Intracranial Treatment in Melanoma Patients with Brain Metastasis Is Associated with Improved Survival in the Era of Immunotherapy and Anti-BRAF Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
Treatments
2.2. Statistical Analysis
2.3. Ethics
3. Results
3.1. Patient Population
3.2. BM and Survival
3.3. BM Patients with or without LT
3.4. Safety of LT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.-J.; et al. Dabrafenib and Trametinib versus Dabrafenib and Placebo for Val600 BRAF-Mutant Melanoma: A Multicentre, Double-Blind, Phase 3 Randomised Controlled Trial. Lancet 2015, 386, 444–451. [Google Scholar] [CrossRef]
- Davies, M.A.; Saiag, P.; Robert, C.; Grob, J.-J.; Flaherty, K.T.; Arance, A.; Chiarion-Sileni, V.; Thomas, L.; Lesimple, T.; Mortier, L.; et al. Dabrafenib plus Trametinib in Patients with BRAFV600-Mutant Melanoma Brain Metastases (COMBI-MB): A Multicentre, Multicohort, Open-Label, Phase 2 Trial. Lancet Oncol. 2017, 18, 863–873. [Google Scholar] [CrossRef]
- Cagney, D.N.; Martin, A.M.; Catalano, P.J.; Redig, A.J.; Lin, N.U.; Lee, E.Q.; Wen, P.Y.; Dunn, I.F.; Bi, W.L.; Weiss, S.E.; et al. Incidence and Prognosis of Patients with Brain Metastases at Diagnosis of Systemic Malignancy: A Population-Based Study. Neuro-Oncol. 2017, 19, 1511–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schouten, L.J.; Rutten, J.; Huveneers, H.A.M.; Twijnstra, A. Incidence of Brain Metastases in a Cohort of Patients with Carcinoma of the Breast, Colon, Kidney, and Lung and Melanoma. Cancer 2002, 94, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination Nivolumab and Ipilimumab or Nivolumab Alone in Melanoma Brain Metastases: A Multicentre Randomised Phase 2 Study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- Long, G.V.; Trefzer, U.; Davies, M.A.; Kefford, R.F.; Ascierto, P.A.; Chapman, P.B.; Puzanov, I.; Hauschild, A.; Robert, C.; Algazi, A.; et al. Dabrafenib in Patients with Val600Glu or Val600Lys BRAF-Mutant Melanoma Metastatic to the Brain (BREAK-MB): A Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2012, 13, 1087–1095. [Google Scholar] [CrossRef]
- Margolin, K.; Ernstoff, M.S.; Hamid, O.; Lawrence, D.; McDermott, D.; Puzanov, I.; Wolchok, J.D.; Clark, J.I.; Sznol, M.; Logan, T.F.; et al. Ipilimumab in Patients with Melanoma and Brain Metastases: An Open-Label, Phase 2 Trial. Lancet Oncol. 2012, 13, 459–465. [Google Scholar] [CrossRef]
- Tawbi, H.A.-H.; Forsyth, P.A.J.; Hodi, F.S.; Lao, C.D.; Moschos, S.J.; Hamid, O.; Atkins, M.B.; Lewis, K.D.; Thomas, R.P.; Glaspy, J.A.; et al. Efficacy and Safety of the Combination of Nivolumab (NIVO) plus Ipilimumab (IPI) in Patients with Symptomatic Melanoma Brain Metastases (CheckMate 204). JCO 2019, 37, 9501. [Google Scholar] [CrossRef]
- Keilholz, U.; Ascierto, P.A.; Dummer, R.; Robert, C.; Lorigan, P.; van Akkooi, A.; Arance, A.; Blank, C.U.; Chiarion Sileni, V.; Donia, M.; et al. ESMO Consensus Conference Recommendations on the Management of Metastatic Melanoma: Under the Auspices of the ESMO Guidelines Committee. Ann. Oncol. 2020, 31, 1435–1448. [Google Scholar] [CrossRef]
- Long, G.V.; Grob, J.-J.; Nathan, P.; Ribas, A.; Robert, C.; Schadendorf, D.; Lane, S.R.; Mak, C.; Legenne, P.; Flaherty, K.T.; et al. Factors Predictive of Response, Disease Progression, and Overall Survival after Dabrafenib and Trametinib Combination Treatment: A Pooled Analysis of Individual Patient Data from Randomised Trials. Lancet Oncol. 2016, 17, 1743–1754. [Google Scholar] [CrossRef]
- Vosoughi, E.; Lee, J.M.; Miller, J.R.; Nosrati, M.; Minor, D.R.; Abendroth, R.; Lee, J.W.; Andrews, B.T.; Leng, L.Z.; Wu, M.; et al. Survival and Clinical Outcomes of Patients with Melanoma Brain Metastasis in the Era of Checkpoint Inhibitors and Targeted Therapies. BMC Cancer 2018, 18, 490. [Google Scholar] [CrossRef] [PubMed]
- Badakhshi, H.; Engeling, F.; Budach, V.; Ghadjar, P.; Zschaeck, S.; Kaul, D. Are Prognostic Indices for Brain Metastases of Melanoma Still Valid in the Stereotactic Era? Radiat. Oncol. 2018, 13, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchsbaum, J.C.; Suh, J.H.; Lee, S.-Y.; Chidel, M.A.; Greskovich, J.F.; Barnett, G.H. Survival by Radiation Therapy Oncology Group Recursive Partitioning Analysis Class and Treatment Modality in Patients with Brain Metastases from Malignant Melanoma: A Retrospective Study. Cancer 2002, 94, 2265–2272. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Jiang, W.; Brown, P.D.; Braunstein, S.; Sneed, P.; Wattson, D.A.; Shih, H.A.; Bangdiwala, A.; Shanley, R.; Lockney, N.A.; et al. Estimating Survival in Melanoma Patients With Brain Metastases: An Update of the Graded Prognostic Assessment for Melanoma Using Molecular Markers (Melanoma-MolGPA). Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 812–816. [Google Scholar] [CrossRef] [Green Version]
- Frinton, E.; Tong, D.; Tan, J.; Read, G.; Kumar, V.; Kennedy, S.; Lim, C.; Board, R.E. Metastatic Melanoma: Prognostic Factors and Survival in Patients with Brain Metastases. J. Neurooncol. 2017, 135, 507–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minniti, G.; Anzellini, D.; Reverberi, C.; Cappellini, G.C.A.; Marchetti, L.; Bianciardi, F.; Bozzao, A.; Osti, M.; Gentile, P.C.; Esposito, V. Stereotactic Radiosurgery Combined with Nivolumab or Ipilimumab for Patients with Melanoma Brain Metastases: Evaluation of Brain Control and Toxicity. J. Immunother. Cancer 2019, 7, 102. [Google Scholar] [CrossRef] [Green Version]
- Tallet, A.V.; Dhermain, F.; Le Rhun, E.; Noël, G.; Kirova, Y.M. Combined Irradiation and Targeted Therapy or Immune Checkpoint Blockade in Brain Metastases: Toxicities and Efficacy. Ann. Oncol. 2017, 28, 2962–2976. [Google Scholar] [CrossRef]
- Modesto, A.; Chira, C.; Sol, J.-C.; Lubrano, V.; Boulinguez, S.; Pagès, C.; Sibaud, V.; Gomez-Roca, C.; Moyal, É.; Meyer, N. Prise En Charge Des Patients Atteints de Métastases Cérébrales de Mélanome. Cancer/Radiothérapie 2019, 23, 147–150. [Google Scholar] [CrossRef]
- Dalmasso, C.; Pagès, C.; Chaltiel, L.; Brun, A.; Sibaud, V.; Boulinguez, S.; Chira, C.; Moyal, E.; Lubrano, V.; Meyer, N.; et al. Survival Estimation of Melanoma Patients with Brain Metastasis Using the Melanoma-MolGPA Score: External Validation from a French Cohort. Melanoma Res. 2020, 30, 472–476. [Google Scholar] [CrossRef]
- Magnuson, W.J.; Lester-Coll, N.H.; Wu, A.J.; Yang, T.J.; Lockney, N.A.; Gerber, N.K.; Beal, K.; Amini, A.; Patil, T.; Kavanagh, B.D.; et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor–Naïve Epidermal Growth Factor Receptor–Mutant Non–Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. JCO 2017, 35, 1070–1077. [Google Scholar] [CrossRef]
- Aboudaram, A.; Modesto, A.; Chaltiel, L.; Gomez-Roca, C.; Boulinguez, S.; Sibaud, V.; Delord, J.-P.; Chira, C.; Delannes, M.; Moyal, E.; et al. Concurrent Radiotherapy for Patients with Metastatic Melanoma and Receiving Anti-Programmed-Death 1 Therapy: A Safe and Effective Combination. Melanoma Res. 2017, 27, 485–491. [Google Scholar] [CrossRef]
- Donia, M.; Kimper-Karl, M.L.; Høyer, K.L.; Bastholt, L.; Schmidt, H.; Svane, I.M. The Majority of Patients with Metastatic Melanoma Are Not Represented in Pivotal Phase III Immunotherapy Trials. Eur. J. Cancer 2017, 74, 89–95. [Google Scholar] [CrossRef]
- Patchell, R.A.; Regine, W.F.; Loeffler, J.S.; Sawaya, R.; Andrews, D.W.; Chin, L.S. Radiosurgery Plus Whole-Brain Radiation Therapy for Brain Metastases. JAMA 2006, 296, 2089–2091. [Google Scholar] [CrossRef]
- Soffietti, R.; Kocher, M.; Abacioglu, U.M.; Villa, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.-D.; Carrie, C.; et al. A European Organisation for Research and Treatment of Cancer Phase III Trial of Adjuvant Whole-Brain Radiotherapy Versus Observation in Patients With One to Three Brain Metastases From Solid Tumors After Surgical Resection or Radiosurgery: Quality-of-Life Results. J. Clin. Oncol. 2013, 31, 65–72. [Google Scholar] [CrossRef]
- Mahajan, A.; Ahmed, S.; McAleer, M.F.; Weinberg, J.S.; Li, J.; Brown, P.; Settle, S.; Prabhu, S.S.; Lang, F.F.; Levine, N.; et al. Post-Operative Stereotactic Radiosurgery versus Observation for Completely Resected Brain Metastases: A Single-Centre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 1040–1048. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Shanley, R.; Luo, X.; Andrews, D.; Werner-Wasik, M.; Valicenti, R.; Bahary, J.-P.; Souhami, L.; Won, M.; Mehta, M. Secondary Analysis of RTOG 9508, a Phase 3 Randomized Trial of Whole-Brain Radiation Therapy Versus WBRT Plus Stereotactic Radiosurgery in Patients With 1-3 Brain Metastases; Poststratified by the Graded Prognostic Assessment (GPA). Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 526–531. [Google Scholar] [CrossRef] [Green Version]
- Mulvenna, P.; Nankivell, M.; Barton, R.; Faivre-Finn, C.; Wilson, P.; McColl, E.; Moore, B.; Brisbane, I.; Ardron, D.; Holt, T.; et al. Dexamethasone and Supportive Care with or without Whole Brain Radiotherapy in Treating Patients with Non-Small Cell Lung Cancer with Brain Metastases Unsuitable for Resection or Stereotactic Radiotherapy (QUARTZ): Results from a Phase 3, Non-Inferiority, Randomised Trial. Lancet 2016, 388, 2004–2014. [Google Scholar] [CrossRef] [Green Version]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villà, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.-D.; Carrie, C.; et al. Adjuvant Whole-Brain Radiotherapy Versus Observation After Radiosurgery or Surgical Resection of One to Three Cerebral Metastases: Results of the EORTC 22952-26001 Study. JCO 2011, 29, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Hong, A.M.; Fogarty, G.B.; Dolven-Jacobsen, K.; Burmeister, B.H.; Lo, S.N.; Haydu, L.E.; Vardy, J.L.; Nowak, A.K.; Dhillon, H.M.; Ahmed, T.; et al. Adjuvant Whole-Brain Radiation Therapy Compared With Observation After Local Treatment of Melanoma Brain Metastases: A Multicenter, Randomized Phase III Trial. J. Clin. Oncol. 2019, 37, 3132–3141. [Google Scholar] [CrossRef]
- Gutzmer, R.; Vordermark, D.; Hassel, J.C.; Krex, D.; Wendl, C.; Schadendorf, D.; Sickmann, T.; Rieken, S.; Pukrop, T.; Höller, C.; et al. Melanoma Brain Metastases—Interdisciplinary Management Recommendations 2020. Cancer Treat. Rev. 2020, 89, 102083. [Google Scholar] [CrossRef]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative Stereotactic Radiosurgery Compared with Whole Brain Radiotherapy for Resected Metastatic Brain Disease (NCCTG N107C/CEC·3): A Multicentre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy versus Standard of Care Palliative Treatment in Patients with Oligometastatic Cancers (SABR-COMET): A Randomised, Phase 2, Open-Label Trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Petrelli, F.; De Stefani, A.; Trevisan, F.; Parati, C.; Inno, A.; Merelli, B.; Ghidini, M.; Bruschieri, L.; Vitali, E.; Cabiddu, M.; et al. Combination of Radiotherapy and Immunotherapy for Brain Metastases: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol./Hematol. 2019, 144, 102830. [Google Scholar] [CrossRef]
- Guénolé, M.; Lucia, F.; Bourbonne, V.; Dissaux, G.; Reygagne, E.; Goasduff, G.; Pradier, O.; Schick, U. Impact of Concomitant Systemic Treatments on Toxicity and Intracerebral Response after Stereotactic Radiotherapy for Brain Metastases. BMC Cancer 2020, 20, 991. [Google Scholar] [CrossRef]
- Gatterbauer, B.; Hirschmann, D.; Eberherr, N.; Untersteiner, H.; Cho, A.; Shaltout, A.; Göbl, P.; Fitschek, F.; Dorfer, C.; Wolfsberger, S.; et al. Toxicity and Efficacy of Gamma Knife Radiosurgery for Brain Metastases in Melanoma Patients Treated with Immunotherapy or Targeted Therapy—A Retrospective Cohort Study. Cancer Med. 2020, 9, 4026–4036. [Google Scholar] [CrossRef] [Green Version]
- Dovedi, S.J.; Cheadle, E.J.; Popple, A.L.; Poon, E.; Morrow, M.; Stewart, R.; Yusko, E.C.; Sanders, C.M.; Vignali, M.; Emerson, R.O.; et al. Fractionated Radiation Therapy Stimulates Antitumor Immunity Mediated by Both Resident and Infiltrating Polyclonal T-Cell Populations When Combined with PD-1 Blockade. Clin. Cancer Res. 2017, 23, 5514–5526. [Google Scholar] [CrossRef] [Green Version]
- Kiess, A.P.; Wolchok, J.D.; Barker, C.A.; Postow, M.A.; Tabar, V.; Huse, J.T.; Chan, T.A.; Yamada, Y.; Beal, K. Stereotactic Radiosurgery for Melanoma Brain Metastases in Patients Receiving Ipilimumab: Safety Profile and Efficacy of Combined Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Skrepnik, T.; Sundararajan, S.; Cui, H.; Stea, B. Improved Time to Disease Progression in the Brain in Patients with Melanoma Brain Metastases Treated with Concurrent Delivery of Radiosurgery and Ipilimumab. OncoImmunology 2017, 6, e1283461. [Google Scholar] [CrossRef] [Green Version]
- Fondazione Melanoma Onlus. A Three Arms Prospective, Randomized Phase II Study to Evaluate the Best Sequential Approach with Combo Immunotherapy (Ipilimumab/Nivolumab) and Combo Target Therapy (LGX818/MEK162) in Patients with Metastatic Melanoma and BRAF Mutation. Available online: Clinicaltrials.gov (accessed on 5 September 2021).
- Schadendorf, D. A Phase II, Open-Label, Randomized-Controlled Trial Evaluating the Efficacy and Safety of a Sequencing Schedule of Cobimetinib Plus Vemurafenib Followed by Immunotherapy with an Anti- PD-L1 Antibody Atezolizumab for the Treatment in Patients with Unresectable or Metastatic BRAF V600 Mutant Melanoma. Available online: Clinicaltrials.gov (accessed on 5 September 2021).
- Koenig, J.L.; Shi, S.; Sborov, K.; Gensheimer, M.F.; Li, G.; Nagpal, S.; Chang, S.D.; Gibbs, I.C.; Soltys, S.G.; Pollom, E.L. Adverse Radiation Effect and Disease Control in Patients Undergoing Stereotactic Radiosurgery and Immune Checkpoint Inhibitor Therapy for Brain Metastases. World Neurosurg. 2019, 126, e1399–e1411. [Google Scholar] [CrossRef]
- Ahmed, K.A.; Abuodeh, Y.A.; Echevarria, M.I.; Arrington, J.A.; Stallworth, D.G.; Hogue, C.; Naghavi, A.O.; Kim, S.; Kim, Y.; Patel, B.G.; et al. Clinical Outcomes of Melanoma Brain Metastases Treated with Stereotactic Radiosurgery and Anti-PD-1 Therapy, Anti-CTLA-4 Therapy, BRAF/MEK Inhibitors, BRAF Inhibitor, or Conventional Chemotherapy. Ann. Oncol. 2016, 27, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Glitza Oliva, I.; Tawbi, H.; Davies, M.A. Melanoma Brain Metastases: Current Areas of Investigation and Future Directions. Cancer J. 2017, 23, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minniti, G.; Scaringi, C.; Paolini, S.; Lanzetta, G.; Romano, A.; Cicone, F.; Osti, M.; Enrici, R.M.; Esposito, V. Single-Fraction Versus Multifraction (3 × 9 Gy) Stereotactic Radiosurgery for Large (>2 Cm) Brain Metastases: A Comparative Analysis of Local Control and Risk of Radiation-Induced Brain Necrosis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Melanoma Institute Australia. A Phase II, Open Label, Randomised, Controlled Trial of Ipilimumab and Nivolumab with Concurrent Intracranial Stereotactic Radiotherapy versus Ipilimumab and Nivolumab Alone in Patients with Melanoma Brain Metastases. Available online: Clinicaltrials.gov (accessed on 5 September 2021).
- University Hospital Tuebingen. An Open Label Phase II Study to Evaluate Safety and Efficacy of Combined Treatment With Ipilimumab and Nivolumab in Patients With Four and More Symptomatic Brain Metastases of Melanoma. Available online: Clinicaltrials.gov (accessed on 30 May 2021).
| Total n = 250 | Patients with BM n = 106 | Patients without BM n = 144 | |
|---|---|---|---|
| Age at 1st metastasis in years | |||
| Median | 66 | 65 | 66 |
| (range) | (27–90) | (27–89) | (28–90) |
| Sex | |||
| Male | 141 (56.4%) | 64 (60.4%) | 77 (53.5%) |
| female | 109 (43.6%) | 42 (39.6%) | 67 (46.5%) |
| Primary site of metastasis | |||
| Limbs | 88 (35.2%) | 43 (40.6%) | 45 (31.3%) |
| Trunk | 66 (26.4%) | 27 (25.5%) | 39 (27.1%) |
| Head or neck | 34 (13.6%) | 15 (14.2%) | 19 (13.2%) |
| Mucosa | 21 (8.4%) | 4 (3.8%) | 17 (11.8%) |
| Unknown | 31 (12.4%) | 15 (14.2%) | 16 (11.1%) |
| Other | 10 (4.0%) | 2 (1.9%) | 8 (5.6%) |
| Molecular characteristics | |||
| BRAF | 105 (42.0%) | 52 (49.1%) | 53 (36.8%) |
| NRAS | 38 (15.2%) | 15 (14.2%) | 23 (16.0%) |
| BRAF + NRAS | 2 (0.8%) | 0 (0.0%) | 2 (1.4%) |
| cKIT | 11 (4.4%) | 6 (5.7%) | 5 (3.5%) |
| BRAF + cKIT | 1 (0.4%) | 1 (0.9%) | 0 (0.0%) |
| Wildtype | 88 (35.2%) | 31 (29.2%) | 57 (36.9%) |
| LDH | |||
| ≤1 ULN | 79 (52.7%) | 32 (50%) | 47 (54.7%) |
| >1 ULN | 71 (47.3%) | 32 (50%) | 39 (45.3%) |
| Missing | 100 | 42 | 58 |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| 12-Month Survival | [95% CI] | p Value | HR | [95% CI] | p Value | |
| Age at first BM | ||||||
| <70 years | 49.6% | [36.6; 61.3] | 1 | |||
| ≥70 years | 20.1% | [9.1; 34.1] | 0.04 | 1.07 | [0.52–2.22] | 0.86 |
| ECOG PS | ||||||
| 2/3/4 | 25.0% | [9.1; 44.9%] | 1 | |||
| 0/1 | 41.8% | [30.5; 52.7] | 0.21 | 0.40 | [0.18–0.88] | 0.02 |
| LDH | ||||||
| >1 ULN | 16.2% | [5.9; 30.9] | 1 | |||
| ≤1 ULN | 57.8% | [37.6; 73.5] | <0.01 | 0.44 | [0.21–0.95] | 0.04 |
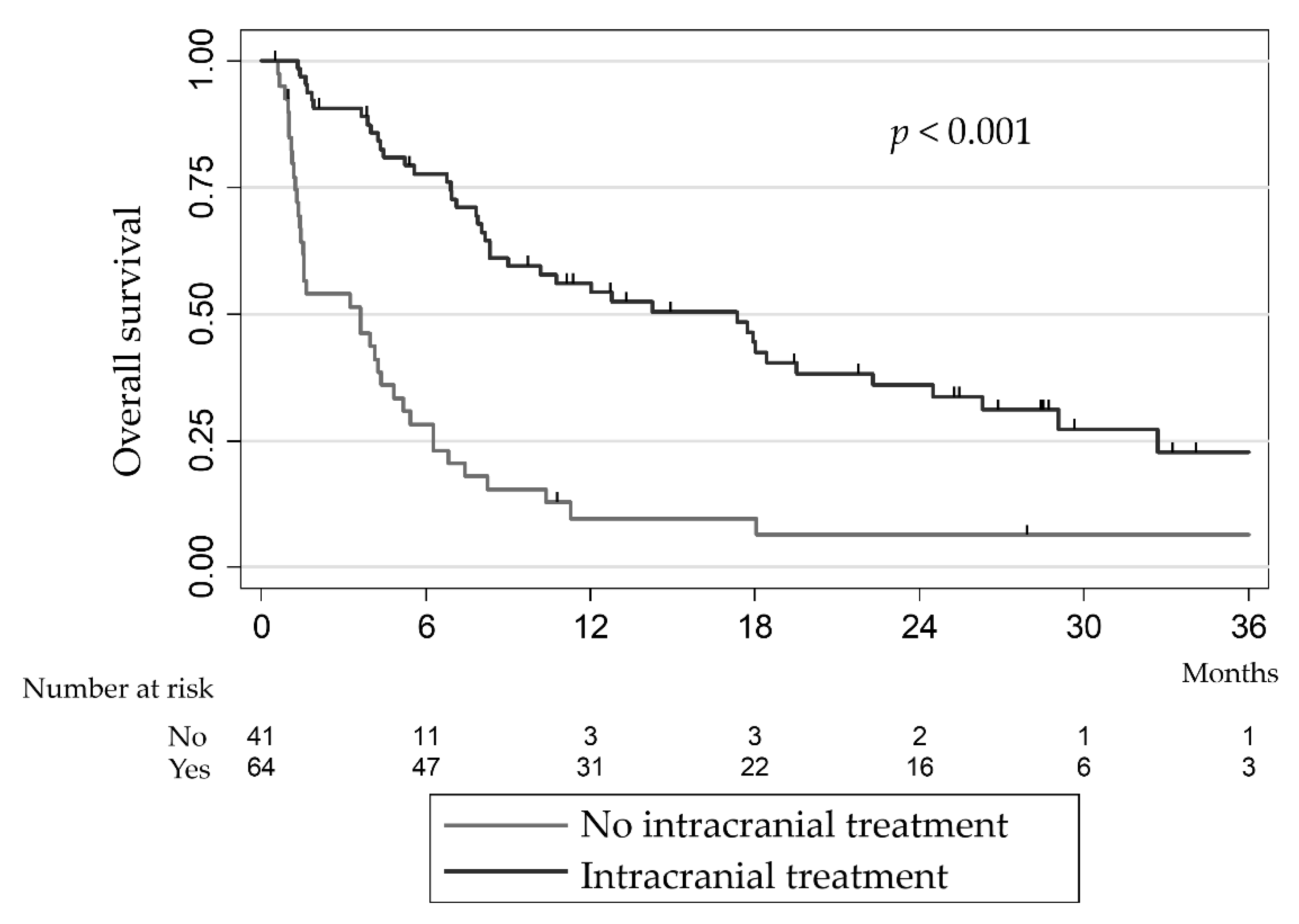
| Intracranial local treatment | ||||||
| No | 9.6% | [2.8; 21.7] | 1 | |||
| Yes | 56.1% | [42.8; 67.5] | <0.01 | 0.21 | [0.10–0.43] | <0.01 |
| Number of BM | ||||||
| ≤3 | 49.6% | [36.4; 61.5] | 1 | |||
| >3 | 22.8% | [11.2; 36.8] | <0.01 | 1.61 | [0.81;3.21] | 0.17 |
| Maximal diameter | ||||||
| ≤10 mm | 50.8% | [30.6; 67.8] | ||||
| >10 mm | 41.6% | [27.4; 55.3] | 0.35 | |||
| BRAF mutation | ||||||
| Absent | 31.2% | [19.1; 44.2] | ||||
| present | 45.6% | [31.4; 58.8] | 0.70 | |||
| Gender | ||||||
| Male | 37.0% | [25.2; 48.8] | ||||
| Female | 41.1% | [25.2; 56.0] | 0.94 | |||
| Patients with BM n = 106 | Patients without Local Treatment n = 42 | Patients with Local Treatment n = 64 | p Value | |
|---|---|---|---|---|
| Age at first BM in years | 66.0 | 68.5 | 65.0 | 0.62 |
| Median (range) | (27.0–90.0) | (27.0–90.0) | (28.0–89.0) | |
| Gender | ||||
| Male | 64 (60.4%) | 28 (66.7%) | 36 (56.3%) | 0.28 |
| Female | 42 (39.6%) | 14 (33.3%) | 28 (43.8%) | |
| Molecular characteristics | ||||
| BRAF | 53 (50%) | 19 (45.2%) | 34 (53.1%) | 0.43 |
| NRAS | 15 (14.2%) | 5 (11.9%) | 10 (15.6%) | 0.59 |
| c-KIT | 7 (6.6%) | 3 (7.1%) | 4 (6.3%) | 1.0 |
| Wildtype | 31 (29.2%) | 16 (38.1%) | 15 (23.4%) | 0.10 |
| LDH | ||||
| ≤1 | 32 (50%) | 8 (29.6%) | 24 (64.9%) | <0.01 |
| >1 | 32 (50%) | 19 (70.4%) | 13 (35.1%) | |
| Missing | 42 | 15 | 27 | |
| ECOG PS (n = 101) | ||||
| 0/1 | 80 (79.2%) | 31 (77.5%) | 49 (80.3%) | 0.7320 |
| 2/3/4 | 21 (20.8%) | 9 (22.5%) | 12 (19.7%) | |
| missing | 5 | 2 | 3 | |
| Extracranial disease at first metastasis | ||||
| No | 5 (4.7%) | 0 | 5 (7.8%) | |
| Yes | 101 (95.3%) | 42 (100%) | 59 (92.2%) | 0.15 |
| Node | 61 (57.5%) | 26 (61.9%) | 35 (54.7%) | 0.46 |
| Liver | 27 (25.5%) | 16 (38.1%) | 11 (17.2%) | 0.02 |
| Lung | 51 (48.1%) | 20 (47.6) | 31 (48.4%) | 0.93 |
| Bone | 20 (18.9%) | 10 (23.8%) | 10 (15.6%) | 0.29 |
| Inaugural * BM | 14 (13.2%) | 2 (4.8%) | 12 (18.8%) | 0.04 |
| Metachronous ** BM | 92 (86.8%) | 40 (95.2%) | 52 (81.3%) | |
| Neurologic symptoms at diagnosis of BM | ||||
| Yes | 31 (29.2%) | 4 (9.5%) | 27 (42.2%) | <0.01 |
| No | 75 (70.8%) | 38 (90.5%) | 37 (57.8) | |
| Number of BM | ||||
| 1 | 45 (42.9%) | 15 (35.7%) | 30 (47.6%) | 0.45 |
| 2–4 | 25 (23.8%) | 12 (28.6%) | 13 (20.6%) | |
| >4 | 35 (33.3%) | 15 (35.7%) | 20 (31.7%) | |
| Missing | 1 | 0 | 1 | |
| Maximal diameter (mm) Median (range) | 13.0 (1.0–60.0) | 11 (1.0–43.0) | 14.5 (1.0–60.0) | 0.08 |
| Previous systemic treatment | ||||
| No | 49 (46.2%) | 12 (28.6%) | 37 (57.8%) | <0.01 |
| Yes | 57 (53.8%) | 30 (71.4%) | 27 (42.2%) | |
| Previous immunotherapy | ||||
| No | 66 (62.3%) | 21 (50%) | 45 (70.3%) | 0.03 |
| Yes | 40 (37.7%) | 21 (50%) | 19 (29.7%) | |
| For BRAF patients: previous iBRAF/iMEK | ||||
| No | 28 (52.8%) | 7 (36.8%) | 21 (61.8%) | 0.08 |
| Yes | 25 (47.2%) | 12 (63.2%) | 13 (38.2%) | |
| Melanoma-molGPA (n = 100) | ||||
| 0–1 | 23 (23.0%) | 9 (22.5%) | 14 (23.3%) | 0.15 |
| 1.5–2 | 51 (51.0%) | 25 (62.5%) | 26 (43.3%) | |
| 2.5–3 | 24 (24.0%) | 6 (15.0%) | 18 (30.0%) | |
| 3.5–4 | 2 (2.0%) | 0 | 2 (3.3%) | |
| Missing | 6 | 2 | 4 | |
| No LT. (n = 42) | SRT (n = 28) | WBRT (n = 21) | Surgery (+/−RT) (n = 16) | |
|---|---|---|---|---|
| No systemic treatment | 12 (28.6%) | 5 (17.9%) | 4 (19.0%) | 2 (12.5%) |
| At least one line of ICI | 21 (50.0%) | 15 (53.6%) | 12 (57.1%) | 13 (61.9%) |
| At least one line of anti-BRAF and/or anti-Mek | 12 (28.6%) | 10 (35.7%) | 9 (42.9%) | 7 (33.3%) |
| At least one line of chemotherapy | 5 (11.9%) | 7 (25.0%) | 5 (23.8%) | 3 (14.2%) |
| Only chemotherapy | 2 (4.8%) | 2 (7.1%) | 2 (9.5%) | 0 (0.0%) |
| Total Radiation Therapy Patients n = 58 | |
|---|---|
| Late toxicities | |
| Radionecrosis | |
| All grade | 6 |
| Grade 1 | 6 |
| Grade 2 | 2 |
| Grade 3 | 3 |
| Treatment for radionecrosis | |
| Steroids | 4 |
| Surgery | 1 |
| Radio-induced Oedema | |
| All grade | 9 |
| Grade 1 | 0 |
| Grade 2 | 4 |
| Grade 3 | 5 |
| Treatment for radio-induced Oedema | |
| Steroids | 9 |
| Anticonvulsant | 9 |
| Haemorrhagic transformation | |
| All grades | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalmasso, C.; Pagès, C.; Chaltiel, L.; Sibaud, V.; Moyal, E.; Chira, C.; Sol, J.C.; Latorzeff, I.; Meyer, N.; Modesto, A. Intracranial Treatment in Melanoma Patients with Brain Metastasis Is Associated with Improved Survival in the Era of Immunotherapy and Anti-BRAF Therapy. Cancers 2021, 13, 4493. https://doi.org/10.3390/cancers13174493
Dalmasso C, Pagès C, Chaltiel L, Sibaud V, Moyal E, Chira C, Sol JC, Latorzeff I, Meyer N, Modesto A. Intracranial Treatment in Melanoma Patients with Brain Metastasis Is Associated with Improved Survival in the Era of Immunotherapy and Anti-BRAF Therapy. Cancers. 2021; 13(17):4493. https://doi.org/10.3390/cancers13174493
Chicago/Turabian StyleDalmasso, Céline, Cécile Pagès, Léonor Chaltiel, Vincent Sibaud, Elisabeth Moyal, Ciprian Chira, Jean Christophe Sol, Igor Latorzeff, Nicolas Meyer, and Anouchka Modesto. 2021. "Intracranial Treatment in Melanoma Patients with Brain Metastasis Is Associated with Improved Survival in the Era of Immunotherapy and Anti-BRAF Therapy" Cancers 13, no. 17: 4493. https://doi.org/10.3390/cancers13174493
APA StyleDalmasso, C., Pagès, C., Chaltiel, L., Sibaud, V., Moyal, E., Chira, C., Sol, J. C., Latorzeff, I., Meyer, N., & Modesto, A. (2021). Intracranial Treatment in Melanoma Patients with Brain Metastasis Is Associated with Improved Survival in the Era of Immunotherapy and Anti-BRAF Therapy. Cancers, 13(17), 4493. https://doi.org/10.3390/cancers13174493






