Optimal Sequencing and Predictive Biomarkers in Patients with Advanced Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
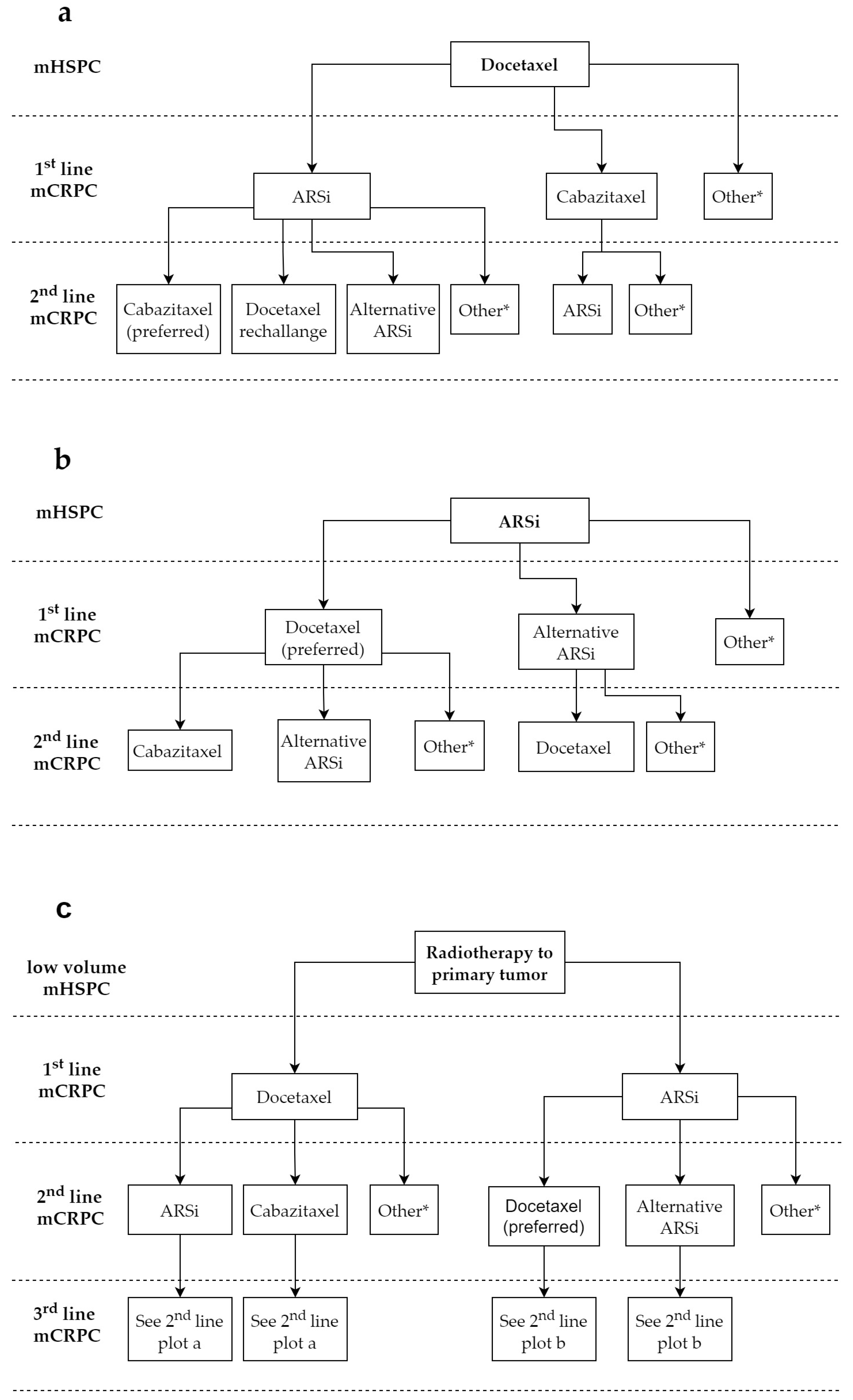
2. Optimal Sequencing in mHSPC, nmCRPC and mCRPC
2.1. Selection of First-Line Treatment
2.1.1. First-Line mHSPC
2.1.2. First-Line nmCRPC
2.1.3. First-Line mCRPC in Patients Pretreated with ADT Monotherapy
2.1.4. First-Line mCRPC in Patients Pretreated with ADT plus Docetaxel or ARSi
2.2. Selection of Subsequent Lines for mCRPC
2.3. Radiopharmaceutical Therapies
2.3.1. The Role of Radium-223
2.3.2. The Advent of Lutetium-177-PSMA-617
2.4. Bone-Targeted Therapies
2.5. Treatment Combinations
3. Predictive Biomarkers and Potential Impact on Treatment Sequence
3.1. DDR Genes
| Biomarker | Source | Drugs | Studies | Phase III Trials |
|---|---|---|---|---|
| DDR (BRCA1/2, ATM, PALB2 and other genes) | PMBC, tumor tissue or ctDNA | Olaparib Rucaparib Talazoparib Niraparib | Phase 2 TOPARP [97] Phase 2 TRITON-2 [98] Phase 2 TALAPRO-1 [99] Phase 2 GALAHAD [100] | PROFOUND [26,83] PROpel [101] * KEYLINK-010 [102] * TRITON-3 [103] * CASPAR [104] * TALAPRO-2 [105] * MAGNITUDE [106] * |
| PTENloss | Tumor tissue | Ipatasertib Capivasertib | Phase 2 A. Martin study [107] Phase 2 ProCAID [108] | IPATential150 [109] |
| AR-V7 | CTCs | ARSi | PROPHECY biomarker study [110] | |
| Molecular subtype Luminal A Luminal B Basal | Tumor tissue | Apalutamide Docetaxel | SPARTAN [111] and TITAN [112] (biomarker analyses) CHAARTED [113] (biomarker analysis) | |
| Others MSI-h/MMRd CDK12 deficiency SPOP mutations RB1 loss TP53 alterations TMPRSS2 | Tumor tissue | ARSi ICI | Explorative analyses |
3.2. AR Pathway
3.3. PTEN Loss and PI3K Alterations
3.4. Basal Versus Luminal Prostate Cancer
3.5. Aggressive-Variant Prostate Cancer
3.6. Other Molecular Biomarkers
3.7. Molecular Biomarkers and Diagnostic Challenges
3.8. PET Tracers as Predictive Biomarkers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kyriakopoulos, C.E.; Chen, Y.-H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, N.; Ali, A.; Ingleby, F.; Hoyle, A.; Amos, C.; Attard, G.; Brawley, C.; Calvert, J.; Chowdhury, S.; Cook, A.; et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: Long-term survival results from the STAMPEDE trial. Ann. Oncol. 2019, 30, 1992–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattrini, C.; Castro, E.; Lozano, R.; Zanardi, E.; Rubagotti, A.; Boccardo, F.; Olmos, D. Current Treatment Options for Metastatic Hormone-Sensitive Prostate Cancer. Cancers 2019, 11, 1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroglu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 686–700. [Google Scholar] [CrossRef]
- James, N.; Rush, H.; Clarke, N.; Attard, G.; Cook, A.; Dearnaley, D.; Gillessen, S.; Hoyle, A.; Jones, R.; Millman, R.; et al. 611O Abiraterone acetate plus prednisolone for hormone-naïve prostate cancer (PCa): Long-term results from metastatic (M1) patients in the STAMPEDE randomised trial (NCT00268476). Ann. Oncol. 2020, 31, S509. [Google Scholar] [CrossRef]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Gomes, A.J.P.D.S.; Given, R.; Soto, J.; Merseburger, A.S.; Özgüroglu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef] [Green Version]
- Gravis, G.; Boher, J.-M.; Joly, F.; Soulié, M.; Albiges, L.; Priou, F.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen Deprivation Therapy (ADT) Plus Docetaxel Versus ADT Alone in Metastatic Non castrate Prostate Cancer: Impact of Metastatic Burden and Long-term Survival Analysis of the Randomized Phase 3 GETUG-AFU15 Trial. Eur. Urol. 2016, 70, 256–262. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef]
- Chi, K.N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; de Santana Gomes, A.J.P.; Given, R.; Juárez, A.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J. Clin. Oncol. 2021, 39, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
- Boevé, L.M.; Hulshof, M.C.; Vis, A.N.; Zwinderman, A.H.; Twisk, J.W.; Witjes, W.P.; Delaere, K.P.; Van Moorselaar, R.J.A.; Verhagen, P.C.; Van Andel, G. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur. Urol. 2018, 75, 410–418. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N. Engl. J. Med. 2020, 383, 1040–1049. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Fizazi, K.; Saad, F.; Shore, N.D.; De Giorgi, U.; Penson, D.F.; Ferreira, U.; Efstathiou, E.; Madziarska, K.; Kolinsky, M.P.; et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2197–2206. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide and Overall Survival in Prostate Cancer. Eur. Urol. 2020, 79, 150–158. [Google Scholar] [CrossRef]
- Berthold, D.R.; Pond, G.R.; Soban, F.; De Wit, R.; Eisenberger, M.; Tannock, I.F. Docetaxel Plus Prednisone or Mitoxantrone Plus Prednisone for Advanced Prostate Cancer: Updated Survival in the TAX 327 Study. J. Clin. Oncol. 2008, 26, 242–245. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; Fizazi, K.; Saad, F.; A Mulders, P.F.; Sternberg, C.N.; Miller, K.; Logothetis, C.J.; Shore, N.D.; Small, E.J.; et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015, 16, 152–160. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Lin, P.; Tombal, B.; Saad, F.; Higano, C.S.; Joshua, A.M.; Parli, T.; Rosbrook, B.; van Os, S.; Beer, T.M. Five-year Survival Prediction and Safety Outcomes with Enzalutamide in Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer from the PREVAIL Trial. Eur. Urol. 2020, 78, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Bono, J.; Bracarda, S.; Sternberg, C.; Chi, K.; Olmos, D.; Sandhu, S.; Massard, C.; Matsubara, N.; Alekseev, B.; Gafanov, R.; et al. LBA4 IPATential150: Phase III study of ipatasertib (ipat) plus abiraterone (abi) vs placebo (pbo) plus abi in metastatic castration-resistant prostate cancer (mCRPC). Ann. Oncol. 2020, 31, S1153–S1154. [Google Scholar] [CrossRef]
- Fizazi, K.; Scher, H.I.; Molina, A.; Logothetis, C.J.; Chi, K.N.; Jones, R.J.; Staffurth, J.N.; North, S.; Vogelzang, N.J.; Saad, F.; et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012, 13, 983–992. [Google Scholar] [CrossRef]
- Bahl, A.; Oudard, S.; Tombal, B.; Özgüroglu, M.; Hansen, S.; Kocak, I.; Gravis, G.; Devin, J.; Shen, L.; De Bono, J.S.; et al. Impact of cabazitaxel on 2-year survival and palliation of tumour-related pain in men with metastatic castration-resistant prostate cancer treated in the TROPIC trial. Ann. Oncol. 2013, 24, 2402–2408. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; De Wit, R.; Mulders, P.; Aragon-Ching, J.; Shore, N.D.; et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.; O’Sullivan, J.; Fosså, S.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Wit, R.; De Bono, J.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Wülfing, C.; Kramer, G.; Eymard, J.-C.; Bamias, A.; Carles, J.; et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef]
- Hussain, M.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 383, 2345–2357. [Google Scholar] [CrossRef]
- Morris, M.J.; De Bono, J.S.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Phase III study of lutetium-177-PSMA-617 in patients with metastatic castration-resistant prostate cancer (VISION). J. Clin. Oncol. 2021, 39, LBA4. [Google Scholar] [CrossRef]
- Sydes, M.; Spears, M.; Mason, M.; Clarke, N.; Dearnaley, D.; de Bono, J.; Attard, G.; Chowdhury, S.; Cross, W.; Gillessen, S.; et al. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: Directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann. Oncol. 2018, 29, 1235–1248. [Google Scholar] [CrossRef] [Green Version]
- Hoyle, A.P.; Ali, A.; James, N.D.; Cook, A.; Parker, C.C.; de Bono, J.S.; Attard, G.; Chowdhury, S.; Cross, W.R.; Dearnaley, D.P.; et al. Abiraterone in “High-” and “Low-risk” Metastatic Hormone-sensitive Prostate Cancer. Eur. Urol. 2019, 76, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Messina, C.; Cattrini, C. From astrology to prostate cancer: What is the role of subgroup analyses? Ann. Oncol. 2020, 31, 437–438. [Google Scholar] [CrossRef]
- Smith, M.R.; Kabbinavar, F.; Saad, F.; Hussain, A.A.; Gittelman, M.M.; Bilhartz, D.D.; Wynne, C.C.; Murray, R.R.; Zinner, N.N.; Schulman, C.; et al. Natural History of Rising Serum Prostate-Specific Antigen in Men With Castrate Nonmetastatic Prostate Cancer. J. Clin. Oncol. 2005, 23, 2918–2925. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N. Engl. J. Med. 2018, 378, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Fizazi, K.; Saad, F.; Rathenborg, P.; Shore, N.; Ferreira, U.; Ivashchenko, P.; Demirhan, E.; Modelska, K.; Phung, D.; et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2018, 378, 2465–2474. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2019, 380, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Fallah, J.; Zhang, L.; Amatya, A.; Gong, Y.; King-Kallimanis, B.; Bhatnagar, V.; Weinstock, C.; Suzman, D.L.; Agrawal, S.; Chang, E.; et al. Survival outcomes in older men with non-metastatic castration-resistant prostate cancer treated with androgen receptor inhibitors: A US Food and Drug Administration pooled analysis of patient-level data from three randomised trials. Lancet Oncol. 2021, 22, 1230–1239. [Google Scholar] [CrossRef]
- Drago, J.Z.; Kantoff, P.W.; Stopsack, K.H. Adverse event profiles of apalutamide, enzalutamide, and darolutamide in SPARTAN, PROSPER, and ARAMIS: How confident are we about which drug is safest? J. Clin. Oncol. 2020, 38, 318. [Google Scholar] [CrossRef]
- Fendler, W.P.; Weber, M.; Iravani, A.; Hofman, M.; Calais, J.; Czernin, J.; Ilhan, H.; Saad, F.; Small, E.J.; Smith, M.R.; et al. Prostate-Specific Membrane Antigen Ligand Positron Emission Tomography in Men with Nonmetastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2019, 25, 7448–7454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Evans, C.P.; Kim, C.-S.; Kimura, G.; et al. Enzalutamide in Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur. Urol. 2016, 71, 151–154. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, S.; Bjartell, A.; Lumen, N.; Maroto, P.; Paiss, T.; Gomez-Veiga, F.; Birtle, A.; Kramer, G.; Kalinka, E.; Spaëth, D.; et al. Real-World Outcomes in First-Line Treatment of Metastatic Castration-Resistant Prostate Cancer: The Prostate Cancer Registry. Target. Oncol. 2020, 15, 301–315. [Google Scholar] [CrossRef]
- Cattrini, C.; Laorden, N.R.; Castro, E.; García-Carbonero, I.; Piulats, J.; Puente, J.; Valderrama, B.; Guzman, J.C.V.; Billalabeitia, E.G.; Barrera, R.M.; et al. Impact of treatment sequence in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): Data from the prospective PROREPAIR-B study. Ann. Oncol. 2019, 30, v345–v346. [Google Scholar] [CrossRef]
- Loriot, Y.; Eymard, J.-C.; Patrikidou, A.; Ileana, E.; Massard, C.; Albiges, L.; Di Palma, M.; Escudier, B.; Fizazi, K. Prior long response to androgen deprivation predicts response to next-generation androgen receptor axis targeted drugs in castration resistant prostate cancer. Eur. J. Cancer 2015, 51, 1946–1952. [Google Scholar] [CrossRef]
- Huillard, O.; Albiges, L.; Eymard, J.-C.; Massard, C.; Di Palma, M.; Escudier, B.J.; Fizazi, K.; Loriot, Y. Efficacy of docetaxel chemotherapy in metastatic prostate cancer (mPC) patients (pts) experiencing early castration resistance (CR). J. Clin. Oncol. 2013, 31, 5075. [Google Scholar] [CrossRef]
- Oudard, S.; Fizazi, K.; Sengeløv, L.; Daugaard, G.; Saad, F.; Hansen, S.; Hjälm-Eriksson, M.; Jassem, J.; Thiery-Vuillemin, A.; Caffo, O.; et al. Cabazitaxel Versus Docetaxel As First-Line Therapy for Patients With Metastatic Castration-Resistant Prostate Cancer: A Randomized Phase III Trial—FIRSTANA. J. Clin. Oncol. 2017, 35, 3189–3197. [Google Scholar] [CrossRef]
- Zhou, T.; Sun, Y.-H.; Zhang, W.; Wu, T.-Y.; Chen, Q.; Shi, X.-L.; Xiao, G.-G.; Zhao, L.; Xu, C.-L. Indirect comparison between abiraterone acetate and enzalutamide for the treatment of metastatic castration-resistant prostate cancer: A systematic review. Asian J. Androl. 2017, 19, 196–202. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Ramaswamy, K.; Huang, A.; Mardekian, J.; Schultz, N.M.; Wang, L.; Sandin, R.; Lechpammer, S.; George, D.J. Survival outcomes in patients with chemotherapy-naive metastatic castration-resistant prostate cancer treated with enzalutamide or abiraterone acetate. Prostate Cancer Prostatic Dis. 2021, 1–9. [Google Scholar] [CrossRef]
- Lavaud, P.; Gravis, G.; Foulon, S.; Joly, F.; Oudard, S.; Priou, F.; Latorzeff, I.; Mourey, L.; Soulié, M.; Delva, R.; et al. Anticancer Activity and Tolerance of Treatments Received Beyond Progression in Men Treated Upfront with Androgen Deprivation Therapy With or Without Docetaxel for Metastatic Castration-naïve Prostate Cancer in the GETUG-AFU 15 Phase 3 Trial. Eur. Urol. 2017, 73, 696–703. [Google Scholar] [CrossRef]
- Annala, M.; Fu, S.; Bacon, J.; Sipola, J.; Iqbal, N.; Ferrario, C.; Ong, M.; Wadhwa, D.; Hotte, S.; Lo, G.; et al. Cabazitaxel versus abiraterone or enzalutamide in poor prognosis metastatic castration-resistant prostate cancer: A multicentre, randomised, open-label, phase II trial. Ann. Oncol. 2021, 32, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Buck, S.A.; Koolen, S.L.; Mathijssen, R.H.; de Wit, R.; van Soest, R.J. Cross-resistance and drug sequence in prostate cancer. Drug Resist. Updat. 2021, 56, 100761. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Borre, M.; Gurney, H.; Loriot, Y.; Andresen-Daniil, C.; Kalleda, R.; Pham, T.; Taplin, M.-E.; PLATO collaborators. Abiraterone Alone or in Combination With Enzalutamide in Metastatic Castration-Resistant Prostate Cancer With Rising Prostate-Specific Antigen During Enzalutamide Treatment. J. Clin. Oncol. 2018, 36, 2639–2646. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, D.J.; Annala, M.; Taavitsainen, S.; Finch, D.L.; Oja, C.; Vergidis, J.; Zulfiqar, M.; Sunderland, K.; A Azad, A.; Kollmannsberger, C.K.; et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: A multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019, 20, 1730–1739. [Google Scholar] [CrossRef]
- de Bono, J.S.; Oudard, S.; Özgüroglu, M.; Hansen, S.; Machiels, J.-P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorente, D.; Mateo, J.; Perez-Lopez, R.; de Bono, J.S.; Attard, G. Sequencing of agents in castration-resistant prostate cancer. Lancet Oncol. 2015, 16, e279–e292. [Google Scholar] [CrossRef]
- de Bono, J.S.; Smith, M.R.; Saad, F.; Rathkopf, D.E.; Mulders, P.F.; Small, E.J.; Shore, N.D.; Fizazi, K.; De Porre, P.; Kheoh, T.; et al. Subsequent Chemotherapy and Treatment Patterns After Abiraterone Acetate in Patients with Metastatic Castration-resistant Prostate Cancer: Post Hoc Analysis of COU-AA-302. Eur. Urol. 2016, 71, 656–664. [Google Scholar] [CrossRef] [Green Version]
- Demirci, U.; Oflazoglu, U.; Kodaz, H.; Ciltas, A.; Kefeli, U.; Akyol, M.; Ozturk, B.; Geredeli, C.; Seker, M.M.; Cihan, S.; et al. Abiraterone acetate (AA) in patients with metastatic castration-resistant prostate cancer (MCRPC) after docetaxel chemotherapy: Multicentric experience of Anatolian Society of Medical Oncology. J. Clin. Oncol. 2014, 32, e16094. [Google Scholar] [CrossRef]
- Loriot, Y.; Bianchini, D.; Ileana, E.; Sandhu, S.; Patrikidou, A.; Pezaro, C.; Albiges, L.; Attard, G.; Fizazi, K.; De Bono, J.; et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann. Oncol. 2013, 24, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Caffo, O.; Basso, U.; Facchini, G.; Gasparro, D.; Alesini, D.; Tucci, M.; Ortega, C.; Fratino, L.; Campadelli, E.; Re, G.L.; et al. Activity of subsequent new drugs (NDs) in post-docetaxel (DOC) failure for metastatic castration-resistant prostate cancer (mCRPC) patients (pts): A multicenter Italian experience. J. Clin. Oncol. 2014, 32, 5089. [Google Scholar] [CrossRef]
- Al Nakouzi, N.; Le Moulec, S.; Albigès, L.; Wang, C.; Beuzeboc, P.; Gross-Goupil, M.; Rouge, T.D.L.M.; Guillot, A.; Gajda, D.; Massard, C.; et al. Cabazitaxel Remains Active in Patients Progressing After Docetaxel Followed by Novel Androgen Receptor Pathway Targeted Therapies. Eur. Urol. 2015, 68, 228–235. [Google Scholar] [CrossRef]
- Oh, W.K.; Cheng, W.Y.; Miao, R.; Vekeman, F.; Gauthier-Loiselle, M.; Duh, M.S.; Drea, E.; Szatrowski, T.P. Real-world outcomes in patients with metastatic castration-resistant prostate cancer receiving second-line chemotherapy versus an alternative androgen receptor-targeted agent (ARTA) following early progression on a first-line ARTA in a US community oncology setting. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 500.e1–500.e9. [Google Scholar] [CrossRef]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.; Goodman, O.B.; Saad, F.; et al. Abiraterone and Increased Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef]
- Hoskin, P.; Sartor, O.; O’Sullivan, J.M.; Johannessen, D.C.; Helle, S.I.; Logue, J.; Bottomley, D.; Nilsson, S.; Vogelzang, N.J.; Fang, F.; et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: A prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014, 15, 1397–1406. [Google Scholar] [CrossRef]
- Sartor, O.; Hoskin, P.; Coleman, R.E.; Nilsson, S.; Vogelzang, N.J.; Petrenciuc, O.; Staudacher, K.; Thuresson, M.; Parker, C. Chemotherapy following radium-223 dichloride treatment in ALSYMPCA. Prostate 2016, 76, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Carles, J.; Gillessen, S.; Heidenreich, A.; Heinrich, D.; Gratt, J.; Lévy, J.; Miller, K.; Nilsson, S.; Petrenciuc, O.; et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: An international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016, 17, 1306–1316. [Google Scholar] [CrossRef]
- Smith, M.; Parker, C.; Saad, F.; Miller, K.; Tombal, B.; Ng, Q.S.; Boegemann, M.; Matveev, V.; Piulats, J.M.; Zucca, L.E.; et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 408–419. [Google Scholar] [CrossRef]
- Gillessen, S.; Choudhury, A.; Rodriguez-Vida, A.; Nole, F.; Diaz, E.G.; Roumeguere, T.A.; Daugaard, G.; Loriot, Y.; Saad, F.; McDermott, R.S.; et al. Decreased fracture rate by mandating bone protecting agents in the EORTC 1333/PEACEIII trial combining Ra223 with enzalutamide versus enzalutamide alone: An updated safety analysis. J. Clin. Oncol. 2021, 39, 5002. [Google Scholar] [CrossRef]
- EMA Xofigo. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/xofigo#overview-section (accessed on 19 July 2021).
- Sadaghiani, M.S.; Sheikhbahaei, S.; Werner, R.A.; Pienta, K.J.; Pomper, M.G.; Solnes, L.B.; Gorin, M.A.; Wang, N.-Y.; Rowe, S.P. A Systematic Review and Meta-analysis of the Effectiveness and Toxicities of Lutetium-177–labeled Prostate-specific Membrane Antigen–targeted Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 2021, 80, 82–94. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; A Pattison, D.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Srinivas, S.; Iagaru, A. To Scan or Not to Scan: An Unnecessary Dilemma for PSMA Radioligand Therapy. J. Nucl. Med. 2021. [Google Scholar] [CrossRef]
- Privé, B.M.; Peters, S.M.; Muselaers, C.H.; van Oort, I.M.; Janssen, M.J.; Sedelaar, J.M.; Konijnenberg, M.W.; Zámecnik, P.; Uijen, M.J.; Schilham, M.G.; et al. Lutetium-177-PSMA-617 in Low-Volume Hormone-Sensitive Metastatic Prostate Cancer: A Prospective Pilot Study. Clin. Cancer Res. 2021, 27, 3595–3601. [Google Scholar] [CrossRef]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Zheng, M. Long-Term Efficacy of Zoledronic Acid for the Prevention of Skeletal Complications in Patients With Metastatic Hormone-Refractory Prostate Cancer. J. Natl. Cancer Inst. 2004, 96, 879–882. [Google Scholar] [CrossRef] [Green Version]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef] [Green Version]
- Francini, E.; Montagnani, F.; Nuzzo, P.V.; Gonzalez-Velez, M.; Alimohamed, N.S.; Rosellini, P.; Moreno-Candilejo, I.; Cigliola, A.; Rubio-Perez, J.; Crivelli, F.; et al. Association of Concomitant Bone Resorption Inhibitors With Overall Survival Among Patients With Metastatic Castration-Resistant Prostate Cancer and Bone Metastases Receiving Abiraterone Acetate With Prednisone as First-Line Therapy. JAMA Netw. Open 2021, 4, e2116536. [Google Scholar] [CrossRef]
- Takvorian, S.U.; Haas, N.B. Use of Bone Resorption Inhibitors in Metastatic Castration-Resistant Prostate Cancer-20 Years Later, and the Answer Is Still Yes. JAMA Netw. Open 2021, 4, e2117159. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Halabi, S.; Ryan, C.J.; Hussain, A.; Vogelzang, N.; Stadler, W.; Hauke, R.J.; Monk, J.P.; Saylor, P.; Bhoopalam, N.; et al. Randomized Controlled Trial of Early Zoledronic Acid in Men With Castration-Sensitive Prostate Cancer and Bone Metastases: Results of CALGB 90202 (Alliance). J. Clin. Oncol. 2014, 32, 1143–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messina, C.; Buzzatti, G.; Dellepiane, C.; Cavo, A.; Tolomeo, F.; Cattrini, C.; Boccardo, F. Genitourinary tumours in the targeted therapies era. Anti-Cancer Drugs 2016, 27, 917–943. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Hussain, M.; Sternberg, C.N.; Fizazi, K.; Yamada, K.S.; Kappeler, C.; Kuss, I.; Tombal, B.F. ARASENS: A phase 3 trial of darolutamide in combination with docetaxel for men with metastatic hormone-sensitive prostate cancer (mHSPC). J. Clin. Oncol. 2018, 36, TPS383. [Google Scholar] [CrossRef]
- Fizazi, K.; Maldonado, X.; Foulon, S.; Roubaud, G.; McDermott, R.S.; Flechon, A.; Tombal, B.F.; Supiot, S.; Berthold, D.R.; Ronchin, P.; et al. A phase 3 trial with a 2x2 factorial design of abiraterone acetate plus prednisone and/or local radiotherapy in men with de novo metastatic castration-sensitive prostate cancer (mCSPC): First results of PEACE-1. J. Clin. Oncol. 2021, 39, 5000. [Google Scholar] [CrossRef]
- Morris, M.J.; Heller, G.; Bryce, A.H.; Armstrong, A.J.; Beltran, H.; Hahn, O.M.; McGary, E.C.; Mehan, P.T.; Goldkorn, A.; Roth, B.J.; et al. Alliance A031201: A phase III trial of enzalutamide (ENZ) versus enzalutamide, abiraterone, and prednisone (ENZ/AAP) for metastatic castration resistant prostate cancer (mCRPC). J. Clin. Oncol. 2019, 37, 5008. [Google Scholar] [CrossRef]
- Rathkopf, D.E.; Efstathiou, E.; Attard, G.; Flaig, T.W.; Franke, F.A.; Goodman, O.B.; Oudard, S.; Steuber, T.; Suzuki, H.; Wu, D.; et al. Final results from ACIS, a randomized, placebo (PBO)-controlled double-blind phase 3 study of apalutamide (APA) and abiraterone acetate plus prednisone (AAP) versus AAP in patients (pts) with chemo-naive metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2021, 39, 9. [Google Scholar] [CrossRef]
- Caffo, O.; Palesandro, E.; Nole, F.; Gasparro, D.; Mucciarini, C.; Aieta, M.; Zagonel, V.; Iacovelli, R.; De Giorgi, U.; Rossetti, S.; et al. A multicentric phase II randomized trial of docetaxel (D) plus enzalutamide (E) versus docetaxel (D) as first-line chemotherapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): CHEIRON study. J. Clin. Oncol. 2019, 37, 148. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.; Chen, Y.-H.; Duan, F.; Jeraj, R.; Luo, J.; Antonarakis, E.S.; Liu, G.; Carducci, M.A. Cabazitaxel with abiraterone versus abiraterone alone randomized trial for extensive disease following docetaxel: The CHAARTED 2 Trial: A trial of the ECOG-ACRIN Cancer Research Group (EA8153). J. Clin. Oncol. 2019, 37, TPS5094. [Google Scholar] [CrossRef]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T.; et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J. Clin. Oncol. 2020, 38, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Fléchon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, S.K.; Ciprotti, M.; et al. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell 2020, 38, 489–499.e3. [Google Scholar] [CrossRef] [PubMed]
- Kessel, A.; McFarland, T.R.; Sayegh, N.; Morton, K.; Sirohi, D.; Kohli, M.; Swami, U.; Nussenzveig, R.; Agarwal, N.; Maughan, B.L. Randomized phase II trial of radium-223 (RA) plus enzalutamide (EZ) versus EZ alone in metastatic castration-refractory prostate cancer (mCRPC): Final efficacy and safety results. J. Clin. Oncol. 2021, 39, 135. [Google Scholar] [CrossRef]
- Cattrini, C.; Zanardi, E.; Vallome, G.; Cavo, A.; Cerbone, L.; Di Meglio, A.; Fabbroni, C.; Latocca, M.; Rizzo, F.; Messina, C.; et al. Targeting androgen-independent pathways: New chances for patients with prostate cancer? Crit. Rev. Oncol. 2017, 118, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Lozano, R.; Castro, E.; Aragón, I.M.; Cendón, Y.; Cattrini, C.; López-Casas, P.P.; Olmos, D. Genetic aberrations in DNA repair pathways: A cornerstone of precision oncology in prostate cancer. Br. J. Cancer 2020, 124, 552–563. [Google Scholar] [CrossRef]
- Messina, C.; Cattrini, C.; Soldato, D.; Vallome, G.; Caffo, O.; Castro, E.; Olmos, D.; Boccardo, F.; Zanardi, E. BRCA Mutations in Prostate Cancer: Prognostic and Predictive Implications. J. Oncol. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Castro, E.; Romero-Laorden, N.; del Pozo, A.; Lozano, R.; Medina, A.; Puente, J.; Piulats, J.M.; Lorente, D.; Saez, M.I.; Morales-Barrera, R.; et al. PROREPAIR-B: A Prospective Cohort Study of the Impact of Germline DNA Repair Mutations on the Outcomes of Patients With Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2019, 37, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- Nicolosi, P.; Ledet, E.; Yang, S.; Michalski, S.; Freschi, B.; O’Leary, E.; Esplin, E.D.; Nussbaum, R.L.; Sartor, O. Prevalence of Germline Variants in Prostate Cancer and Implications for Current Genetic Testing Guidelines. JAMA Oncol. 2019, 5, 523–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abida, W.; Armenia, J.; Gopalan, A.; Brennan, R.; Walsh, M.; Barron, D.; Danila, D.; Rathkopf, D.; Morris, M.; Slovin, S.; et al. Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precis. Oncol. 2017, 1, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Goh, C.; Olmos, D.; Saunders, E.; Leongamornlert, D.; Tymrakiewicz, M.; Mahmud, N.; Dadaev, T.; Govindasami, K.; Guy, M.; et al. Germline BRCA Mutations Are Associated With Higher Risk of Nodal Involvement, Distant Metastasis, and Poor Survival Outcomes in Prostate Cancer. J. Clin. Oncol. 2013, 31, 1748–1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, E.; Goh, C.; Leongamornlert, D.; Saunders, E.; Tymrakiewicz, M.; Dadaev, T.; Govindasami, K.; Guy, M.; Ellis, S.; Frost, D.; et al. Effect of BRCA Mutations on Metastatic Relapse and Cause-specific Survival After Radical Treatment for Localised Prostate Cancer. Eur. Urol. 2015, 68, 186–193. [Google Scholar] [CrossRef]
- Cattrini, C.; Mejorada, R.L.; Conteduca, V.; Ruiz-Vico, M.; Lolkema, M.; Lorente, D.; Sandhu, S.; Laorden, N.R.; Loriot, Y.; Azad, A.; et al. 692TiP BRCA2men: An international, multicentre, observational and ambispective study to validate the predictive value of germline BRCA2 mutations for selecting the first-line of treatment in metastatic castration-resistant prostate cancer (mCRPC). Ann. Oncol. 2020, 31, S547–S548. [Google Scholar] [CrossRef]
- Mateo, J.; Carreira, S.; Sandhu, S.; Miranda, S.; Mossop, H.; Perez-Lopez, R.; Rodrigues, D.N.; Robinson, D.; Omlin, A.; Tunariu, N.; et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N. Engl. J. Med. 2015, 373, 1697–1708. [Google Scholar] [CrossRef]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Sautois, B.; Vogelzang, N.J.; Bambury, R.M.; Voog, E.; et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J. Clin. Oncol. 2020, 38, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Mehra, N.; Higano, C.S.; Saad, F.; Buttigliero, C.; van Oort, I.M.; Mata, M.; Chen, H.-C.; Healy, C.G.; Czibere, A.; et al. TALAPRO-1: Phase II study of talazoparib (TALA) in patients (pts) with DNA damage repair alterations (DDRm) and metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2021, 39, 93. [Google Scholar] [CrossRef]
- Smith, M.; Sandhu, S.; Kelly, W.; Scher, H.; Efstathiou, E.; Lara, P.; Yu, E.; George, D.; Chi, K.; Saad, F.; et al. Pre-specified interim analysis of GALAHAD: A phase II study of niraparib in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and biallelic DNA-repair gene defects (DRD). Ann. Oncol. 2019, 30, v884–v885. [Google Scholar] [CrossRef]
- Study on Olaparib Plus Abiraterone as First-Line Therapy in Men with Metastatic Castration-Resistant Prostate Cancer. ClinicalTrials.gov AstraZeneca, Multicenter Study. Available online: https://clinicaltrials.gov/ct2/show/NCT03732820 (accessed on 7 September 2021).
- Yu, E.Y.; Park, S.H.; Huang, Y.-H.; Bennamoun, M.; Xu, L.; Kim, J.; Antonarakis, E.S. Phase III study of pembrolizumab (pembro) plus olaparib versus enzalutamide (enza) or abiraterone acetate (abi) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) who progressed on chemotherapy: KEYLYNK-010. J. Clin. Oncol. 2020, 38, TPS256. [Google Scholar] [CrossRef]
- Ryan, C.J.; Abida, W.; Bryce, A.H.; Balar, A.V.; Dumbadze, I.; Given, R.W.; Morris, D.; Petrylak, D.P.; Redfern, C.H.; Scher, H.I.; et al. TRITON3: An international, randomized, open-label, phase III study of the PARP inhibitor rucaparib vs. physician’s choice of therapy for patients with metastatic castration-resistant prostate cancer (mCRPC) associated with homologous recombination deficiency (HRD). J. Clin. Oncol. 2018, 36, TPS389. [Google Scholar] [CrossRef]
- Rao, A.; Ryan, C.J.; VanderWeele, D.J.; Heller, G.; Lewis, L.D.; Watt, C.; Chen, R.C.; Grubb, R.; Hahn, O.M.; Beltran, H.; et al. CASPAR (Alliance A031902): A randomized, phase III trial of enzalutamide (ENZ) with rucaparib (RUCA)/placebo (PBO) as a novel therapy in first-line metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2021, 39, TPS181. [Google Scholar] [CrossRef]
- Agarwal, N.; Shore, N.D.; Dunshee, C.; Karsh, L.I.; Azad, A.; Fay, A.P.; Carles, J.; Sullivan, B.; Di Santo, N.; Elmeliegy, M.; et al. TALAPRO-2: A placebo-controlled phase III study of talazoparib (TALA) plus enzalutamide (ENZA) for patients with first-line metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, TPS264. [Google Scholar] [CrossRef]
- Chi, K.N.; Rathkopf, D.E.; Attard, G.; Smith, M.R.; Efstathiou, E.; Olmos, D.; Small, E.J.; Lee, J.Y.; Ricci, D.S.; Simon, J.S.; et al. A phase III randomized, placebo-controlled, double-blind study of niraparib plus abiraterone acetate and prednisone versus abiraterone acetate and prednisone in patients with metastatic prostate cancer (MAGNITUDE). J. Clin. Oncol. 2020, 38, TPS5588. [Google Scholar] [CrossRef]
- De Bono, J.S.; De Giorgi, U.; Rodrigues, D.N.; Massard, C.; Bracarda, S.; Font, A.; Arija, J.A.A.; Shih, K.C.; Radavoi, G.D.; Xu, N.; et al. Randomized Phase II Study Evaluating Akt Blockade with Ipatasertib, in Combination with Abiraterone, in Patients with Metastatic Prostate Cancer with and without PTEN Loss. Clin. Cancer Res. 2018, 25, 928–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crabb, S.J.; Griffiths, G.; Marwood, E.; Dunkley, D.; Downs, N.; Martin, K.; Light, M.; Northey, J.; Wilding, S.; Whitehead, A.; et al. Pan-AKT Inhibitor Capivasertib With Docetaxel and Prednisolone in Metastatic Castration-Resistant Prostate Cancer: A Randomized, Placebo-Controlled Phase II Trial (ProCAID). J. Clin. Oncol. 2021, 39, 190–201. [Google Scholar] [CrossRef]
- Sweeney, C.; Bracarda, S.; Sternberg, C.N.; Chi, K.N.; Olmos, D.; Sandhu, S.; Massard, C.; Matsubara, N.; Alekseev, B.; Parnis, F.; et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2021, 398, 131–142. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Halabi, S.; Luo, J.; Nanus, D.M.; Giannakakou, P.; Szmulewitz, R.Z.; Danila, D.C.; Healy, P.; Anand, M.; Rothwell, C.J.; et al. Prospective Multicenter Validation of Androgen Receptor Splice Variant 7 and Hormone Therapy Resistance in High-Risk Castration-Resistant Prostate Cancer: The PROPHECY Study. J. Clin. Oncol. 2019, 37, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Graff, J.N.; Hadaschik, B.A.; Oudard, S.; Mainwaring, P.N.; Bhaumik, A.; Gormley, M.; Londhe, A.; Thomas, S.; Lopez-Gitlitz, A.; et al. Molecular determinants of prostate specific antigen (PSA) kinetics and clinical response to apalutamide (APA) in patients (pts) with nonmetastatic castration-resistant prostate cancer (nmCRPC) in SPARTAN. J. Clin. Oncol. 2020, 38, 5521. [Google Scholar] [CrossRef]
- Feng, F.Y.; Thomas, S.; Aguilar-Bonavides, C.; Gormley, M.; Agarwal, N.; Attard, G.; Wyatt, A.W.; Davicioni, E.; Ricci, D.S.; Lopez-Gitlitz, A.; et al. Molecular determinants of outcome for metastatic castration-sensitive prostate cancer (mCSPC) with addition of apalutamide (APA) or placebo (PBO) to androgen deprivation therapy (ADT) in TITAN. J. Clin. Oncol. 2020, 38, 5535. [Google Scholar] [CrossRef]
- Hamid, A.; Wang, X.V.; Chen, Y.-H.; Feng, F.Y.; Den, R.B.; Attard, G.; Van Allen, E.M.; Huang, H.-C.; Karns, A.; Dittamore, R.; et al. Luminal B subtype as a predictive biomarker of docetaxel benefit for newly diagnosed metastatic hormone sensitive prostate cancer (mHSPC): A correlative study of E3805 CHAARTED. J. Clin. Oncol. 2020, 38, 162. [Google Scholar] [CrossRef]
- De Bono, J.S.; Fizazi, K.; Saad, F.; Shore, N.D.; Roubaud, G.; Ozguroglu, M.; Penel, N.; Matsubara, N.; Mehra, N.; Procopio, G.; et al. PROfound: Efficacy of olaparib (ola) by prior taxane use in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and homologous recombination repair (HRR) gene alterations. J. Clin. Oncol. 2020, 38, 134. [Google Scholar] [CrossRef]
- Wallis, C.J.D.; Klaassen, Z.; Jackson, W.C.; Dess, R.T.; Reichert, Z.R.; Sun, Y.; Spratt, D.E. Olaparib vs Cabazitaxel in Metastatic Castration-Resistant Prostate Cancer. JAMA Netw. Open 2021, 4, e2110950. [Google Scholar] [CrossRef]
- Reason, T.; McCrea, C.; Hettle, R.; Ghate, S.; Poehlein, C.H.; Olmos, D. Indirect treatment comparison of the efficacy of olaparib 300 mg tablets BID and cabazitaxel 25 mg/m2 every 3 weeks plus daily prednisolone and granulocyte colony-stimulating factor in the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2021, 39, 5051. [Google Scholar] [CrossRef]
- De Bono, J.S.; Matsubara, N.; Penel, N.; Mehra, N.; Kolinsky, M.P.; Bompas, E.; Feyerabend, S.; Gravis, G.; Joung, J.Y.; Nishimura, K.; et al. Exploratory gene-by-gene analysis of olaparib in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): PROfound. J. Clin. Oncol. 2021, 39, 126. [Google Scholar] [CrossRef]
- Carreira, S.; Porta, N.; Arce-Gallego, S.; Seed, G.; Llop-Guevara, A.; Bianchini, D.; Rescigno, P.; Paschalis, A.; Bertan, C.; Baker, C.; et al. Biomarkers Associating with PARP Inhibitor Benefit in Prostate Cancer in the TOPARP-B Trial. Cancer Discov. 2021. [Google Scholar] [CrossRef] [PubMed]
- Markowski, M.C.; Antonarakis, E.S. BRCA1 Versus BRCA2 and PARP Inhibitor Sensitivity in Prostate Cancer: More Different Than Alike? J. Clin. Oncol. 2020, 38, 3735–3739. [Google Scholar] [CrossRef] [PubMed]
- Taza, F.; Holler, A.E.; Adra, N.; Ashkar, R.; Sokolova, A.; Kessel, A.; Nafissi, N.; Barata, P.C.; Assed Bastos, D.; Smaletz, O.; et al. Differential activity of PARP inhibitors in BRCA1- versus BRCA2-altered metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2021, 39. [Google Scholar] [CrossRef]
- Clarke, N.; Wiechno, P.; Alekseev, B.; Sala, N.; Jones, R.; Kocak, I.; Chiuri, V.E.; Jassem, J.; Fléchon, A.; Redfern, C.; et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018, 19, 975–986. [Google Scholar] [CrossRef]
- De Laere, B.; van Dam, P.J.; Whitington, T.; Mayrhofer, M.; Diaz, E.H.; Van den Eynden, G.; Vandebroek, J.; Del-Favero, J.; Van Laere, S.; Dirix, L.; et al. Comprehensive Profiling of the Androgen Receptor in Liquid Biopsies from Castration-resistant Prostate Cancer Reveals Novel Intra-AR Structural Variation and Splice Variant Expression Patterns. Eur. Urol. 2017, 72, 192–200. [Google Scholar] [CrossRef]
- Wyatt, A.W.; Azad, A.A.; Volik, S.V.; Annala, M.; Beja, K.; McConeghy, B.; Haegert, A.; Warner, E.W.; Mo, F.; Brahmbhatt, S.; et al. Genomic Alterations in Cell-Free DNA and Enzalutamide Resistance in Castration-Resistant Prostate Cancer. JAMA Oncol. 2016, 2, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, V.; Wetterskog, D.; Sharabiani, M.T.A.; Grande, E.; Fernandez-Perez, M.P.; Jayaram, A.; Salvi, S.; Castellano, D.; Romanel, A.; Lolli, C.; et al. Androgen receptor gene status in plasma DNA associates with worse outcome on enzalutamide or abiraterone for castration-resistant prostate cancer: A multi-institution correlative biomarker study. Ann. Oncol. 2017, 28, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, V.; Jayaram, A.; Romero-Laorden, N.; Wetterskog, D.; Salvi, S.; Gurioli, G.; Scarpi, E.; Castro, E.; Marin-Aguilera, M.; Lolli, C.; et al. Plasma Androgen Receptor and Docetaxel for Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2018, 75, 368–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conteduca, V.; Wetterskog, D.; Castro, E.; Scarpi, E.; Romero-Laorden, N.; Gurioli, G.; Jayaram, A.; Lolli, C.; Schepisi, G.; Wingate, A.; et al. Plasma androgen receptor and response to adapted and standard docetaxel regimen in castration-resistant prostate cancer: A multicenter biomarker study. Eur. J. Cancer 2021, 152, 49–59. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef] [Green Version]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Nakazawa, M.; Nadal, R.; Paller, C.J.; Denmeade, S.R.; Carducci, M.A.; et al. Androgen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol. 2015, 1, 582–591. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, M.; Lu, C.; Chen, Y.; Paller, C.J.; Carducci, M.A.; Eisenberger, M.A.; Luo, J.; Antonarakis, E.S. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann. Oncol. 2015, 26, 1859–1865. [Google Scholar] [CrossRef]
- Onstenk, W.; Sieuwerts, A.M.; Kraan, J.; Van, M.; Nieuweboer, A.J.; Mathijssen, R.H.; Hamberg, P.; Meulenbeld, H.J.; De Laere, B.; Dirix, L.Y.; et al. Efficacy of Cabazitaxel in Castration-resistant Prostate Cancer Is Independent of the Presence of AR-V7 in Circulating Tumor Cells. Eur. Urol. 2015, 68, 939–945. [Google Scholar] [CrossRef]
- Taplin, M.-E.; Antonarakis, E.S.; Ferrante, K.J.; Horgan, K.; Blumenstein, B.A.; Saad, F.; Luo, J.; De Bono, J.S. Clinical factors associated with AR-V7 detection in ARMOR3-SV, a randomized trial of galeterone (Gal) vs enzalutamide (Enz) in men with AR-V7+ metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2017, 35, 5005. [Google Scholar] [CrossRef]
- Scher, H.I.; Lu, D.; Schreiber, N.A.; Louw, J.; Graf, R.P.; Vargas, H.A.; Johnson, A.; Jendrisak, A.; Bambury, R.; Danila, D.; et al. Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker With Outcomes and Survival in Castration-Resistant Prostate Cancer. JAMA Oncol. 2016, 2, 1441–1449. [Google Scholar] [CrossRef]
- Cattrini, C.; Rubagotti, A.; Zinoli, L.; Cerbone, L.; Zanardi, E.; Capaia, M.; Barboro, P.; Boccardo, F. Role of Circulating Tumor Cells (CTC), Androgen Receptor Full Length (AR-FL) and Androgen Receptor Splice Variant 7 (AR-V7) in a Prospective Cohort of Castration-Resistant Metastatic Prostate Cancer Patients. Cancers 2019, 11, 1365. [Google Scholar] [CrossRef] [Green Version]
- Prostate Cancer NCCN Guidelines. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 26 July 2021).
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraldeschi, R.; Nava Rodrigues, D.; Riisnaes, R.; Miranda, S.; Figueiredo, I.; Rescigno, P.; Ravi, P.; Pezaro, C.; Omlin, A.; Lorente, D.; et al. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur. Urol. 2014, 67, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Sweeney, C.; Bracarda, S.; Sternberg, C.N.; Chi, K.N.; Olmos, D.; Sandhu, S.K.; Massard, C.; Matsubara, N.; Harle-Yge, M.-L.; et al. Biomarker analysis of the phase III IPATential150 trial of first-line ipatasertib (Ipat) plus abiraterone (Abi) in metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 182. [Google Scholar] [CrossRef]
- Bono, J.S.D.; Sweeney, C.; Bracarda, S.; Sternberg, C.N.; Chi, K.N.; Olmos, D.; Sandhu, S.K.; Massard, C.; Matsubara, N.; Garcia, J.; et al. PI3K/AKT pathway biomarkers analysis from the phase III IPATential150 trial of ipatasertib plus abiraterone in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2021, 39, 13. [Google Scholar] [CrossRef]
- Zhao, S.G.; Chang, S.L.; Erho, N.; Yu, M.; Lehrer, J.; Alshalalfa, M.; Speers, C.; Cooperberg, M.R.; Kim, W.; Ryan, C.J.; et al. Associations of Luminal and Basal Subtyping of Prostate Cancer With Prognosis and Response to Androgen Deprivation Therapy. JAMA Oncol. 2017, 3, 1663–1672. [Google Scholar] [CrossRef]
- Montironi, R.; Cimadamore, A.; Lopez-Beltran, A.; Scarpelli, M.; Aurilio, G.; Santoni, M.; Massari, F.; Cheng, L. Morphologic, Molecular and Clinical Features of Aggressive Variant Prostate Cancer. Cells 2020, 9, 1073. [Google Scholar] [CrossRef]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.; Varambally, S.; et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosquera, J.M.; Beltran, H.; Park, K.; MacDonald, T.Y.; Robinson, B.D.; Tagawa, S.T.; Perner, S.; Bismar, T.A.; Erbersdobler, A.; Dhir, R.; et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia 2013, 15, 1-IN4. [Google Scholar] [CrossRef] [Green Version]
- Nyquist, M.D.; Corella, A.; Coleman, I.; De Sarkar, N.; Kaipainen, A.; Ha, G.; Gulati, R.; Ang, L.; Chatterjee, P.; Lucas, J.; et al. Combined TP53 and RB1 Loss Promotes Prostate Cancer Resistance to a Spectrum of Therapeutics and Confers Vulnerability to Replication Stress. Cell Rep. 2020, 31, 107669. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, A.M.; Harzstark, A.L.; Corn, P.G.; Wen, S.; Araujo, J.C.; Tu, S.M.; Pagliaro, L.C.; Kim, J.; Millikan, R.E.; Ryan, C.; et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Cancer Res. 2013, 19, 3621–3630. [Google Scholar] [CrossRef] [Green Version]
- Vlachostergios, P.J.; Puca, L.; Beltran, H. Emerging Variants of Castration-Resistant Prostate Cancer. Curr. Oncol. Rep. 2017, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Conteduca, V.; Oromendia, C.; Eng, K.W.; Bareja, R.; Sigouros, M.; Molina, A.; Faltas, B.M.; Sboner, A.; Mosquera, J.M.; Elemento, O.; et al. Clinical features of neuroendocrine prostate cancer. Eur. J. Cancer 2019, 121, 7–18. [Google Scholar] [CrossRef]
- Beltran, H.; Oromendia, C.; Danila, D.C.; Montgomery, B.; Hoimes, C.; Szmulewitz, R.Z.; Vaishampayan, U.; Armstrong, A.J.; Stein, M.; Pinski, J.; et al. A Phase II Trial of the Aurora Kinase A Inhibitor Alisertib for Patients with Castration-resistant and Neuroendocrine Prostate Cancer: Efficacy and Biomarkers. Clin. Cancer Res. 2018, 25, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spetsieris, N.; Boukovala, M.; Patsakis, G.; Alafis, I.; Efstathiou, E. Neuroendocrine and Aggressive-Variant Prostate Cancer. Cancers 2020, 12, 3792. [Google Scholar] [CrossRef]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Cieslik, M.; Lonigro, R.J.; Vats, P.; Reimers, M.A.; Cao, X.; Ning, Y.; Wang, L.; Kunju, L.P.; de Sarkar, N.; et al. Inactivation of CDK12 Delineates a Distinct Immunogenic Class of Advanced Prostate Cancer. Cell 2018, 173, 1770–1782.e14. [Google Scholar] [CrossRef] [Green Version]
- Antonarakis, E.S.; Isaacsson Velho, P.; Fu, W.; Wang, H.; Agarwal, N.; Sacristan Santos, V.; Maughan, B.L.; Pili, R.; Adra, N.; Sternberg, C.N.; et al. CDK12-Altered Prostate Cancer: Clinical Features and Therapeutic Outcomes to Standard Systemic Therapies, Poly (ADP-Ribose) Polymerase Inhibitors, and PD-1 Inhibitors. JCO Precis. Oncol. 2020, 370–381. [Google Scholar] [CrossRef]
- Boysen, G.; Rodrigues, D.N.; Rescigno, P.; Seed, G.; Dolling, D.; Riisnaes, R.; Crespo, M.; Zafeiriou, Z.; Sumanasuriya, S.; Bianchini, D.; et al. SPOP-Mutated/CHD1-Deleted Lethal Prostate Cancer and Abiraterone Sensitivity. Clin. Cancer Res. 2018, 24, 5585–5593. [Google Scholar] [CrossRef] [Green Version]
- Nava Rodrigues, D.; Casiraghi, N.; Romanel, A.; Crespo, M.; Miranda, S.; Rescigno, P.; Figueiredo, I.; Riisnaes, R.; Carreira, S.; Sumanasuriya, S.; et al. RB1 Heterogeneity in Advanced Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2018, 25, 687–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abida, W.; Cyrta, J.; Heller, G.; Prandi, D.; Armenia, J.; Coleman, I.; Cieslik, M.; Benelli, M.; Robinson, D.; Van Allen, E.M.; et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 11428–11436. [Google Scholar] [CrossRef] [Green Version]
- Hamid, A.A.; Gray, K.P.; Shaw, G.; MacConaill, L.E.; Evan, C.; Bernard, B.; Loda, M.; Corcoran, N.M.; Van Allen, E.M.; Choudhury, A.D.; et al. Compound Genomic Alterations of TP53, PTEN, and RB1 Tumor Suppressors in Localized and Metastatic Prostate Cancer. Eur. Urol. 2019, 76, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, A.W.; Annala, M.; Aggarwal, R.; Beja, K.; Feng, F.; Youngren, J.; Foye, A.; Lloyd, P.; Nykter, M.; Beer, T.M.; et al. Concordance of Circulating Tumor DNA and Matched Metastatic Tissue Biopsy in Prostate Cancer. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, K.N.; Barnicle, A.; Sibilla, C.; Lai, Z.; Corcoran, C.; Williams, J.A.; Barrett, J.C.; Adelman, C.A.; Qiu, P.; Easter, A.; et al. Concordance of BRCA1, BRCA2 (BRCA), and ATM mutations identified in matched tumor tissue and circulating tumor DNA (ctDNA) in men with metastatic castration-resistant prostate cancer (mCRPC) screened in the PROfound study. J. Clin. Oncol. 2021, 39, 26. [Google Scholar] [CrossRef]
- Herberts, C.; Wyatt, A.W. Technical and biological constraints on ctDNA-based genotyping. Trends cancer 2021. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Seed, G.; Bertan, C.; Rescigno, P.; Dolling, D.; Figueiredo, I.; Miranda, S.; Nava Rodrigues, D.; Gurel, B.; Clarke, M.; et al. Genomics of lethal prostate cancer at diagnosis and castration resistance. J. Clin. Investig. 2020, 130, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Frantellizzi, V.; Chiaravalloti, A.; Pontico, M.; De Feo, M.S.; Corica, F.; Montebello, M.; Schillaci, O.; De Vincentis, G.; Bagni, O. Prognostic and Theranostic Applications of Positron Emission Tomography for a Personalized Approach to Metastatic Castration-Resistant Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 3036. [Google Scholar] [CrossRef]
- Caroli, P.; De Giorgi, U.; Scarpi, E.; Fantini, L.; Moretti, A.; Galassi, R.; Celli, M.; Conteduca, V.; Rossi, L.; Bianchi, E.; et al. Prognostic value of 18F-choline PET/CT metabolic parameters in patients with metastatic castration-resistant prostate cancer treated with abiraterone or enzalutamide. Eur. J. Nucl. Med. Mol. Imaging 2017, 45, 348–354. [Google Scholar] [CrossRef]
- Bauckneht, M.; Capitanio, S.; Donegani, M.I.; Zanardi, E.; Miceli, A.; Murialdo, R.; Raffa, S.; Tomasello, L.; Vitti, M.; Cavo, A.; et al. Role of Baseline and Post-Therapy 18F-FDG PET in the Prognostic Stratification of Metastatic Castration-Resistant Prostate Cancer (mCRPC) Patients Treated with Radium-223. Cancers 2019, 12, 31. [Google Scholar] [CrossRef] [Green Version]
- Filippi, L.; Spinelli, G.P.; Chiaravalloti, A.; Schillaci, O.; Equitani, F.; Bagni, O. Prognostic Value of (18)F-Choline PET/CT in Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Radium-223. Biomedicines 2020, 8, 555. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhy, S.U.; Karzai, F.; Marte, J.L.; Bilusic, M.; McMahon, S.; Strauss, J.; Couvillon, A.; Williams, M.; Hankin, A.; Steinberg, S.M.; et al. PSA progression compared to radiographic or clinical progression in metastatic castration-resistant prostate cancer patients treated with enzalutamide. J. Clin. Oncol. 2020, 38, 105. [Google Scholar] [CrossRef]
| Setting | Name of the Trial | Population | Exp Arm | Control Arm | N Exp/ Cont | Primary Endpoint | FU (months) | mOS (months) Exp/Contr | HR (95% CI) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| mHSPC | GETUG-AFU 15 | L/H volume | Doce + ADT | ADT | 192/193 | OS | 83.9 | 62.1/48.6 | 0.88 (0.68–1.14) | [9] |
| CHAARTED | L/H volume | Doce + ADT | ADT | 397/393 | OS | 53.7 | 57.6/47.2 | 0.72 (0.59–0.89) | [1] | |
| STAMPEDE (arm C) | L/H volume | Doce + ADT | ADT | 362/724 | OS | 78.2 | 59.1/43.1 | 0.81 (0.69–0.95) | [2] | |
| ENZAMET | L/H volume | Enza + ADT ± doce | NSAA + ADT ± doce | 563/562 | OS | 34 | NE/NE | 0.67 (0.52–0.86) | [6] | |
| ARCHES | L/H volume; prior doce allowed | Enza + ADT | Placebo + ADT | 574/576 | rPFS | 14.4 | NE/NE | 0.81 (0.53–1.25) | [10] | |
| TITAN | L/H volume; prior doce allowed | Apa + ADT | Placebo + ADT | 525/527 | OS and rPFS | 44 | NE/52.2 | 0.65 (0.53–0.79) | [11] | |
| LATITUDE | High risk | AA + P + ADT | Placebo + P + ADT | 597/602 | OS | 51.8 | 53.3/36.5 | 0.66 (0.56–0.78) | [4] | |
| STAMPEDE (arm G) | L/H risk L/H volume | AA + P + ADT | Placebo + P + ADT | 501/502 | OS | 73.2 | 79.2/45.6 | 0.60 (0.50–0.71) | [5] | |
| STAMPEDE (arm H) | L/H volume | RT to prostate + ADT | ADT | 1032/1029 | OS | 37 | 42.5/41.6 | 0.92 (0.80–1.06) | [8] | |
| HORRAD | PSA > 20ng/mL and bone lesions | RT to prostate + ADT | ADT | 216/216 | OS | 47 | 45/43 | 0.90 (0.70–1.14) | [12] | |
| nmCRPC | ARAMIS | PSA doubling time ≤ 10 months and basal PSA ≥ 2 ng/mL | Daro + ADT | Placebo + ADT | 955/554 | MFS | 29 | NE/NE | 0.69 (0.53–0.88) | [13] |
| PROSPER | Enza + ADT | Placebo + ADT | 933/468 | MFS | 48 | 67/56.3 | 0.73 (0.61–0.89) | [14] | ||
| SPARTAN | Apa + ADT | Placebo + ADT | 806/401 | MFS | 52 | 73.9/59.9 | 0.78 (0.64–0.96) | [15] | ||
| TAX 327 | With or without symptoms | Doce + P | Mitoxantrone + P | 335/337 | OS | NA | 19.2/16.3 | 0.79 (0.67–0.93) | [16] | |
| 1st line mCRPC | COU-AA-302 | A/midly symptomatic pre-doce; no visceral mtx | AA + P + ADT | Placebo + P + ADT | 546/542 | rPFS, OS | 49.2 | 34.7/30.3 | 0.81 (0.70–0.93) | [17] |
| PREVAIL | A/midly symptomatic pre-doce | Enza + ADT | Placebo + ADT | 872/845 | rPFS, OS | 69 | 36/31 | 0.83 (0.75–0.93) | [18] | |
| IMPACT | A/midly symptomatic pre-/post-doce; Gleason ≤ 7; no visceral mtx | Sipuleucel-T + ADT | Placebo + ADT | 341/171 | OS | 34.1 | 25.8/21.7 | 0.78 (0.61–0.98) | [19] | |
| IPAtential150 | A/midly symptomatic | AA + P + ipatasertib | AA + P + placebo | 547/554 | (bio)rPFS | 19 | NE/NE | NE | [20] | |
| ≥2nd line mCRPC | COU-AA-301 | Post-doce | AA + P | Placebo + P | 797/398 | OS | 20.2 | 15.8/11.2 | 0.74 (0.64–0.86) | [21] |
| TROPIC | Post-doce | Cabazitaxel + P | Mitoxantrone + P | 378/377 | OS | 25.5 | NA/NA | 0.72 (0.61–0.84) | [22] | |
| AFFIRM | Post-doce | Enza | Placebo | 800/399 | OS | 14.4 | 18.4/13.6 | 0.63 (0.53–0.75) | [23] | |
| ALSYMPCA | Pre- and post-doce or unfit for doce; bone mtx and no visceral mtx | Radium-223 | Placebo | 614/307 | OS | NA | 14.9/11.3 | 0.70 (0.58–0.83) | [24] | |
| CARD | Post-doce and post-ARSi | Cabazitaxel | AA+P/Enza | 129/126 | IPFS | 9.2 | 13.6/11 | 0.64 (0.46–0.89) | [25] | |
| PROFOUND | Post-ARSi and pre-/post-taxane | Olaparib | AA+P/Enza | 162/83 * | (bio)IPFS | 21 | 19.1/14.7* | 0.69 (0.50–0.97) * | [26] | |
| VISION | Post-ARSi and 1–2 taxanes | LuPSMA | Standard of care | 551/280 | rPFS, OS | 20.9 | 15.3/11.3 | 0.62 (0.52–0.74) | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattrini, C.; España, R.; Mennitto, A.; Bersanelli, M.; Castro, E.; Olmos, D.; Lorente, D.; Gennari, A. Optimal Sequencing and Predictive Biomarkers in Patients with Advanced Prostate Cancer. Cancers 2021, 13, 4522. https://doi.org/10.3390/cancers13184522
Cattrini C, España R, Mennitto A, Bersanelli M, Castro E, Olmos D, Lorente D, Gennari A. Optimal Sequencing and Predictive Biomarkers in Patients with Advanced Prostate Cancer. Cancers. 2021; 13(18):4522. https://doi.org/10.3390/cancers13184522
Chicago/Turabian StyleCattrini, Carlo, Rodrigo España, Alessia Mennitto, Melissa Bersanelli, Elena Castro, David Olmos, David Lorente, and Alessandra Gennari. 2021. "Optimal Sequencing and Predictive Biomarkers in Patients with Advanced Prostate Cancer" Cancers 13, no. 18: 4522. https://doi.org/10.3390/cancers13184522
APA StyleCattrini, C., España, R., Mennitto, A., Bersanelli, M., Castro, E., Olmos, D., Lorente, D., & Gennari, A. (2021). Optimal Sequencing and Predictive Biomarkers in Patients with Advanced Prostate Cancer. Cancers, 13(18), 4522. https://doi.org/10.3390/cancers13184522









