrenson, R.; Lao, C.; Elwood, M.; Brown, C.; Sarfati, D.; Campbell, I. Urban rural differences in breast cancer in New Zealand. Int. J. Environ. Res. Public Health 2016, 13, 1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Census Bureau QuickFacts: New Mexico. Available online: https://www.census.gov/quickfacts/NM (accessed on 5 May 2021).
- Map of Health Professional Shortage Areas: Primary Care, by County, 2019—Rural Health Information Hub. Available online: https://www.ruralhealthinfo.org/charts/5?state=NM (accessed on 27 April 2020).
- Tremblay, D.; Latreille, J.; Bilodeau, K.; Samson, A.; Roy, L.; L’Italien, M.-F.; Mimeault, C. Improving the Transition From Oncology to Primary Care Teams: A Case for Shared Leadership. J. Oncol. Pract. 2016, 12, 1012–1019. [Google Scholar] [CrossRef]
- USDA ERS—Rural-Urban Commuting Area Codes. Available online: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/ (accessed on 30 June 2020).
- CDC—BRFSS—Survey Data & Documentation. Available online: https://www.cdc.gov/brfss/data_documentation/index.htm (accessed on 27 April 2020).
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Cancer in New Mexico. New Mexico Tumor Registry. 2019. Available online: https://nmtrweb.unm.edu/_pdf/new-mexico-cancer-report-2019.pdf (accessed on 5 May 2020).
- Rutledge, T.L.; Kano, M.; Guest, D.; Sussman, A.; Kinney, A.Y. Optimizing endometrial cancer follow-up and survivorship care for rural and other underserved women: Patient and provider perspectives. Gynecol. Oncol. 2017, 145, 334–339. [Google Scholar] [CrossRef]
- Nekhlyudov, L. Integrating primary care in cancer survivorship programs: Models of care for a growing patient population. Oncologist 2014, 19, 579–582. [Google Scholar] [CrossRef] [Green Version]
- Henley, S.J.; Jemal, A. Rural Cancer Control: Bridging the Chasm in Geographic Health Inequity. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1248–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawfik, B.; Adler Jaffe, S.; Mohler, L.; Oomen-Hajagos, J.; Gil, I.S.; Chamberlain, R.; Gagnon, S.; Kano, M.; Gundelach, A.; Ryan, S.R.; et al. Developing a survivorship care plan (SCP) delivery process for patients and primary care providers serving poor, rural, and minority patients with cancer. Support. Care Cancer 2021, 29, 5021–5028. [Google Scholar] [CrossRef]
- Howell, D.; Hack, T.F.; Oliver, T.K.; Chulak, T.; Mayo, S.; Aubin, M.; Chasen, M.; Earle, C.C.; Friedman, A.J.; Green, E.; et al. Survivorship services for adult cancer populations: A pan-Canadian guideline. Curr. Oncol. 2011, 18, e265–e281. [Google Scholar] [CrossRef] [Green Version]
- Mayer, D.K.; Alfano, C.M. Personalized Risk-Stratified Cancer Follow-Up Care: Its Potential for Healthier Survivors, Happier Clinicians, and Lower Costs. J. Natl. Cancer Inst. 2019, 111, 442–448. [Google Scholar] [CrossRef]
- Freund, K.M.; Battaglia, T.A.; Calhoun, E.; Dudley, D.J.; Fiscella, K.; Paskett, E.; Raich, P.C.; Roetzheim, R.G.; Patient Navigation Research Program Group. National Cancer Institute Patient Navigation Research Program: Methods, protocol, and measures. Cancer 2008, 113, 3391–3399. [Google Scholar] [CrossRef]
- Ferrell, B.R.; Winn, R. Medical and nursing education and training opportunities to improve survivorship care. J. Clin. Oncol. 2006, 24, 5142–5148. [Google Scholar] [CrossRef]
- Shaw, T.; Yates, P.; Moore, B.; Ash, K.; Nolte, L.; Krishnasamy, M.; Nicholson, J.; Rynderman, M.; Avery, J.; Jefford, M. Development and evaluation of an online educational resource about cancer survivorship for cancer nurses: A mixed-methods sequential study. Eur. J. Cancer Care (Engl.) 2017, 26, e12576. [Google Scholar] [CrossRef] [PubMed]
- Taplin, S.H.; Clauser, S.; Rodgers, A.B.; Breslau, E.; Rayson, D. Interfaces Across the Cancer Continuum Offer Opportunities to Improve the Process of Care. J. Natl. Cancer Inst. Monogr. 2010, 2010, 104. [Google Scholar] [CrossRef]
- Deshler, A.M.B.; Fee-Schroeder, K.C.; Dowdy, J.L.; Mettler, T.A.; Novotny, P.; Zhao, X.; Frost, M.H. A patient orientation program at a comprehensive cancer center. Oncol. Nurs. Forum 2006, 33, 569–578. [Google Scholar] [CrossRef]
- Nekhlyudov, L.; Mollica, M.A.; Jacobsen, P.B.; Mayer, D.K.; Shulman, L.N.; Geiger, A.M. Developing a Quality of Cancer Survivorship Care Framework: Implications for Clinical Care, Research, and Policy. J. Natl. Cancer Inst. 2019, 111, 1120–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, C.; Craig, J.; Eggert, J.; Bailey-Dorton, C. Implementing and measuring the impact of patient navigation at a comprehensive community cancer center. Oncol. Nurs. Forum 2010, 37, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hook, A.; Ware, L.; Siler, B.; Packard, A. Breast cancer navigation and patient satisfaction: Exploring a community-based patient navigation model in a rural setting. Oncol. Nurs. Forum 2012, 39, 379–385. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 150) | ≤30 miles (n = 98) | >30 miles (n = 52) | |
|---|---|---|---|
| Age, Mean, SD | 59.2, 11.3 | 59.0, 11.4 | 59.7, 11.1 |
| Race | |||
| White | 102 (68.0) | 73 (74.5) | 29 (55.8) |
| American Indian | 23 (15.3) | 4 (4.1) | 19 (36.5) |
| Other/Mixed | 25 (16.7) | 21 (21.4) | 4 (7.7) |
| Hispanic | 62 (41.6) | 46 (46.9) | 16 (31.4) |
| Language | |||
| English | 136 (91.3) | 90 (92.8) | 46 (88.5) |
| Spanish | 6 (4.0) | 5 (5.2) | 1 (1.9) |
| Another language | 7 (4.7) | 2 (2.1) | 5 (9.6) |
| Out of State | 10 (6.7) | 0 (0) | 10 (19.2) |
| Education | |||
| Less than high school | 12 (8.0) | 9 (9.1) | 3 (5.7) |
| High school | 40 (26.7) | 28 (28.6) | 12 (23.1) |
| Some college | 27 (18.0) | 16 (16.3) | 11 (21.2) |
| 2-year college degree | 25 (16.7) | 14 (14.3) | 11 (21.2) |
| 4-year college degree | 22 (14.7) | 17 (17.3) | 5 (9.6) |
| Postgraduate degree | 24 (16.0) | 14 (14.3) | 10 (19.2) |
| HH Income | |||
| Less than USD 9999 | 16 (10.7) | 11 (11.2) | 5 (9.6) |
| USD 10,000–USD 24,999 | 39 (26.0) | 29 (29.6) | 10 (19.2) |
| USD 25,000–USD 49,999 | 38 (25.3) | 20 (20.4) | 18 (34.6) |
| USD 50,000–USD 74,999 | 22 (14.7) | 14 (14.3) | 8 (15.4) |
| More than USD 75,000 | 29 (19.3) | 22 (22.4) | 7 (13.5) |
| Prefer not to answer | 6 (4.0) | 2 (2.0) | 4 (7.7) |
| Cancer Type | |||
| Endometrial | 59 (39.3) | 25 (25.5) | 34 (65.4) |
| Cervical | 19 (12.7) | 13 (13.3) | 6 (11.5) |
| Breast | 71 (47.3) | 59 (60.2) | 12 (23.1) |
| Vulvar | 1 (0.7) | 1 (1.0) | 0 (0) |
| Rural Residence | 39 (26.0) | 0 (0) | 39 (75.0) |
| Construct | Total (n = 150) | ≤30 miles (n = 98) | >30 miles (n = 52) | |
|---|---|---|---|---|
| 2 (2.0) | Number of comorbidities | |||
| 0 | 33 (22.0) | 23 (23.5) | 10 (19.2) | |
| 1 | 50 (33.3) | 32 (32.7) | 18 (34.6) | |
| 2 | 32 (21.3) | 21 (21.4) | 11 (21.2) | |
| 3+ | 35 (23.3) | 22 (22.4) | 13 (25.0) | |
| Prediabetes | 19 (12.7) | 11 (11.2) | 8 (15.4) | |
| Diabetes | 32 (21.3) | 19 (19.4) | 13 (25.0) | |
| Hypertension | 46 (30.7) | 26 (26.5) | 20 (38.5) | |
| Stroke | 4 (2.7) | 2 (2.0) | 2 (3.8) | |
| Angina or coronary heart disease | 4 (2.7) | 4 (4.1) | 0 (0) | |
| Heart attack | 4 (2.7) | 3 (3.1) | 1 (1.9) | |
| Asthma | 21 (14.0) | 12 (12.2) | 9 (17.3) | |
| Arthritis, Rheumatoid Arthritis, Gout, Lupus, or Fibromyalgia | 38 (25.3) | 23 (23.5) | 15 (28.8) | |
| Depressive disorders | 33 (22.0) | 24 (24.5) | 9 (17.3) | |
| Kidney disease | 4 (2.7) | 4 (4.1) | 0 (0) | |
| Chronic Obstructive Pulmonary Disease, emphysema, or chronic bronchitis | 4 (2.7) | 4 (4.1) | 0 (0) | |
| Any other types of cancer | 10 (6.7) | 4 (4.1) | 6 (11.5) | |
| Other disease | 26 (17.3) | 19 (19.4) | 7 (13.5) | |
| Current smoker (cigarettes) | ||||
| Every day | 10 (6.7) | 9 (9.2) | 1 (1.9) | |
| Some days | 2 (1.3) | 0 (0) | ||
| Not at all | 138 (92.0) | 87 (88.8) | 51 (98.1) | |
| Ever had a colonoscopy | 97 (76.4) | 61 (73.5) | 36 (81.8) | |
| Ever had a blood stool test using a home kit | 49 (38.6) | 28 (33.7) | 21 (47.7) | |
| Ever had a mammogram | 61 (92.4) | 27 (90.0) | 34 (94.4) | |
| Primary Health Care Utilization: | Have PCP | 132 (88.0) | 89 (90.8) | 43 (82.7) |
| PCP Type | ||||
| Family practice/internal medicine doctor | 111 (84.7) | 76 (85.4) | 35 (83.3) | |
| OB-GYN | 3 (2.3) | 2 (2.2) | 1 (2.4) | |
| Nurse Practitioner/Physician’s Assistant | 16 (12.2) | 11 (12.4) | 5 (11.9) | |
| Other | 1 (0.8) | 0 (0) | 1 (2.4) | |
| PCP visits in past 5 years | ||||
| Monthly | 13 (8.7) | 8 (8.2) | 5 (9.6) | |
| 3–4 times per year | 73 (48.7) | 45 (45.9) | 28 (53.8) | |
| Once per year | 51 (34.0) | 37 (37.8) | 14 (26.9) | |
| Every few years | 13 (8.7) | 8 (8.2) | 5 (9.6) | |
| Travel time to PCP | ||||
| Less than 30 min | 107 (79.9) | 77 (85.6) | 30 (68.2) | |
| 30 min to 1 h | 16 (11.9) | 9 (10.0) | 7 (15.9) | |
| 1 h to 1.5 h | 3 (2.2) | 2 (2.2) | 1 (2.3) | |
| 1.5 to 2 h | 1 (0.7) | 1 (1.1) | 0 (0) | |
| 2 to 2.5 h | 2 (1.5) | 0 (0) | 2 (4.5) | |
| More than 2.5 h | 5 (3.7) | 1 (1.1) | 4 (9.1) | |
| Cancer Care Access and Perceived Burden (Today’s Appointment): | Distress: Arranging Appointment (Scale: 1–100) | |||
| None (0) | 86 (58.1) | 56 (58.3) | 30 (57.7) | |
| Low (1–33) | 53 (35.8) | 33 (34.4) | 20 (38.5) | |
| Medium (34–66) | 5 (3.4) | 3 (3.1) | 2 (3.8) | |
| High (67–100) | 4 (2.7) | 4 (4.2) | 0 (0) | |
| Distress: Travel to Appointment (Scale: 1–100) | ||||
| None (0) | 79 (53.0) | 50 (51.0) | 29 (56.9) | |
| Low (1–33) | 53 (35.6) | 38 (38.8) | 15 (29.4) | |
| Medium (34–66) | 13 (8.7) | 8 (8.2) | 5 (9.8) | |
| High (67–100) | 4 (2.7) | 2 (2.0) | 2 (3.9) | |
| Missed work to come to appointment | 38 (25.9) | 22 (22.9) | 16 (31.4) | |
| Family member or friend missed work | 23 (15.4) | 8 (8.2) | 15 (28.8) | |
| Overnight stay for appointment | 22 (14.8) | 0 (0) | 22 (43.1) | |
| Transportation to appointment | ||||
| Drove self | 86 (57.3) | 62 (63.3) | 24 (46.2) | |
| Driven by a family member/friend | 56 (37.3) | 31 (31.6) | 25 (48.1) | |
| Ride service | 6 (4.0) | 3 (3.1) | 3 (5.8) | |
| Public transportation | 2 (1.3) | 2 (2.0) | 0 (0) | |
| Receipt of Survivorship Care Document: | Ever received instructions about where to return or who to see for routine cancer check-ups after completing treatment for cancer | |||
| No | 15 (10.0) | 10 (10.2) | 5 (9.6) | |
| Yes | 133 (88.7) | 87 (88.8) | 46 (88.5) | |
| Do not know/Not sure | 2 (1.3) | 1 (1.0) | 1 (1.9) | |
| Ever given a written summary of all the cancer treatments received | ||||
| No | 69 (46.0) | 44 (44.9) | 25 (48.1) | |
| Yes | 63 (42.0) | 42 (42.9) | 21 (40.4) | |
| Don not know/Not sure | 18 (12.0) | 12 (12.2) | 6 (11.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kano, M.; Chen, L.; Boyce, T.; Gomez, R.; Gundelach, A.C.; Jaffe, S.A.; Sussman, A.L.; Dayao, Z.R.; Lobo, J.; Pestak, C.R.; et al. Characterizing Low-Risk Breast and Gynecological Cancer Patients for Transition into an Oncology/Primary Care Coordinated Care Model: Findings from a Survey of Diverse Survivors in a Rural U.S. State. Cancers 2021, 13, 4428. https://doi.org/10.3390/cancers13174428
Kano M, Chen L, Boyce T, Gomez R, Gundelach AC, Jaffe SA, Sussman AL, Dayao ZR, Lobo J, Pestak CR, et al. Characterizing Low-Risk Breast and Gynecological Cancer Patients for Transition into an Oncology/Primary Care Coordinated Care Model: Findings from a Survey of Diverse Survivors in a Rural U.S. State. Cancers. 2021; 13(17):4428. https://doi.org/10.3390/cancers13174428
Chicago/Turabian StyleKano, Miria, Lu Chen, Tawny Boyce, Ricardo Gomez, Amy C. Gundelach, Shoshana Adler Jaffe, Andrew L. Sussman, Zoneddy R. Dayao, Jolene Lobo, Claire R. Pestak, and et al. 2021. "Characterizing Low-Risk Breast and Gynecological Cancer Patients for Transition into an Oncology/Primary Care Coordinated Care Model: Findings from a Survey of Diverse Survivors in a Rural U.S. State" Cancers 13, no. 17: 4428. https://doi.org/10.3390/cancers13174428





