Review of Myeloma Therapies and Their Potential for Oral and Maxillofacial Side Effects
Abstract
:Simple Summary
Abstract
1. Introduction
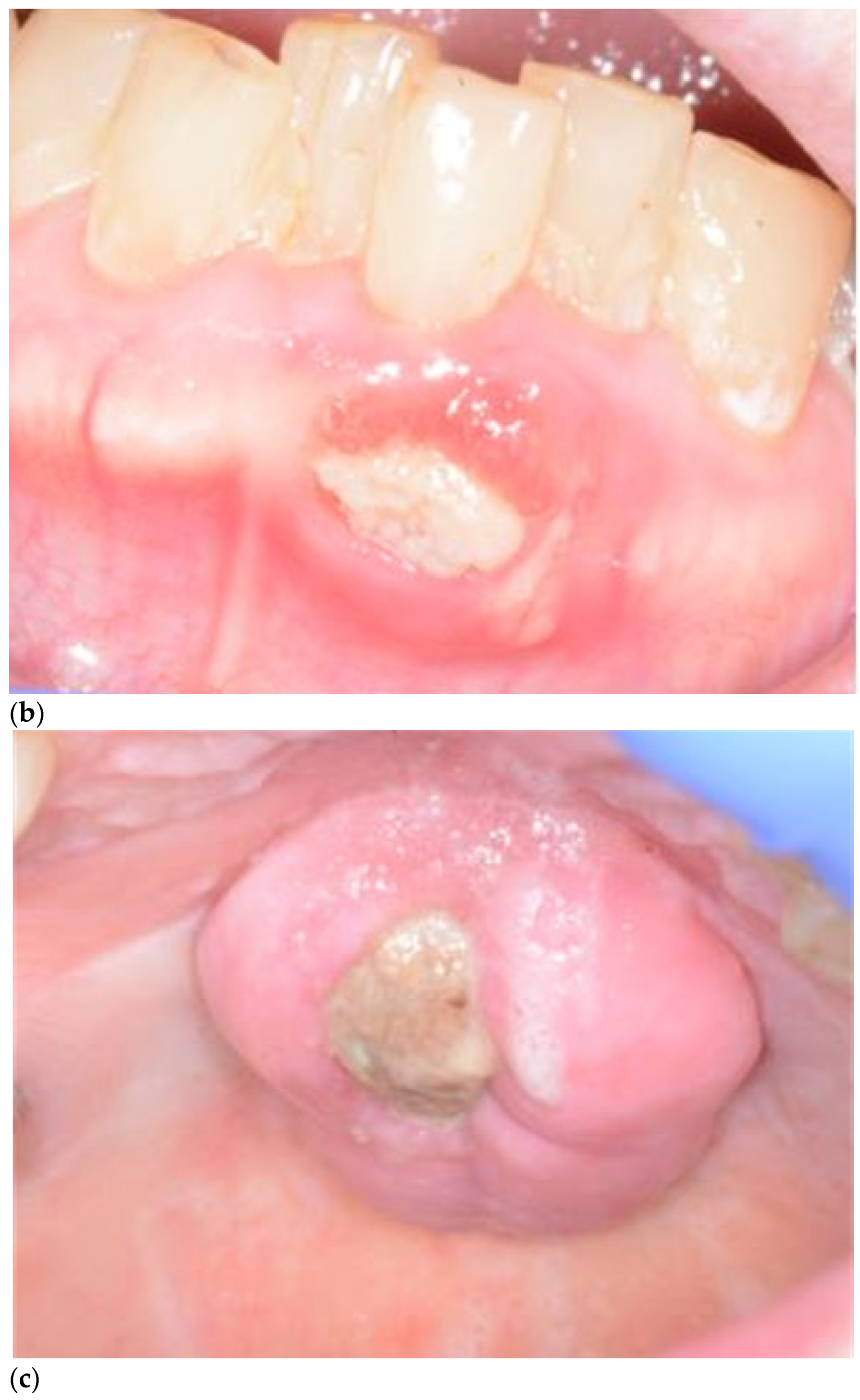
2. Definition and Diagnosis of MRONJ
3. Incidence
4. Pathogenesis
5. Risk Factors for MRONJ
5.1. Medications
5.1.1. Bisphosphonates (BP)
5.1.2. Monoclonal Antibodies: Denosumab
5.1.3. Immunomodulators (IMiDs): Thalidomide, Lenalidomide, Pomalidomide
5.1.4. Steroids
5.2. Procedures
5.3. Comorbidities, Vitamin D Deficiency, Other Medical, Age, Gender
5.4. Oral Health, Inflammation, and Infection
5.5. Anatomical Factors
5.6. Genetic Factors
6. Staging
7. Prevention
- Extraction of teeth with poor restorative or periodontal prognosis
- Extraction of teeth that are non-functional and pose a future infection risk
- Remedial dental work to stabilise the dentition
- Improve or replace ill-fitting prostheses
- Instruction on correct oral hygiene methods including the use of interproximal cleaning and high-fluoride toothpaste
- Dietary counselling on caries risk, reducing sugary drinks and snacks
- Discussion about modifiable risk factors including tobacco and alcohol cessation
8. Management of MRONJ
8.1. Conservative
8.2. Surgical
8.3. Antimicrobial
9. Emerging Therapies for MRONJ
9.1. Teriparatide
9.2. Hyperbaric Oxygen Therapy (HBO)
9.3. Plasma Rich Fibrin (PRF), Photo Biomodulation (Low-Level-Laser Therapy), Antimicrobial Photodynamic Therapy
9.4. Pentoxifylline and Tocopherol
9.5. Mesenchymal Stem Cell Therapy
10. Conclusions
- MRONJ is common in MM patients with an incidence of between 4.9–20.5%. As MM is the second most common haematological malignancy there are a significant number of patients at risk
- Duration of exposure to antiresorptive therapy is a major risk factor
- Prevention is better than a cure
- MRONJ requires expert management with a multidisciplinary team
Author Contributions
Funding
Conflicts of Interest
References
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Fusco, V.; Cabras, M.; Erovigni, F.; Dell’Acqua, A.; Arduino, P.G.; Pentenero, M.; Appendino, P.; Basano, L.; Ferrera, F.; Fasciolo, A.; et al. A multicenter observational study on Medication-Related Osteonecrosis of the Jaw (MRONJ) in advanced cancer and myeloma patients of a cancer network in North-Western Italy. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e466–e473. [Google Scholar] [CrossRef] [PubMed]
- Yarom, N.; Shapiro, C.L.; Peterson, D.E.; Van Poznak, C.H.V.; Bohlke, K.; Ruggiero, S.L.; Migliorati, C.A.; Khan, A.; Morrison, A.; Anderson, H.; et al. Medication-Related Osteonecrosis of the Jaw: MASCC/ISOO/ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 2270–2290. [Google Scholar] [CrossRef]
- Walter, C.; Al-Nawas, B.; Frickhofen, N.; Gamm, H.; Beck, J.; Reinsch, L.; Blum, C.; Grötz, K.A.; Wagner, W. Prevalence of bisphosphonate associated osteonecrosis of the jaws in multiple myeloma patients. Head Face Med. 2010, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Morgan, G.J.; Davies, F.E.; Gregory, W.M.; Cocks, K.; Bell, S.E.; Szubert, A.J.; Navarro-Coy, N.; Drayson, M.; Owen, R.G.; Feyler, S.; et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): A randomised controlled trial. Lancet 2010, 376, 1989–1999. [Google Scholar] [CrossRef] [Green Version]
- Stopeck, A.T.; Fizazi, K.; Body, J.J.; Brown, J.E.; Carducci, M.; Diel, I.; Fujiwara, Y.; Martín, M.; Paterson, A.; Tonkin, K.; et al. Safety of long-term denosumab therapy: Results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Support. Care Cancer 2017, 24, 447–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stopeck, A.T.; Warner, D.J. Response to letter to the Editors—Safety of long-term denosumab therapy. Support. Care Cancer 2016, 25, 353–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavrokokki, T.; Cheng, A.; Stein, B.; Goss, A. Nature and Frequency of Bisphosphonate-Associated Osteonecrosis of the Jaws in Australia. J. Oral Maxillofac. Surg. 2007, 65, 415–423. [Google Scholar] [CrossRef]
- Ring, E.S.; Lawson, M.A.; Snowden, J.A.; Jolley, I.; Chantry, A.D. New agents in the Treatment of Myeloma Bone Disease. Calcif. Tissue Int. 2018, 102, 196–209. [Google Scholar] [CrossRef] [Green Version]
- Morgan, G.; Davies, F.; Gregory, W.M.; Bell, S.E.; Szubert, A.J.; Cook, G.; Drayson, M.; Owen, R.G.; Ross, F.M.; Jackson, G.H.; et al. Long-term Follow-up of MRC Myeloma IX Trial: Survival Outcomes with Bisphosphonate and Thalidomide Treatment. Clin. Cancer Res. 2013, 19, 6030–6038. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.-S.; Lee, J.-I.; Yoon, H.-J.; Min, C.-K.; Lee, S.-H. Medication-related osteonecrosis of the jaw: A preliminary retrospective study of 130 patients with multiple myeloma. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 1. [Google Scholar] [CrossRef] [Green Version]
- Kajizono, M.; Sada, H.; Sugiura, Y.; Soga, Y.; Kitamura, Y.; Matsuoka, J.; Sendo, T. Incidence and Risk Factors of Osteonecrosis of the Jaw in Advanced Cancer Patients after Treatment with Zoledronic Acid or Denosumab: A Retrospective Cohort Study. Biol. Pharm. Bull. 2015, 38, 1850–1855. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, M.; Kuroshima, S.; Sawase, T. Clinical considerations for medication-related osteonecrosis of the jaw: A comprehensive literature review. Int. J. Implant. Dent. 2021, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, G.; Skoglund, I.; Rythén, M. The rise and fall of the alveolar process: Dependency of teeth and metabolic aspects. Arch. Oral Biol. 2018, 96, 195–200. [Google Scholar] [CrossRef]
- Nair, S.P.; Meghji, S.; Wilson, M.; Reddi, K.; White, P.; Henderson, B. Bacterially induced bone destruction: Mechanisms and misconceptions. Infect. Immun. 1996, 64, 2371–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabst, A.M.; Ziebart, T.; Koch, F.P.; Taylor, K.Y.; Al-Nawas, B.; Walter, C. The influence of bisphosphonates on viability, migration, and apoptosis of human oral keratinocytes—in vitro study. Clin. Oral Investig. 2012, 16, 87–93. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Dimopoulos, M.A. Myeloma bone disease: From biology findings to treatment approaches. Blood 2019, 133, 1534–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melton, L.J., 3rd; Kyle, R.A.; Achenbach, S.J.; Oberg, A.L.; Rajkumar, S.V. Fracture risk with multiple myeloma: A population-based study. J. Bone Miner. Res. 2005, 20, 487–493. [Google Scholar] [CrossRef]
- Terpos, E.; Berenson, J.; Cook, R.J.; Lipton, A.; Coleman, R.E. Prognostic variables for survival and skeletal complications in patients with multiple myeloma osteolytic bone disease. Leukemia 2010, 24, 1043–1049. [Google Scholar] [CrossRef]
- Otto, S.; Schreyer, C.; Hafner, S.; Mast, G.; Ehrenfeld, M.; Stürzenbaum, S.; Pautke, C. Bisphosphonate-related osteonecrosis of the jaws—Characteristics, risk factors, clinical features, localization and impact on oncological treatment. J. Cranio-Maxillofac. Surg. 2012, 40, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Von Moos, R.; Body, J.J.; Egerdie, B.; Stopeck, A.; Brown, J.; Fallowfield, L.; Patrick, D.L.; Cleeland, C.; Damyanov, D.; Palazzo, F.S.; et al. Pain and analgesic use associated with skeletal-related events in patients with advanced cancer and bone metastases. Support. Care Cancer 2016, 24, 1327–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Yu, Y.; Shi, Y.; Li, M.; Yang, C.; Wang, S. Combined Administration of Bisphosphonates, Chemotherapeutic Agents, and/or Targeted Drugs Increases the Risk for Stage 3 Medication-Related Osteonecrosis of the Jaw: A 4-Year Retrospective Study. BioMed Res. Int. 2020, 2020, 5847429. [Google Scholar] [CrossRef] [PubMed]
- Teoh, L.; Moses, G.; Nguyen, A.P.; McCullough, M.J. Medication-related osteonecrosis of the jaw: Analysing the range of implicated drugs from the Australian database of adverse event notifications. Br. J. Clin. Pharmacol. 2021, 87, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Antonelli, A.; Chiarella, E.; Baudi, F.; Barni, T.; Di Vito, A. The Case of Medication-Related Osteonecrosis of the Jaw Addressed from a Pathogenic Point of View. Innovative Therapeutic Strategies: Focus on the Most Recent Discoveries on Oral Mesenchymal Stem Cell-Derived Exosomes. Pharmaceuticals 2020, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.; Boissier, S.; Filleur, S.; Guglielmi, J.; Cabon, F.; Colombel, M.; Clézardin, P. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002, 62, 6538–6544. [Google Scholar]
- Vincenzi, B.; Santini, D.; Dicuonzo, G.; Battistoni, F.; Gavasci, M.; La Cesa, A.; Grilli, C.; Virzì, V.; Gasparro, S.; Rocci, L.; et al. Zoledronic Acid-Related Angiogenesis Modifications and Survival in Advanced Breast Cancer Patients. J. Interf. Cytokine Res. 2005, 25, 144–151. [Google Scholar] [CrossRef]
- Santini, D.; Vincenzi, B.; Dicuonzo, G.; Avvisati, G.; Massacesi, C.; Battistoni, F.; Gavasci, M.; Rocci, L.; Tirindelli, M.C.; Altomare, V.; et al. Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin. Cancer Res. 2003, 9, 2893–2897. [Google Scholar]
- Fantasia, J.E. The Role of Antiangiogenic Therapy in the Development of Osteonecrosis of the Jaw. Oral Maxillofac. Surg. Clin. N. Am. 2015, 27, 547–553. [Google Scholar] [CrossRef]
- Cheng, C.; Wentworth, K.; Shoback, D.M. New Frontiers in Osteoporosis Therapy. Annu. Rev. Med. 2020, 71, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Raje, N.; Terpos, E.; Willenbacher, W.; Shimizu, K.; Garcia-Sanz, R.; Durie, B.; Legieć, W.; Krejčí, M.; Laribi, K.; Zhu, L.; et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018, 19, 370–381. [Google Scholar] [CrossRef]
- Terpos, E.; Raje, N.; Croucher, P.; Garcia-Sanz, R.; Leleu, X.; Pasteiner, W.; Wang, Y.; Glennane, A.; Canon, J.; Pawlyn, C. Denosumab compared with zoledronic acid on PFS in multiple myeloma: Exploratory results of an international phase 3 study. Blood Adv. 2021, 5, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Brown, J.E.; Van Poznak, C.; Ibrahim, T.; Stemmer, S.M.; Stopeck, A.T.; Diel, I.J.; Takahashi, S.; Shore, N.; Henry, D.H.; et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: Integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann. Oncol. 2012, 23, 1341–1347. [Google Scholar] [CrossRef]
- Bekker, P.J.; Holloway, D.L.; Rasmussen, A.S.; Murphy, R.; Martin, S.W.; Leese, P.T.; Holmes, G.B.; Dunstan, C.; DePaoli, A.M. A Single-Dose Placebo-Controlled Study of AMG 162, a Fully Human Monoclonal Antibody to RANKL, in Postmenopausal Women. J. Bone Miner. Res. 2004, 19, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G.G.; Xia, Z.; Dunford, J.E.; Oppermann, U.; Kwaasi, A.; Hulley, P.A.; Kavanagh, K.L.; Triffitt, J.T.; Lundy, M.W.; Phipps, R.J.; et al. Bisphosphonates: An Update on Mechanisms of Action and How These Relate to Clinical Efficacy. Ann. N. Y. Acad. Sci. 2007, 1117, 209–257. [Google Scholar] [CrossRef]
- Gedmintas, L.; Solomon, D.H.; Kim, S. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: A systematic review and meta-analysis. J. Bone Miner. Res. 2013, 28, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Nieves, J.W.; Bilezikian, J.P.; Lane, J.M.; Einhorn, T.; Wang, Y.; Steinbuch, M.; Cosman, F. Fragility fractures of the hip and femur: Incidence and patient characteristics. Osteoporos. Int. 2010, 21, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Mashiba, T.; Hirano, T.; Turner, C.H.; Forwood, M.R.; Johnston, C.C.; Burr, D.B. Suppressed Bone Turnover by Bisphosphonates Increases Microdamage Accumulation and Reduces Some Biomechanical Properties in Dog Rib. J. Bone Miner. Res. 2000, 15, 613–620. [Google Scholar] [CrossRef]
- Dickinson, M.; Prince, H.M.; Kirsa, S.; Zannettino, A.; Gibbs, S.D.J.; Mileshkin, L.; O’Grady, J.; Seymour, J.F.; Szer, J.; Horvath, N.; et al. Osteonecrosis of the jaw complicating bisphosphonate treatment for bone disease in multiple myeloma: An overview with recommendations for prevention and treatment. Intern. Med. J. 2009, 39, 304–316. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.J.; Loughnan, M.S.; Flynn, E.; Folkman, J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 4082–4085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.X.; Kortuem, K.M.; Stewart, A.K. Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk. Lymphoma 2013, 54, 683–687. [Google Scholar] [CrossRef]
- Jan, M.; Sperling, A.S.; Ebert, B.L. Cancer therapies based on targeted protein degradation—Lessons learned with lenalidomide. Nat. Rev. Clin. Oncol. 2021, 18, 401–417. [Google Scholar] [CrossRef]
- Mateos, M.-V.; de la Calle, V.G. Lenalidomide as maintenance for every newly diagnosed patient with multiple myeloma. Lancet Oncol. 2019, 20, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Krönke, J.; Udeshi, N.D.; Narla, A.; Grauman, P.; Hurst, S.N.; McConkey, M.; Svinkina, T.; Heckl, D.; Comer, E.; Li, X.; et al. Lenalidomide Causes Selective Degradation of IKZF1 and IKZF3 in Multiple Myeloma Cells. Science 2014, 343, 301–305. [Google Scholar] [CrossRef] [Green Version]
- King, R.; Tanna, N.; Patel, V. Medication-related osteonecrosis of the jaw unrelated to bisphosphonates and denosumab—a review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 289–299. [Google Scholar] [CrossRef]
- Fliefel, R.; Tröltzsch, M.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 568–585. [Google Scholar] [CrossRef]
- McGowan, K.; Acton, C.; Ivanovski, S.; Johnson, N.W.; Ware, R.S. Systemic comorbidities are associated with medication-related osteonecrosis of the jaws: Case–control study. Oral Dis. 2019, 25, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Caldas, R.J.; Antunes, H.S.; Pegoraro, C.D.O.R.; Guedes, F.R.; Santos, P.S.D.S. Oral health condition in cancer patients under bisphosphonate therapy. Support. Care Cancer 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Wazzan, T.; Kashtwari, D.; Almaden, W.F.; Gong, Y.; Chen, Y.; Moreb, J.; Katz, J. Radiographic bone loss and the risk of medication-related osteonecrosis of the jaw (MRONJ) in multiple myeloma patients-A retrospective case control study. Spéc. Care Dent. 2018, 38, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Thumbigere-Math, V.; Sabino, M.C.; Gopalakrishnan, R.; Huckabay, S.; Dudek, A.Z.; Basu, S.; Hughes, P.J.; Michalowicz, B.S.; Leach, J.W.; Swenson, K.K.; et al. Bisphosphonate-Related Osteonecrosis of the Jaw: Clinical Features, Risk Factors, Management, and Treatment Outcomes of 26 Patients. J. Oral Maxillofac. Surg. 2009, 67, 1904–1913. [Google Scholar] [CrossRef]
- Marx, R.E.; Sawatari, Y.; Fortin, M.; Broumand, V. Bisphosphonate-Induced Exposed Bone (Osteonecrosis/Osteopetrosis) of the Jaws: Risk Factors, Recognition, Prevention, and Treatment. J. Oral Maxillofac. Surg. 2005, 63, 1567–1575. [Google Scholar] [CrossRef]
- Iwata, E.; Akashi, M.; Kishimoto, M.; Kusumoto, J.; Hasegawa, T.; Furudoi, S.; Komori, T. Meaning and Limitation of Cortical Bone Width Measurement with DentaScan in Medication-Related Osteonecrosis of the Jaws. Kobe J. Med. Sci. 2016, 62, E114–E119. [Google Scholar]
- Fung, P.; Nicoletti, P.; Shen, Y.; Porter, S.; Fedele, S. Pharmacogenetics of Bisphosphonate-associated Osteonecrosis of the Jaw. Oral Maxillofac. Surg. Clin. N. Am. 2015, 27, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Group OaDE. Therapeutic Guidelines: Oral and Dental—Version 3, 3rd ed.; Therapeutic Guidelines Limited: Melbourne, VIC, Australia, 2019. [Google Scholar]
- Dal Prá, K.; Lemos, C.; Okamoto, R.; Soubhia, A.; Pellizzer, E. Efficacy of the C-terminal telopeptide test in predicting the development of bisphosphonate-related osteonecrosis of the jaw: A systematic review. Int. J. Oral Maxillofac. Surg. 2017, 46, 151–156. [Google Scholar] [CrossRef] [Green Version]
- El-Rabbany, M.; Sgro, A.; Lam, D.K.; Shah, P.S.; Azarpazhooh, A. Effectiveness of treatments for medication-related osteonecrosis of the jaw: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2017, 148, 584–594.e2. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, G.; Saponaro, G.; Di Nardo, F.; Moro, A.; Boniello, R.; Cervelli, D.; Marianetti, T.; Palazzoni, G.; Pelo, S. Clinical Experience with Spiramycin in Bisphosphonate-Associated Osteonecrosis of the Jaw. Int. J. Immunopathol. Pharmacol. 2010, 23, 619–626. [Google Scholar] [CrossRef]
- Russmueller, G.; Seemann, R.; Weiss, K.; Stadler, V.; Speiss, M.; Perisanidis, C.; Fuereder, T.; Willinger, B.; Sulzbacher, I.; Steininger, C. The association of medication-related osteonecrosis of the jaw with Actinomyces spp. infection. Sci. Rep. 2016, 6, 31604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisi, M.; Karapetsa, D.; Gennai, S.; Ramaglia, L.; Graziani, F.; Gabriele, M. Conservative surgical treatment of medication related osteonecrosis of the jaw (MRONJ) lesions in patients affected by osteoporosis exposed to oral bisphosphonates: 24 months follow-up. J. Cranio-Maxillofac. Surg. 2018, 46, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, Y.; Iwasaki, A.; Nakai, F.; Mashiba, T.; Miyake, M. A comparative effectiveness pilot study of teriparatide for medication-related osteonecrosis of the jaw: Daily versus weekly administration. Osteoporos. Int. 2019, 31, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Sim, I.-W.; Borromeo, G.L.; Tsao, C.; Hardiman, R.; Hofman, M.S.; Hjelle, C.P.; Siddique, M.; Cook, G.J.R.; Seymour, J.F.; Ebeling, P.R. Teriparatide Promotes Bone Healing in Medication-Related Osteonecrosis of the Jaw: A Placebo-Controlled, Randomized Trial. J. Clin. Oncol. 2020, 38, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-G.; Won, E.-J.; Choi, H.-W.; Kim, H.-R.; Choi, H.J.; Park, H.-R.; Shin, J.-H.; Suh, S.-P.; Ryang, D.-W.; Shin, M.-G. Serum Parathyroid Hormone Is a New Potential Risk Factor in Multiple Myeloma. BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Freiberger, J.J.; Padilla-Burgos, R.; McGraw, T.; Suliman, H.B.; Kraft, K.H.; Stolp, B.W.; Moon, R.E.; Piantadosi, C.A. What Is the Role of Hyperbaric Oxygen in the Management of Bisphosphonate-Related Osteonecrosis of the Jaw: A Randomized Controlled Trial of Hyperbaric Oxygen as an Adjunct to Surgery and Antibiotics. J. Oral Maxillofac. Surg. 2012, 70, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.; Leeson, R.; Nissan, J.; Olate, S.; De Castro, C.H.B.C.; Acocella, A.; Lalli, A. A Systematic Review of Oxygen Therapy for the Management of Medication-Related Osteonecrosis of the Jaw (MRONJ). Appl. Sci. 2019, 9, 1026. [Google Scholar] [CrossRef] [Green Version]
- Beth-Tasdogan, N.H.; Mayer, B.; Hussein, H.; Zolk, O. Interventions for managing medication-related osteonecrosis of the jaw (MRONJ). Cochrane Database Syst. Rev. 2017, 10, CD012432. [Google Scholar] [CrossRef]
- Tenore, G.; Zimbalatti, A.; Rocchetti, F.; Graniero, F.; Gaglioti, D.; Mohsen, A.; Caputo, M.; Lollobrigida, M.; Lamazza, L.; De Biase, A.; et al. Management of Medication-Related Osteonecrosis of the Jaw (MRONJ) Using Leukocyte- and Platelet-Rich Fibrin (L-PRF) and Photobiomodulation: A Retrospective Study. J. Clin. Med. 2020, 9, 3505. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.B.B.; Camilotti, R.S.; Ponte, M.E. Efficacy of laser therapy in the management of bisphosphonate-related osteonecrosis of the jaw (BRONJ): A systematic review. Lasers Med. Sci. 2016, 31, 1261–1272. [Google Scholar] [CrossRef]
- Tartaroti, N.C.; Marques, M.M.; Naclério-Homem, M.D.G.; Migliorati, C.A.; Deboni, M.C.Z. Antimicrobial photodynamic and photobiomodulation adjuvant therapies for prevention and treatment of medication-related osteonecrosis of the jaws: Case series and long-term follow-up. Photodiagnosis Photodyn. Ther. 2020, 29, 101651. [Google Scholar] [CrossRef] [PubMed]
- Delanian, S.; Chatel, C.; Porcher, R.; Depondt, J.; Lefaix, J.-L. Complete Restoration of Refractory Mandibular Osteoradionecrosis by Prolonged Treatment with a Pentoxifylline-Tocopherol-Clodronate Combination (PENTOCLO): A Phase II Trial. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 832–839. [Google Scholar] [CrossRef]
- Owosho, A.A.; Estilo, C.L.; Huryn, J.M.; Yom, S.K. Pentoxifylline and tocopherol in the management of cancer patients with medication-related osteonecrosis of the jaw: An observational retrospective study of initial case series. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 455–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, M.S.; Wicknick, F.W.; Epstein, J.B.; Berenson, J.R.; Gorsky, M. Management of bisphosphonate-associated osteonecrosis: Pentoxifylline and tocopherol in addition to antimicrobial therapy. An initial case series. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, 593–596. [Google Scholar] [CrossRef]
- Magremanne, M.; Reychler, H. Pentoxifylline and Tocopherol in the Treatment of Yearly Zoledronic Acid–Related Osteonecrosis of the Jaw in a Corticosteroid-Induced Osteoporosis. J. Oral Maxillofac. Surg. 2014, 72, 334–337. [Google Scholar] [CrossRef]
- Cavalcante, R.C.; Tomasetti, G. Pentoxifylline and tocopherol protocol to treat medication-related osteonecrosis of the jaw: A systematic literature review. J. Cranio-Maxillofac. Surg. 2020, 48, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, W.C.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Lombard, T.; Neirinckx, V.; Rogister, B.; Gilon, Y.; Wislet, S. Medication-Related Osteonecrosis of the Jaw: New Insights into Molecular Mechanisms and Cellular Therapeutic Approaches. Stem Cells Int. 2016, 2016, 8768162. [Google Scholar] [CrossRef] [Green Version]
- Kikuiri, T.; Kim, I.; Yamaza, T.; Akiyama, K.; Zhang, Q.; Li, Y.; Chen, C.; Chen, W.; Wang, S.; Le, A.D.; et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J. Bone Miner. Res. 2010, 25, 1668–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Xu, J.; Mao, L.; Liu, Y.; Gao, R.; Zheng, Z.; Chen, W.; Le, A.; Shi, S.; Wang, S. Allogeneic Mesenchymal Stem Cell Therapy for Bisphosphonate-Related Jaw Osteonecrosis in Swine. Stem Cells Dev. 2013, 22, 2047–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Indication for Bisphosphonate Therapy | Risk of Spontaneous ONJ | Risk Following Dental Extraction |
|---|---|---|
| All patients | 0.05–0.10% | 0.37–0.8% |
| Osteoporosis | 0.01–0.14% | 0.09–0.34% |
| Paget’s disease | 0.26–1.8% | 2.1–13.5% |
| Malignancy | 0.88–1.15% | 6.67–9.1% |
| Stage | Clinical Signs/Symptoms | Imaging Findings | Management |
|---|---|---|---|
| 0 | Nil exposed bone Non-specific clinical findings/symptoms Mobile teeth, periapical or periodontal fistula where dental cause is excluded | Non-specific radiographic changes including sclerotic alveolar bone, defined extraction socket, alveolar bone loss in absence of periodontal bone disease, altered trabecular bone appearance, dense bone or absence of bone in extraction sockets, osteosclerosis of alveolar bone, thickening of lamina dura that obscures periodontal ligament space. | Systemic antimicrobial treatment Systemic pain relief Optimise oral/dental health and hygiene |
| 1 | Bone exposure/fistula to bone, nil suppuration, asymptomatic | Radiographic findings as stage 0 may be present localised to alveolar bone | Anti-bacterial mouthwash Clinical follow-up every 3 months Optimise oral/dental health and hygiene |
| 2 | Bone exposure/necrosis with associated pain, infection, erythema, +/− purulent discharge | Radiographic findings as stage 0 may be present localised to alveolar bone | Anti-bacterial mouthwash Symptomatic treatment with oral antibiotics Pain management Local debridement to relieve soft tissue trauma Optimise oral/dental health and hygiene |
| 3 | Exposed and necrotic bone or fistula that probes to bone with associated pain, and infection AND necrotic bone extending beyond the alveolar process to either the inferior border of mandible or ramus, or zygoma of the maxilla resulting in pathological fracture, oral antral or oral nasal communication, or osteolysis extending to the inferior border of the mandible or floor of the maxillary sinus | Osteosclerosis/osteolysis of adjacent bony structures, pathologic fracture, osteolysis extending to maxillary sinus or nasal floor | Anti-bacterial mouthwash Symptomatic treatment with oral antibiotics Pain management Surgical debridement or resection/reconstruction Optimise oral/dental health and hygiene |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaumont, S.; Harrison, S.; Er, J. Review of Myeloma Therapies and Their Potential for Oral and Maxillofacial Side Effects. Cancers 2021, 13, 4479. https://doi.org/10.3390/cancers13174479
Beaumont S, Harrison S, Er J. Review of Myeloma Therapies and Their Potential for Oral and Maxillofacial Side Effects. Cancers. 2021; 13(17):4479. https://doi.org/10.3390/cancers13174479
Chicago/Turabian StyleBeaumont, Sophie, Simon Harrison, and Jeremy Er. 2021. "Review of Myeloma Therapies and Their Potential for Oral and Maxillofacial Side Effects" Cancers 13, no. 17: 4479. https://doi.org/10.3390/cancers13174479
APA StyleBeaumont, S., Harrison, S., & Er, J. (2021). Review of Myeloma Therapies and Their Potential for Oral and Maxillofacial Side Effects. Cancers, 13(17), 4479. https://doi.org/10.3390/cancers13174479






