The Translocator Protein (TSPO) Genetic Polymorphism A147T Is Associated with Worse Survival in Male Glioblastoma Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. SNP Selection and Genotyping
2.3. GlioVis Analysis
2.4. Statistical Analysis
3. Results
3.1. TSPO Expression Is Upregulated in GBM Patient Samples
3.2. Patients Characteristics in CCF and CWRU Datasets
3.3. High Frequency of TSPO rs6971 Polymorphism in the CCF and CWRU Datasets
3.4. The TSPO rs6971 Polymorphism Is Associated with Worse Overall Survival in GBM Patients Stratified by Biological Sex and Treatment
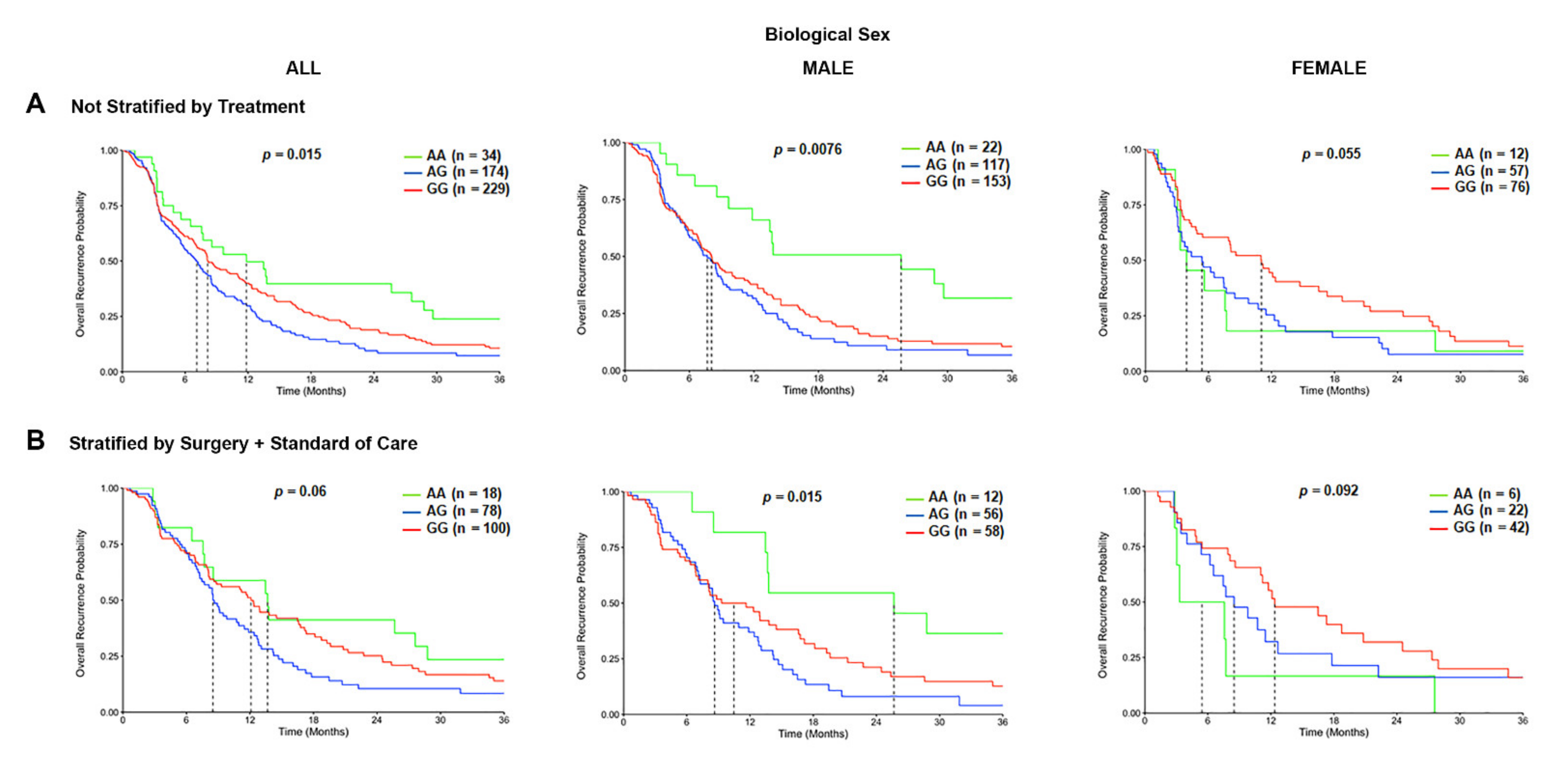
3.5. The TSPO rs6971 Polymorphism Is Associated with Worse Progression-Free Survival in GBM Patients Stratified by Biological Sex and Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013-2017. Neuro. Oncol. 2020, 22, iv1–iv96. [Google Scholar] [CrossRef]
- Gittleman, H.; Ostrom, Q.T.; Stetson, L.C.; Waite, K.; Hodges, T.R.; Wright, C.H.; Wright, J.; Rubin, J.B.; Berens, M.E.; Lathia, J.; et al. Sex is an important prognostic factor for glioblastoma but not for nonglioblastoma. Neurooncol. Pract. 2019, 6, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Suter, R.K.; Rodriguez-Blanco, J.; Ayad, N.G. Epigenetic pathways and plasticity in brain tumors. Neurobiol. Dis. 2020, 145, 105060. [Google Scholar] [CrossRef] [PubMed]
- Colwell, N.; Larion, M.; Giles, A.J.; Seldomridge, A.N.; Sizdahkhani, S.; Gilbert, M.R.; Park, D.M. Hypoxia in the glioblastoma microenvironment: Shaping the phenotype of cancer stem-like cells. Neuro. Oncol. 2017, 19, 887–896. [Google Scholar] [CrossRef]
- Lauko, A.; Lo, A.; Ahluwalia, M.S.; Lathia, J.D. Cancer cell heterogeneity & plasticity in glioblastoma and brain tumors. Semin. Cancer Biol. 2021. [Google Scholar] [CrossRef]
- Locarno, C.V.; Simonelli, M.; Carenza, C.; Capucetti, A.; Stanzani, E.; Lorenzi, E.; Persico, P.; Della Bella, S.; Passoni, L.; Mavilio, D.; et al. Role of myeloid cells in the immunosuppressive microenvironment in gliomas. Immunobiology 2020, 225, 151853. [Google Scholar] [CrossRef]
- Giampazolias, E.; Tait, S.W. Mitochondria and the hallmarks of cancer. FEBS J. 2016, 283, 803–814. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapere, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends. Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Casellas, P.; Galiegue, S.; Basile, A.S. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem. Int. 2002, 40, 475–486. [Google Scholar] [CrossRef]
- Hirsch, T.; Decaudin, D.; Susin, S.A.; Marchetti, P.; Larochette, N.; Resche-Rigon, M.; Kroemer, G. PK11195, a ligand of the mitochondrial benzodiazepine receptor, facilitates the induction of apoptosis and reverses Bcl-2-mediated cytoprotection. Exp. Cell. Res. 1998, 241, 426–434. [Google Scholar] [CrossRef]
- Rupprecht, R.; Papadopoulos, V.; Rammes, G.; Baghai, T.C.; Fan, J.; Akula, N.; Groyer, G.; Adams, D.; Schumacher, M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug. Discov. 2010, 9, 971–988. [Google Scholar] [CrossRef]
- Veenman, L.; Papadopoulos, V.; Gavish, M. Channel-like functions of the 18-kDa translocator protein (TSPO): Regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr. Pharm. Des. 2007, 13, 2385–2405. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.K.; Guilarte, T.R. Translocator protein 18 kDa (TSPO): Molecular sensor of brain injury and repair. Pharmacol. Ther. 2008, 118, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Guilarte, T.R. TSPO in diverse CNS pathologies and psychiatric disease: A critical review and a way forward. Pharmacol. Ther. 2019, 194, 44–58. [Google Scholar] [CrossRef]
- Banati, R.B.; Newcombe, J.; Gunn, R.N.; Cagnin, A.; Turkheimer, F.; Heppner, F.; Price, G.; Wegner, F.; Giovannoni, G.; Miller, D.H.; et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: Quantitative in vivo imaging of microglia as a measure of disease activity. Brain 2000, 123, 2321–2337. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.R.; Cagnin, A.; Turkheimer, F.E.; Miller, C.C.; Shaw, C.E.; Brooks, D.J.; Leigh, P.N.; Banati, R.B. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: An [11C](R)-PK11195 positron emission tomography study. Neurobiol. Dis. 2004, 15, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, J.M.; Wang, Y.; Munro, C.A.; Ma, S.; Yue, C.; Chen, S.; Airan, R.; Kim, P.K.; Adams, A.V.; Garcia, C.; et al. Neuroinflammation and brain atrophy in former NFL players: An in vivo multimodal imaging pilot study. Neurobiol. Dis. 2015, 74, 58–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amhaoul, H.; Hamaide, J.; Bertoglio, D.; Reichel, S.N.; Verhaeghe, J.; Geerts, E.; Van Dam, D.; De Deyn, P.P.; Kumar-Singh, S.; Katsifis, A.; et al. Brain inflammation in a chronic epilepsy model: Evolving pattern of the translocator protein during epileptogenesis. Neurobiol. Dis. 2015, 82, 526–539. [Google Scholar] [CrossRef] [Green Version]
- Pavese, N.; Gerhard, A.; Tai, Y.F.; Ho, A.K.; Turkheimer, F.; Barker, R.A.; Brooks, D.J.; Piccini, P. Microglial activation correlates with severity in Huntington disease: A clinical and PET study. Neurology 2006, 66, 1638–1643. [Google Scholar] [CrossRef]
- Werry, E.L.; Bright, F.M.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kril, J.J.; Kassiou, M. Recent Developments in TSPO PET Imaging as A Biomarker of Neuroinflammation in Neurodegenerative Disorders. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Roncaroli, F.; Durrenberger, P.F.; Coope, D.J.; Karabatsou, K.; Hinz, R.; Thompson, G.; Turkheimer, F.E.; Janczar, K.; Du Plessis, D.; et al. The 18-kDa mitochondrial translocator protein in human gliomas: An 11C-(R)PK11195 PET imaging and neuropathology study. J. Nucl. Med. 2015, 56, 512–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Z.; Herholz, K.; Gerhard, A.; Roncaroli, F.; Du Plessis, D.; Jackson, A.; Turkheimer, F.; Hinz, R. [(1)(1)C]-(R)PK11195 tracer kinetics in the brain of glioma patients and a comparison of two referencing approaches. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1406–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasnain, N.; Mustafa, R.M.; Bakhshi, S.K.; Shamim, M.S. Efficacy of Positron Emission Tomography in distinguishing brain tumours from inflammation. J. Pak. Med. Assoc. 2020, 70, 2291–2293. [Google Scholar] [PubMed]
- Zinnhardt, B.; Muther, M.; Roll, W.; Backhaus, P.; Jeibmann, A.; Foray, C.; Barca, C.; Doring, C.; Tavitian, B.; Dolle, F.; et al. TSPO imaging-guided characterization of the immunosuppressive myeloid tumor microenvironment in patients with malignant glioma. Neuro. Oncol. 2020, 22, 1030–1043. [Google Scholar] [CrossRef]
- Riond, J.; Mattei, M.G.; Kaghad, M.; Dumont, X.; Guillemot, J.C.; Le Fur, G.; Caput, D.; Ferrara, P. Molecular cloning and chromosomal localization of a human peripheral-type benzodiazepine receptor. Eur. J. Biochem. 1991, 195, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; McCabe, R.T.; Rennert, H.; Budarf, M.L.; Sayegh, R.; Emanuel, B.S.; Skolnick, P.; Strauss, J.F., 3rd. The human "peripheral-type" benzodiazepine receptor: Regional mapping of the gene and characterization of the receptor expressed from cDNA. DNA Cell Biol. 1992, 11, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Giatzakis, C.; Papadopoulos, V. Differential utilization of the promoter of peripheral-type benzodiazepine receptor by steroidogenic versus nonsteroidogenic cell lines and the role of Sp1 and Sp3 in the regulation of basal activity. Endocrinology 2004, 145, 1113–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernassau, J.M.; Reversat, J.L.; Ferrara, P.; Caput, D.; Lefur, G. A 3D model of the peripheral benzodiazepine receptor and its implication in intra mitochondrial cholesterol transport. J. Mol. Graph. 1993, 11, 236–244. [Google Scholar] [CrossRef]
- Li, H.; Papadopoulos, V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology 1998, 139, 4991–4997. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, V.M.; Bader, S.; Sudria-Lopez, D.; Siebert, R.; Brandl, C.; Nothdurfter, C.; Weber, B.H.F.; Rupprecht, R.; Wetzel, C.H. Effects of genetic variants in the TSPO gene on protein structure and stability. PLoS One 2018, 13, e0195627. [Google Scholar] [CrossRef]
- Owen, D.R.; Fan, J.; Campioli, E.; Venugopal, S.; Midzak, A.; Daly, E.; Harlay, A.; Issop, L.; Libri, V.; Kalogiannopoulou, D.; et al. TSPO mutations in rats and a human polymorphism impair the rate of steroid synthesis. Biochem J. 2017, 474, 3985–3999. [Google Scholar] [CrossRef] [Green Version]
- Lacapere, J.J.; Papadopoulos, V. Peripheral-type benzodiazepine receptor: Structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids 2003, 68, 569–585. [Google Scholar] [CrossRef]
- Prossin, A.R.; Chandler, M.; Ryan, K.A.; Saunders, E.F.; Kamali, M.; Papadopoulos, V.; Zollner, S.; Dantzer, R.; McInnis, M.G. Functional TSPO polymorphism predicts variance in the diurnal cortisol rhythm in bipolar disorder. Psychoneuroendocrinology 2018, 89, 194–202. [Google Scholar] [CrossRef]
- Costa, B.; Pini, S.; Gabelloni, P.; Da Pozzo, E.; Abelli, M.; Lari, L.; Preve, M.; Lucacchini, A.; Cassano, G.B.; Martini, C. The spontaneous Ala147Thr amino acid substitution within the translocator protein influences pregnenolone production in lymphomonocytes of healthy individuals. Endocrinology 2009, 150, 5438–5445. [Google Scholar] [CrossRef] [Green Version]
- Costa, B.; Pini, S.; Martini, C.; Abelli, M.; Gabelloni, P.; Landi, S.; Muti, M.; Gesi, C.; Lari, L.; Cardini, A.; et al. Ala147Thr substitution in translocator protein is associated with adult separation anxiety in patients with depression. Psychiatr. Genet. 2009, 19, 110–111. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yamada, K.; Iwayama, Y.; Toyota, T.; Furukawa, A.; Takimoto, T.; Terayama, H.; Iwahashi, K.; Takei, N.; Minabe, Y.; et al. Evidence that variation in the peripheral benzodiazepine receptor (PBR) gene influences susceptibility to panic disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141B, 222–226. [Google Scholar] [CrossRef]
- Colasanti, A.; Owen, D.R.; Grozeva, D.; Rabiner, E.A.; Matthews, P.M.; Craddock, N.; Young, A.H. Bipolar Disorder is associated with the rs6971 polymorphism in the gene encoding 18 kDa Translocator Protein (TSPO). Psychoneuroendocrinology 2013, 38, 2826–2829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Pozzo, E.; Costa, B.; Martini, C. Translocator protein (TSPO) and neurosteroids: Implications in psychiatric disorders. Curr. Mol. Med. 2012, 12, 426–442. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Zheng, Y.; Garavito, R.M.; Ferguson-Miller, S. Protein structure. Crystal structures of translocator protein (TSPO) and mutant mimic of a human polymorphism. Science 2015, 347, 555–558. [Google Scholar] [CrossRef] [Green Version]
- Owen, D.R.; Yeo, A.J.; Gunn, R.N.; Song, K.; Wadsworth, G.; Lewis, A.; Rhodes, C.; Pulford, D.J.; Bennacef, I.; Parker, C.A.; et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J. Cereb. Blood Flow Metab. 2012, 32, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Rojas, C.; Stathis, M.; Coughlin, J.M.; Pomper, M.; Slusher, B.S. The Low-Affinity Binding of Second Generation Radiotracers Targeting TSPO is Associated with a Unique Allosteric Binding Site. J. Neuroimmune. Pharmacol. 2018, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, H.; Kononen, J.; Haapasalo, H.; Helen, P.; Sallinen, P.; Harjuntausta, T.; Helin, H.; Alho, H. Expression of peripheral-type benzodiazepine receptor and diazepam binding inhibitor in human astrocytomas: Relationship to cell proliferation. Cancer Res. 1995, 55, 2691–2695. [Google Scholar] [PubMed]
- Vlodavsky, E.; Soustiel, J.F. Immunohistochemical expression of peripheral benzodiazepine receptors in human astrocytomas and its correlation with grade of malignancy, proliferation, apoptosis and survival. J. Neurooncol. 2007, 81, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Unterrainer, M.; Fleischmann, D.F.; Vettermann, F.; Ruf, V.; Kaiser, L.; Nelwan, D.; Lindner, S.; Brendel, M.; Wenter, V.; Stocklein, S.; et al. TSPO PET, tumour grading and molecular genetics in histologically verified glioma: A correlative (18)F-GE-180 PET study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1368–1380. [Google Scholar] [CrossRef]
- Cai, L.; Kirchleitner, S.V.; Zhao, D.; Li, M.; Tonn, J.C.; Glass, R.; Kalin, R.E. Glioblastoma Exhibits Inter-Individual Heterogeneity of TSPO and LAT1 Expression in Neoplastic and Parenchymal Cells. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [Green Version]
- Bowman, R.L.; Wang, Q.; Carro, A.; Verhaak, R.G.; Squatrito, M. GlioVis data portal for visualization and analysis of brain tumor expression datasets. Neuro. Oncol. 2017, 19, 139–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; de Carvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2018, 33, 152. [Google Scholar] [CrossRef] [Green Version]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416. [Google Scholar] [CrossRef] [Green Version]
- Ostrom, Q.T.; Rubin, J.B.; Lathia, J.D.; Berens, M.E.; Barnholtz-Sloan, J.S. Females have the survival advantage in glioblastoma. Neuro. Oncol. 2018, 20, 576–577. [Google Scholar] [CrossRef]
- Trifiletti, D.M.; Alonso, C.; Grover, S.; Fadul, C.E.; Sheehan, J.P.; Showalter, T.N. Prognostic Implications of Extent of Resection in Glioblastoma: Analysis from a Large Database. World Neurosurg. 2017, 103, 330–340. [Google Scholar] [CrossRef]
- Roncaroli, F.; Su, Z.; Herholz, K.; Gerhard, A.; Turkheimer, F.E. TSPO expression in brain tumours: Is TSPO a target for brain tumour imaging? Clin. Transl. Imaging. 2016, 4, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noushmehr, H.; Weisenberger, D.J.; Diefes, K.; Phillips, H.S.; Pujara, K.; Berman, B.P.; Pan, F.; Pelloski, C.E.; Sulman, E.P.; Bhat, K.P.; et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 2010, 17, 510–522. [Google Scholar] [CrossRef] [Green Version]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, K.P.L.; Balasubramaniyan, V.; Vaillant, B.; Ezhilarasan, R.; Hummelink, K.; Hollingsworth, F.; Wani, K.; Heathcock, L.; James, J.D.; Goodman, L.D.; et al. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell 2013, 24, 331–346. [Google Scholar] [CrossRef] [Green Version]
- Garofano, L.; Migliozzi, S.; Oh, Y.T.; D’Angelo, F.; Najac, R.D.; Ko, A.; Frangaj, B.; Caruso, F.P.; Yu, K.; Yuan, J.; et al. Pathway-based classification of glioblastoma uncovers a mitochondrial subtype with therapeutic vulnerabilities. Nat. Cancer 2021, 2, 141–156. [Google Scholar] [CrossRef]
- Veenman, L.; Levin, E.; Weisinger, G.; Leschiner, S.; Spanier, I.; Snyder, S.H.; Weizman, A.; Gavish, M. Peripheral-type benzodiazepine receptor density and in vitro tumorigenicity of glioma cell lines. Biochem. Pharmacol. 2004, 68, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Rechichi, M.; Salvetti, A.; Chelli, B.; Costa, B.; Da Pozzo, E.; Spinetti, F.; Lena, A.; Evangelista, M.; Rainaldi, G.; Martini, C.; et al. TSPO over-expression increases motility, transmigration and proliferation properties of C6 rat glioma cells. Biochim. Biophys. Acta 2008, 1782, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Levin, E.; Premkumar, A.; Veenman, L.; Kugler, W.; Leschiner, S.; Spanier, I.; Weisinger, G.; Lakomek, M.; Weizman, A.; Snyder, S.H.; et al. The peripheral-type benzodiazepine receptor and tumorigenicity: Isoquinoline binding protein (IBP) antisense knockdown in the C6 glioma cell line. Biochemistry 2005, 44, 9924–9935. [Google Scholar] [CrossRef] [PubMed]
- Daniele, S.; Taliani, S.; Da Pozzo, E.; Giacomelli, C.; Costa, B.; Trincavelli, M.L.; Rossi, L.; La Pietra, V.; Barresi, E.; Carotenuto, A.; et al. Apoptosis therapy in cancer: The first single-molecule co-activating p53 and the translocator protein in glioblastoma. Sci. Rep. 2014, 4, 4749. [Google Scholar] [CrossRef] [Green Version]
- Bode, J.; Veenman, L.; Caballero, B.; Lakomek, M.; Kugler, W.; Gavish, M. The 18 kDa translocator protein influences angiogenesis, as well as aggressiveness, adhesion, migration, and proliferation of glioblastoma cells. Pharmacogenet. Genomics 2012, 22, 538–550. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, D.; Wang, H.; Cai, M.; Li, C.; Zhang, X.; Chen, H.; Hu, Y.; Zhang, X.; Ying, M.; et al. TSPO deficiency induces mitochondrial dysfunction, leading to hypoxia, angiogenesis, and a growth-promoting metabolic shift toward glycolysis in glioblastoma. Neuro Oncol. 2020, 22, 240–252. [Google Scholar] [CrossRef]
- Kugler, W.; Veenman, L.; Shandalov, Y.; Leschiner, S.; Spanier, I.; Lakomek, M.; Gavish, M. Ligands of the mitochondrial 18 kDa translocator protein attenuate apoptosis of human glioblastoma cells exposed to erucylphosphohomocholine. Cell Oncol. 2008, 30, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Bader, S.; Wolf, L.; Milenkovic, V.M.; Gruber, M.; Nothdurfter, C.; Rupprecht, R.; Wetzel, C.H. Differential effects of TSPO ligands on mitochondrial function in mouse microglia cells. Psychoneuroendocrinology 2019, 106, 65–76. [Google Scholar] [CrossRef]
- Gavish, M.; Veenman, L. Regulation of Mitochondrial, Cellular, and Organismal Functions by TSPO. Adv. Pharmacol. 2018, 82, 103–136. [Google Scholar] [CrossRef]
- Vaht, M. Variation rs6971 in the Translocator Protein Gene (TSPO) is Associated with Aggressiveness and Impulsivity but Not with Anxiety in a Population-Representative Sample of Young Adults. J. Genet. Psychol. 2021, 182, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Yague, J.G.; Lavaque, E.; Carretero, J.; Azcoitia, I.; Garcia-Segura, L.M. Aromatase, the enzyme responsible for estrogen biosynthesis, is expressed by human and rat glioblastomas. Neurosci. Lett. 2004, 368, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Jiang, Y.; Wei, W.; Cong, P.; Ding, Y.; Xiang, L.; Wu, K. Androgen receptor signaling regulates growth of glioblastoma multiforme in men. Tumour Biol. 2015, 36, 967–972. [Google Scholar] [CrossRef]
- Bao, D.; Cheng, C.; Lan, X.; Xing, R.; Chen, Z.; Zhao, H.; Sun, J.; Wang, Y.; Niu, C.; Zhang, B.; et al. Regulation of p53wt glioma cell proliferation by androgen receptor-mediated inhibition of small VCP/p97-interacting protein expression. Oncotarget 2017, 8, 23142–23154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalcman, N.; Canello, T.; Ovadia, H.; Charbit, H.; Zelikovitch, B.; Mordechai, A.; Fellig, Y.; Rabani, S.; Shahar, T.; Lossos, A.; et al. Androgen receptor: A potential therapeutic target for glioblastoma. Oncotarget 2018, 9, 19980–19993. [Google Scholar] [CrossRef] [Green Version]



| Characteristics | Overall N = 441 1 | G/G Variant N = 232 1 | A/G Heterozygous N = 175 1 | A/A Wild Type N = 34 1 | p-Value 2 |
|---|---|---|---|---|---|
| Age at Diagnosis | 61 (53, 70) | 61 (53, 70) | 61 (54, 70) | 54 (46, 67) | 0.056 |
| Sex | >0.9 | ||||
| Female | 145 (33%) | 76 (33%) | 57 (33%) | 12 (35%) | |
| Male | 296 (67%) | 156 (67%) | 118 (67%) | 22 (65%) | |
| Surgery | 0.9 | ||||
| Gross Total Resection | 152 (49%) | 79 (49%) | 59 (48%) | 14 (48%) | |
| Near Total Resection | 73 (23%) | 37 (23%) | 31 (25%) | 5 (17%) | |
| Subtotal Resection | 88 (28%) | 46 (28%) | 32 (26%) | 10 (34%) | |
| Unknown | 128 | 70 | 53 | 5 | |
| Standard of Care | 03 | ||||
| No | 66 (15%) | 36 (16%) | 28 (16%) | 2 (5.9%) | |
| Yes | 375 (85%) | 196 (84%) | 147 (84%) | 32 (94%) | |
| KPS | >0.9 | ||||
| <=70 | 62 (14%) | 32 (14%) | 25 (15%) | 5 (15%) | |
| 70+ | 370 (86%) | 195 (86%) | 147 (85%) | 28 (85%) | |
| Unknown | 9 | 5 | 3 | 1 | |
| Overall Survival (Months) | 14 (7, 26) | 13 (7, 27) | 12 (6, 20) | 25 (16, 52) | <0.001 |
| Vital Status | 0.009 | ||||
| Alive | 42 (9.6%) | 31 (13%) | 8 (4.6%) | 3 (8.8%) | |
| Deceased | 397 (90%) | 200 (87%) | 166 (95%) | 31 (91%) | |
| Unknown | 2 | 1 | 1 | 0 | |
| Progression Free Survival | 6 (3, 12) | 7 (3, 13) | 5 (3, 10) | 9 (4, 27) | 0.003 |
| Recurrence | 0.5 | ||||
| No | 114 (26%) | 60 (26%) | 48 (28%) | 6 (18%) | |
| Yes | 323 (74%) | 169 (74%) | 126 (72%) | 28 (82%) | |
| Unknown | 4 | 3 | 1 | 0 |
| Characteristic | Overall N = 131 1 | G/G Variant N = 54 1 | A/G Heterozygous N = 67 1 | A/A Wild Type N = 10 1 | p-Value 2 |
|---|---|---|---|---|---|
| Age at Diagnosis | 64 (55, 69) | 64 (55, 70) | 62 (56, 68) | 62 (50, 71) | 0.5 |
| Sex | 0.014 | ||||
| Female | 47 (36%) | 12 (22%) | 29 (43%) | 6 (60%) | |
| Male | 84 (64%) | 42 (78%) | 38 (57%) | 4 (40%) | |
| Surgery | 0.7 | ||||
| Gross Total Resection | 75 (59%) | 33 (62%) | 37 (57%) | 5 (50%) | |
| Subtotal Resection | 53 (41%) | 20 (38%) | 28 (43%) | 5 (50%) | |
| Unknown | 3 | 1 | 2 | 0 | |
| Standard of Care | 0.7 | ||||
| No | 45 (37%) | 20 (39%) | 21 (33%) | 4 (44%) | |
| Yes | 78 (63%) | 31 (61%) | 42 (67%) | 5 (56%) | |
| Unknown | 8 | 3 | 4 | 1 | |
| KPS | 0.7 | ||||
| ≤70 | 56 (60%) | 23 (61%) | 29 (62%) | 4 (44%) | |
| 70+ | 38 (40%) | 15 (39%) | 18 (38%) | 5 (56%) | |
| Unknown | 37 | 16 | 20 | 1 | |
| Overall Survival (Months) | 12 (5, 21) | 10 (5, 16) | 13 (6, 22) | 18 (9, 24) | 0.4 |
| Vital Status | 0.7 | ||||
| Alive | 3 (2.3%) | 2 (3.7%) | 1 (1.5%) | 0 (0%) | |
| Deceased | 128 (98%) | 52 (96%) | 66 (99%) | 10 (100%) | |
| Progression Free Survival | 8 (4, 12) | 6 (4, 10) | 10 (5, 13) | 9 (5, 17) | 0.11 |
| Unknown | 57 | 22 | 32 | 3 | |
| Recurrence | 0.6 | ||||
| No | 56 (43%) | 22 (41%) | 31 (47%) | 3 (30%) | |
| Yes | 74 (57%) | 32 (59%) | 35 (53%) | 7 (70%) | |
| Unknown | 1 | 0 | 1 | 0 |
| Characteristic | Overall N = 572 1 | CCF Dataset N = 411 1 | CWRU Dataset N = 131 1 | p-Value 2 |
|---|---|---|---|---|
| Age at Diagnosis | 62 (53, 70) | 61 (53, 70) | 64 (55, 69) | 0.2 |
| Sex | 0.6 | |||
| Female | 197 (34%) | 150 (33%) | 47 (36%) | |
| Male | 385 (66%) | 301 (67%) | 84 (64%) | |
| Race | 0.9 | |||
| Black/African-American | 12 (2.8%) | 4 (3.1%) | ||
| White | 408 (94%) | 127 (97%) | ||
| rs6971 SNP Status | 0.056 | |||
| G/G variant | 286 (50%) | 232 (53%) | 54 (41%) | |
| A/G heterozygous | 242 (42%) | 175 (40%) | 67 (51%) | |
| A/A wild type | 44 (7.7%) | 34 (7.7%) | 10 (7.6%) | |
| Unknown | 10 | 10 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troike, K.M.; Acanda de la Rocha, A.M.; Alban, T.J.; Grabowski, M.M.; Otvos, B.; Cioffi, G.; Waite, K.A.; Barnholtz Sloan, J.S.; Lathia, J.D.; Guilarte, T.R.; et al. The Translocator Protein (TSPO) Genetic Polymorphism A147T Is Associated with Worse Survival in Male Glioblastoma Patients. Cancers 2021, 13, 4525. https://doi.org/10.3390/cancers13184525
Troike KM, Acanda de la Rocha AM, Alban TJ, Grabowski MM, Otvos B, Cioffi G, Waite KA, Barnholtz Sloan JS, Lathia JD, Guilarte TR, et al. The Translocator Protein (TSPO) Genetic Polymorphism A147T Is Associated with Worse Survival in Male Glioblastoma Patients. Cancers. 2021; 13(18):4525. https://doi.org/10.3390/cancers13184525
Chicago/Turabian StyleTroike, Katie M., Arlet M. Acanda de la Rocha, Tyler J. Alban, Matthew M. Grabowski, Balint Otvos, Gino Cioffi, Kristin A. Waite, Jill S. Barnholtz Sloan, Justin D. Lathia, Tomás R. Guilarte, and et al. 2021. "The Translocator Protein (TSPO) Genetic Polymorphism A147T Is Associated with Worse Survival in Male Glioblastoma Patients" Cancers 13, no. 18: 4525. https://doi.org/10.3390/cancers13184525
APA StyleTroike, K. M., Acanda de la Rocha, A. M., Alban, T. J., Grabowski, M. M., Otvos, B., Cioffi, G., Waite, K. A., Barnholtz Sloan, J. S., Lathia, J. D., Guilarte, T. R., & Azzam, D. J. (2021). The Translocator Protein (TSPO) Genetic Polymorphism A147T Is Associated with Worse Survival in Male Glioblastoma Patients. Cancers, 13(18), 4525. https://doi.org/10.3390/cancers13184525







