Facile Generation of Potent Bispecific Fab via Sortase A and Click Chemistry for Cancer Immunotherapy
Abstract
:Simple Summary
Abstract
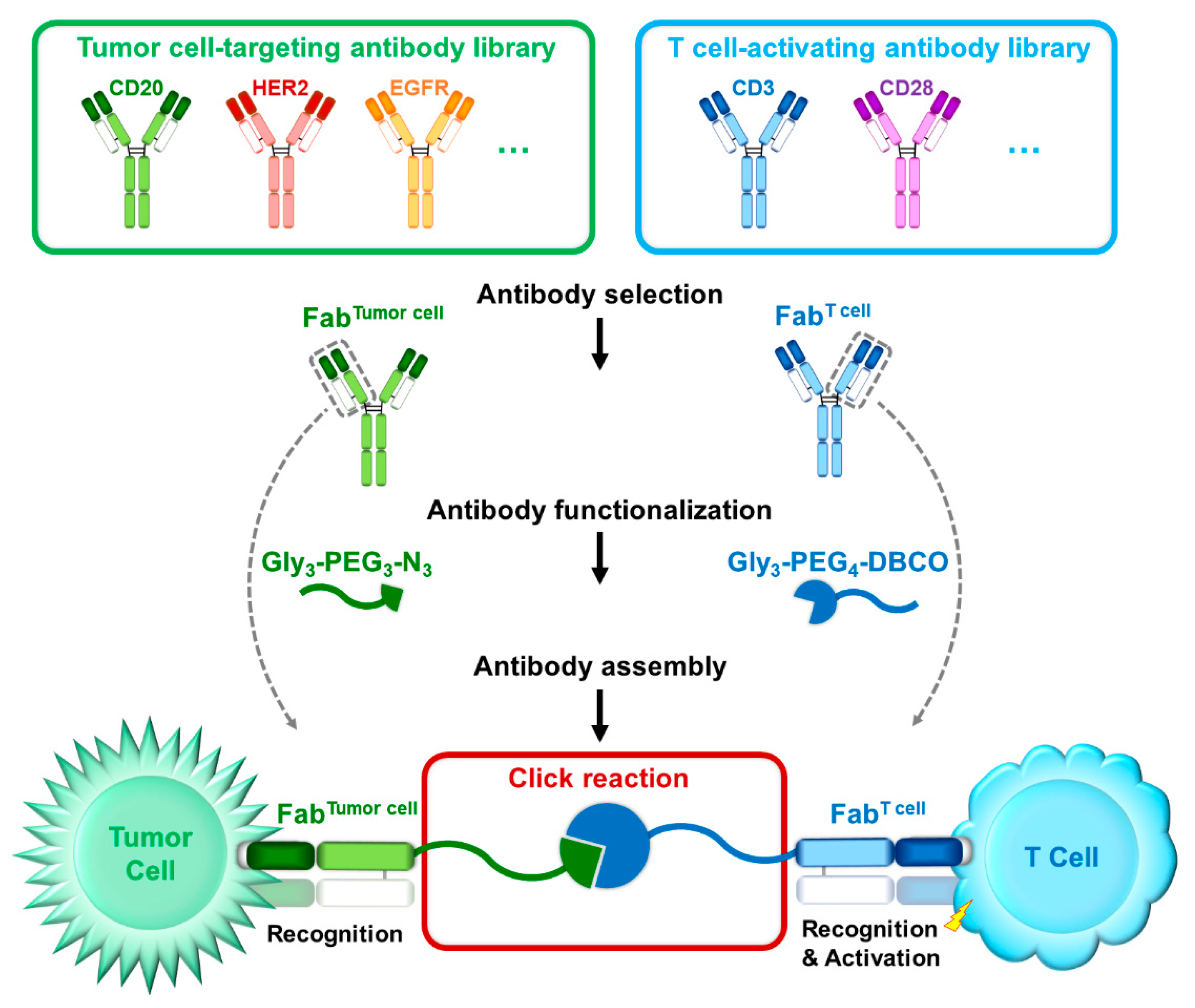
1. Introduction
2. Materials and Methods
2.1. Reagents and Cell Lines
2.2. Sortase A-Mediated Click Handle Installation
2.3. Click Chemistry Mediated Generation of Bispecific Fab (BiFab)
2.4. Flow Cytometry
2.5. Preparation of Active T Cells (ATC) from Peripheral Blood Mononuclear Cells (PBMC)
2.6. Cell Apoptosis
2.7. T Cell Activation
2.8. In Vivo Antitumor Activity of BiFab in Mouse Xenograft Model
2.9. Statistical Analysis
3. Results
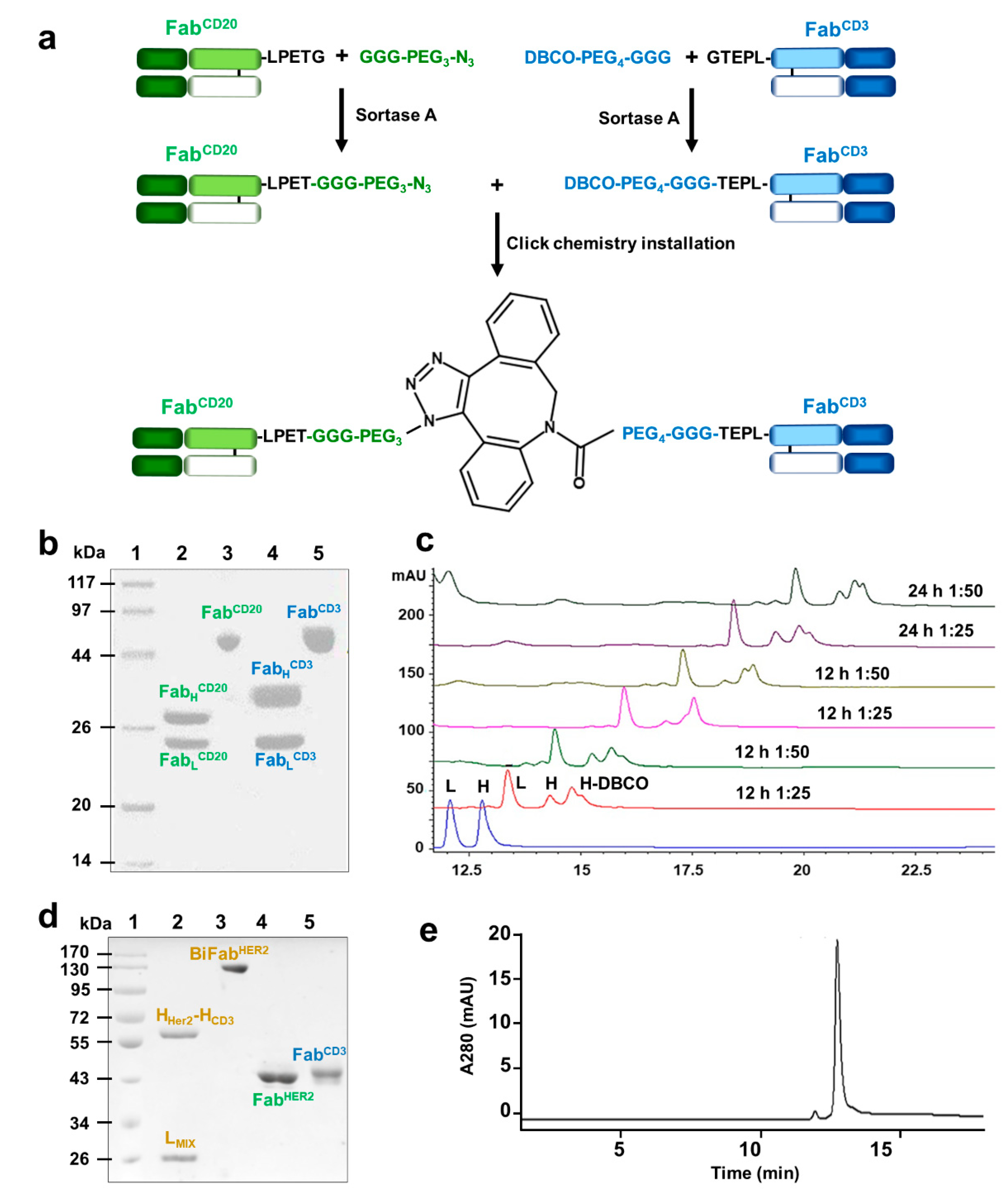
3.1. Generation of Bispecific Fab via Sortase-Mediated Transpeptidation and Click Chemistry
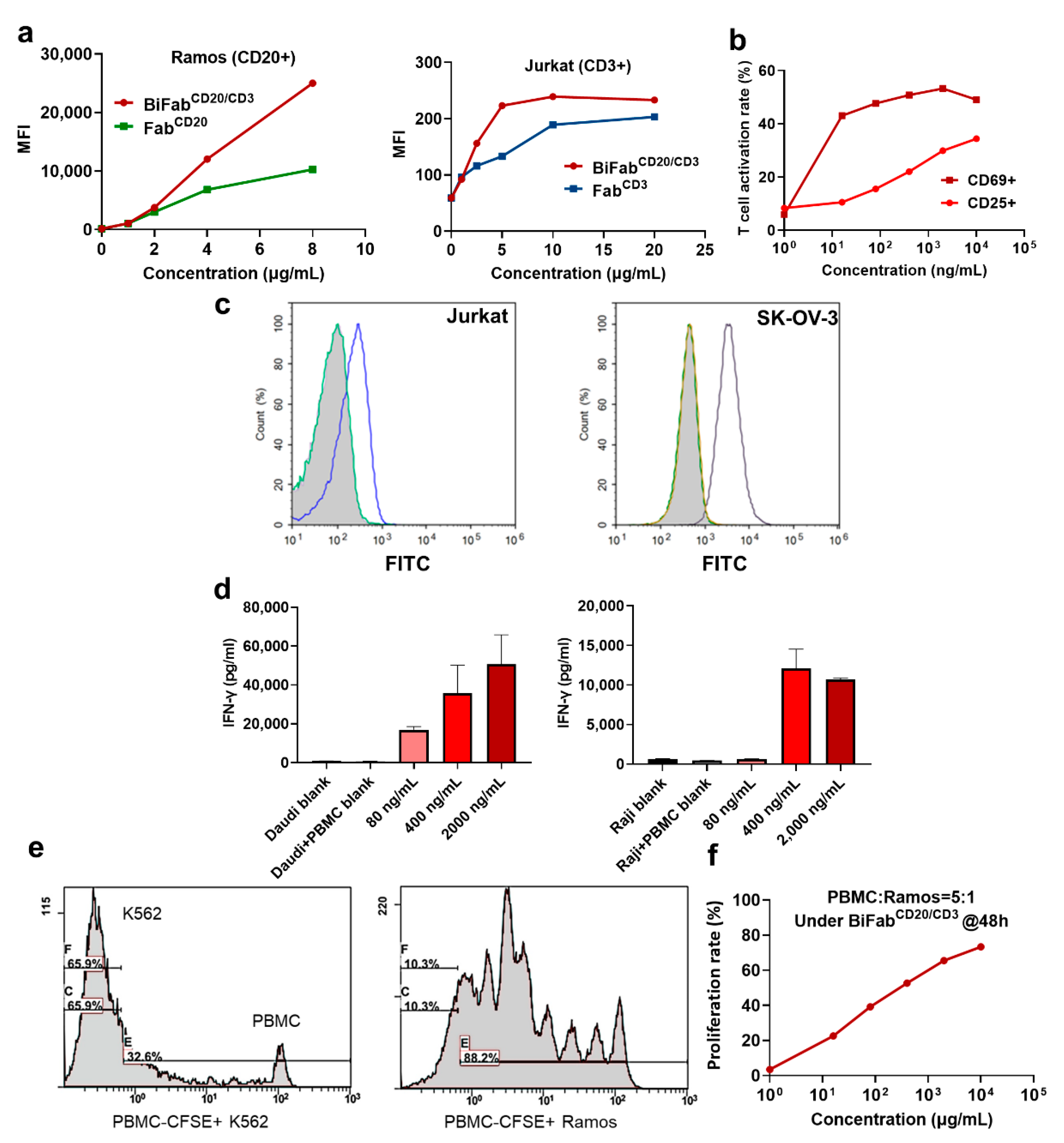
3.2. The Binding Ability of BiFabs with Target and Effector Cells
3.3. BiFab Efficiently and Specifically Induced Cytokine Release and Proliferation of T Cells
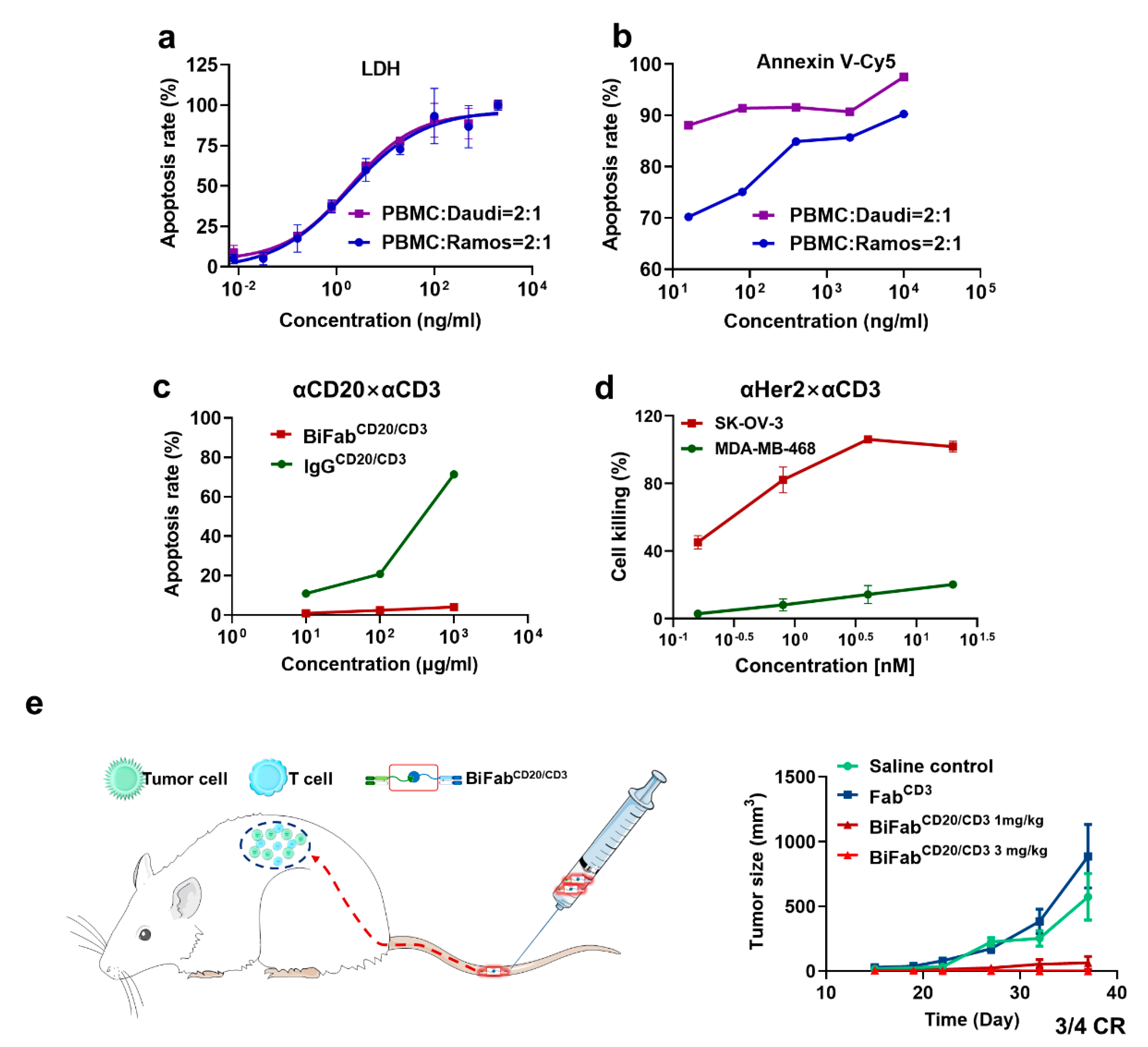
3.4. BiFabs Redirected T Cells to Kill Target Tumor Cells
3.5. BiFabCD20/CD3 Eliminated B-Cell Lymphoma in Xenograft Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Goebeler, M.-E.; Bargou, R.C. T cell-engaging therapies—BiTEs and beyond. Nat. Rev. Clin. Oncol. 2020, 17, 418–434. [Google Scholar] [CrossRef]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef]
- Singh, A.K.; McGuirk, J.P. CAR T cells: Continuation in a revolution of immunotherapy. Lancet Oncol. 2020, 21, e168–e178. [Google Scholar] [CrossRef]
- Viardot, A.; Bargou, R. Bispecific antibodies in haematological malignancies. Cancer Treat. Rev. 2018, 65, 87–95. [Google Scholar] [CrossRef]
- Slaney, C.Y.; Wang, P.; Darcy, P.K.; Kershaw, M.H. CARs versus BiTEs: A Comparison between T Cell–Redirection Strategies for Cancer Treatment. Cancer Discov. 2018, 8, 924–934. [Google Scholar] [CrossRef] [Green Version]
- Blanco, B.; Domínguez-Alonso, C.; Álvarez-Vallina, L. Bispecific immunomodulatory antibodies for cancer immunotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 6, 3–17. [Google Scholar] [CrossRef]
- Li, J.; Stagg, N.J.; Johnston, J.; Harris, M.J.; Menzies, S.A.; DiCara, D.; Clark, V.; Hristopoulos, M.; Cook, R.; Slaga, D.; et al. Membrane-Proximal Epitope Facilitates Efficient T Cell Synapse Formation by Anti-FcRH5/CD3 and Is a Requirement for Myeloma Cell Killing. Cancer Cell 2017, 31, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Wolf, E.; Hofmeister, R.; Kufer, P.; Schlereth, B.; Baeuerle, P.A. BiTEs: Bispecific antibody constructs with unique anti-tumor activity. Drug Discov. Today 2005, 10, 1237–1244. [Google Scholar] [CrossRef]
- Nagorsen, D.; Kufer, P.; Baeuerle, P.A.; Bargou, R. Blinatumomab: A historical perspective. Pharmacol. Ther. 2012, 136, 334–342. [Google Scholar] [CrossRef]
- Moore, P.A.; Zhang, W.; Rainey, G.J.; Burke, S.; Li, H.; Huang, L.; Gorlatov, S.; Veri, M.C.; Aggarwal, S.; Yang, Y.; et al. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood 2011, 117, 4542–4551. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Lam, C.-Y.K.; Long, V.; Widjaja, L.; Yang, Y.; Li, H.; Jin, L.; Burke, S.; Gorlatov, S.; Brown, J.; et al. MGD011, A CD19 x CD3 Dual-Affinity Retargeting Bi-specific Molecule Incorporating Extended Circulating Half-life for the Treatment of B-Cell Malignancies. Clin. Cancer Res. 2017, 23, 1506–1518. [Google Scholar] [CrossRef] [Green Version]
- Kipriyanov, S.M.; Moldenhauer, G.; Schuhmacher, J.; Cochlovius, B.; Von der Lieth, C.-W.; Matys, E.R.; Little, M. Bispecific tandem diabody for tumor therapy with improved antigen binding and pharmacokinetics. J. Mol. Biol. 1999, 293, 41–56. [Google Scholar] [CrossRef]
- Reusch, U.; Duell, J.; Ellwanger, K.; Herbrecht, C.; Knackmuss, S.H.; Fucek, I.; Eser, M.; McAleese, F.; Molkenthin, V.; Le Gall, F.; et al. A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19 + tumor cells. MAbs 2015, 7, 584–604. [Google Scholar] [CrossRef] [Green Version]
- Quintero-Hernández, V.; Juárez-González, V.R.; Ortíz-León, M.; Sánchez, R.; Possani, L.D.; Becerril, B. The change of the scFv into the Fab format improves the stability and in vivo toxin neutralization capacity of recombinant antibodies. Mol. Immunol. 2007, 44, 1307–1315. [Google Scholar] [CrossRef]
- Cheng, M.; Ahmed, M.; Xu, H.; Cheung, N.-K.V. Structural design of disialoganglioside GD2 and CD3-bispecific antibodies to redirect T cells for tumor therapy. Int. J. Cancer 2015, 136, 476–486. [Google Scholar] [CrossRef] [Green Version]
- Ha, J.-H.; Kim, J.-E.; Kim, Y.-S. Immunoglobulin Fc Heterodimer Platform Technology: From Design to Applications in Therapeutic Antibodies and Proteins. Front. Immunol. 2016, 7, 394. [Google Scholar] [CrossRef] [Green Version]
- Kontermann, R.E. Dual targeting strategies with bispecific antibodies. MAbs 2012, 4, 182–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitta, T.; Yagita, H.; Azuma, T.; Sato, K.; Okumura, K. Bispecific F(ab′)2 monomer prepared with anti-CD3 and anti-tumor monoclonal antibodies is most potent in induction of cytolysis of human T cells. Eur. J. Immunol. 1989, 19, 1437–1441. [Google Scholar] [CrossRef]
- Zhu, Z.; Lewis, G.D.; Carter, P. Engineering high affinity humanized anti-p185HER2/anti-CD3 bispecific F(ab’)2 for efficient lysis of p185HER2 overexpressing tumor cells. Int. J. Cancer 1995, 62, 319–324. [Google Scholar] [CrossRef]
- Graziano, R.F.; Guptill, P. Chemical production of bispecific antibodies. Methods Mol. Biol. (Clifton N. J.) 2004, 283, 71–85. [Google Scholar] [CrossRef]
- Kim, C.H.; Axup, J.Y.; Dubrovska, A.; Kazane, S.A.; Hutchins, B.A.; Wold, E.D.; Smider, V.V.; Schultz, P.G. Synthesis of Bispecific Antibodies using Genetically Encoded Unnatural Amino Acids. J. Am. Chem. Soc. 2012, 134, 9918–9921. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Zhou, Q.; Deshmukh, V.; Phull, H.; Ma, J.; Tardif, V.; Naik, R.R.; Bouvard, C.; Zhang, Y.; Choi, S.; et al. Targeting Human C-Type Lectin-like Molecule-1 (CLL1) with a Bispecific Antibody for Immunotherapy of Acute Myeloid Leukemia. Angew. Chem. Int. Ed. 2014, 53, 9841–9845. [Google Scholar] [CrossRef] [Green Version]
- Ramadoss, N.S.; Schulman, A.D.; Choi, S.; Rodgers, D.T.; Kazane, S.A.; Kim, C.H.; Lawson, B.R.; Young, T.S. An Anti-B Cell Maturation Antigen Bispecific Antibody for Multiple Myeloma. J. Am. Chem. Soc. 2015, 137, 5288–5291. [Google Scholar] [CrossRef]
- Pan, L.; Zhao, W.; Lai, J.; Ding, D.; Zhang, Q.; Yang, X.; Huang, M.; Jin, S.; Xu, Y.; Zeng, S.; et al. Sortase A-Generated Highly Potent Anti-CD20-MMAE Conjugates for Efficient Elimination of B-Lineage Lymphomas. Small 2017, 13, 1602267. [Google Scholar] [CrossRef]
- Pishesha, N.; Ingram, J.R.; Ploegh, H.L. Sortase A: A Model for Transpeptidation and Its Biological Applications. Annu. Rev. Cell Dev. Biol. 2018, 34, 163–188. [Google Scholar] [CrossRef]
- Wagner, K.; Kwakkenbos, M.J.; Claassen, Y.B.; Maijoor, K.; Böhne, M.; van der Sluijs, K.F.; Witte, M.D.; van Zoelen, D.J.; Cornelissen, L.A.; Beaumont, T.; et al. Bispecific antibody generated with sortase and click chemistry has broad antiinfluenza virus activity. Proc. Natl. Acad. Sci. USA 2014, 111, 16820–16825. [Google Scholar] [CrossRef] [Green Version]
- Becer, C.R.; Hoogenboom, R.; Schubert, U.S. Click chemistry beyond metal-catalyzed cycloaddition. Angew. Chem. Int. Ed Engl. 2009, 48, 4900–4908. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, W.; Bai, X.; Jin, S.; Li, Y.; Qiu, C.; Pan, L.; Ding, D.; Xu, Y.; Zhou, Z.; et al. High antitumor activity of Sortase A-generated anti-CD20 antibody fragment drug conjugates. Eur. J. Pharm. Sci. 2019, 134, 81–92. [Google Scholar] [CrossRef]
- Woodle, E.S.; Thistlethwaite, J.R.; Jolliffe, L.K.; Zivin, R.A.; Collins, A.; Adair, J.R.; Bodmer, M.; Athwal, D.; Alegre, M.L.; Bluestone, J.A. Humanized OKT3 antibodies: Successful transfer of immune modulating properties and idiotype expression. J. Immunol. 1992, 148, 2756–2763. [Google Scholar]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, S.; Zhao, W.; Liu, W.; Ding, D.; Zhou, J.; Chen, S. A Versatile Chemo-Enzymatic Conjugation Approach Yields Homogeneous and Highly Potent Antibody-Drug Conjugates. Int. J. Mol. Sci. 2017, 18, 2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P.W.H.I. Bispecific antibodies: A mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Borlak, J.; Länger, F.; Spanel, R.; Schöndorfer, G.; Dittrich, C. Immune-mediated liver injury of the cancer therapeutic antibody catumaxomab targeting EpCAM, CD3 and Fcγ receptors. Oncotarget 2016, 7, 28059–28074. [Google Scholar] [CrossRef]
- Wilke, A.C.; Gökbuget, N. Clinical applications and safety evaluation of the new CD19 specific T-cell engager antibody construct blinatumomab. Expert Opin. Drug Saf. 2017, 16, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.L.; Ellerman, D.; Mathieu, M.; Hristopoulos, M.; Chen, X.; Li, Y.; Yan, X.; Clark, R.; Reyes, A.; Stefanich, E.; et al. Anti-CD20/CD3 T cell–dependent bispecific antibody for the treatment of B cell malignancies. Sci. Transl. Med. 2015, 7, 287ra70. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.J.; Olson, K.; Haber, L.J.; Varghese, B.; Duramad, P.; Tustian, A.D.; Oyejide, A.; Kirshner, J.R.; Canova, L.; Menon, J.; et al. A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Sci. Rep. 2016, 5, 17943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanglmaier, M.; Faltin, M.; Ruf, P.; Bodenhausen, A.; Schröder, P.; Lindhofer, H. Bi20 (fBTA05), a novel trifunctional bispecific antibody (anti-CD20 × anti-CD3), mediates efficient killing of B-cell lymphoma cells even with very low CD20 expression levels. Int. J. Cancer 2008, 123, 1181–1189. [Google Scholar] [CrossRef]
- Pan, L.; Cao, C.; Run, C.; Zhou, L.; Chou, J.J. DNA-Mediated Assembly of Multispecific Antibodies for T Cell Engaging and Tumor Killing. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2020, 7, 1900973. [Google Scholar] [CrossRef]
- Lum, L.G.; Thakur, A.; Liu, Q.; Deol, A.; Al-Kadhimi, Z.; Ayash, L.; Abidi, M.H.; Pray, C.; Tomaszewski, E.N.; Steele, P.A.; et al. CD20-targeted T cells after stem cell transplantation for high risk and refractory non-Hodgkin’s lymphoma. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2013, 19, 925–933. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Liu, W.; Jin, S.; Zhao, W.; Xu, Y.; Zhou, Z.; Chen, S.; Pan, L. Facile Generation of Potent Bispecific Fab via Sortase A and Click Chemistry for Cancer Immunotherapy. Cancers 2021, 13, 4540. https://doi.org/10.3390/cancers13184540
Bai X, Liu W, Jin S, Zhao W, Xu Y, Zhou Z, Chen S, Pan L. Facile Generation of Potent Bispecific Fab via Sortase A and Click Chemistry for Cancer Immunotherapy. Cancers. 2021; 13(18):4540. https://doi.org/10.3390/cancers13184540
Chicago/Turabian StyleBai, Xuefei, Wenhui Liu, Shijie Jin, Wenbin Zhao, Yingchun Xu, Zhan Zhou, Shuqing Chen, and Liqiang Pan. 2021. "Facile Generation of Potent Bispecific Fab via Sortase A and Click Chemistry for Cancer Immunotherapy" Cancers 13, no. 18: 4540. https://doi.org/10.3390/cancers13184540





