Next-Generation Sequencing-Directed Therapy in Patients with Metastatic Breast Cancer in Routine Clinical Practice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ethics Statement
2.3. Next-Generation Sequencing
2.4. Multidisciplinary Tumor Board
2.5. Response Assessment and Clinical Outcomes
3. Results
3.1. Patient Characteristics
3.2. Genetic Alterations Identified by Next-Generation Sequencing
3.3. Actionable Alterations Identified by Next-Generation Sequencing (NGS) and NGS-Based Therapy Options with Evaluation of On-Label and Off-Label Treatment
3.4. Tumor Board Recommendations and Next-Generation Sequencing-Based Therapy
3.5. Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desmedt, C.; Voet, T.; Sotiriou, C.; Campbell, P.J. Next-generation sequencing in breast cancer. Curr. Opin. Oncol. 2012, 24, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, L.; Kim, E.S.; Liu, D.; Schrag, D. Precision oncology: Who, how, what, when, and when not? Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 160–169. [Google Scholar] [CrossRef]
- Mayer, E.L.; Burstein, H.J. Chemotherapy for metastatic breast cancer. Hematol. Oncol. Clin. N. Am. 2007, 21, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, T.J.; Meier, J.B.; Jansen, V.M. Current landscape of targeted therapies for hormone-receptor positive, HER2 negative metastatic breast cancer. Front. Oncol. 2018, 8, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponnusankar, S.; Mohan, A. Newer therapies for the treatment of metastatic breast cancer: A clinical update. Indian J. Pharm. Sci. 2013, 75, 251. [Google Scholar] [CrossRef] [Green Version]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J. Clin. Oncol. 2020, 38, 1000. [Google Scholar] [CrossRef]
- Caulfield, S.E.; Davis, C.C.; Byers, K.F. Olaparib: A novel therapy for metastatic breast cancer in patients with a BRCA1/2 mutation. J. Adv. Pract. Oncol. 2019, 10, 167–174. [Google Scholar]
- Kuemmel, S.; Harrach, H.; Schmutzler, R.K.; Kostara, A.; Ziegler-Löhr, K.; Dyson, M.H.; Chiari, O.; Reinisch, M. Olaparib for metastatic breast cancer in a patient with a germline PALB2 variant. NPJ Breast Cancer 2020, 6, 31. [Google Scholar] [CrossRef]
- Radovich, M.; Kiel, P.J.; Nance, S.M.; Niland, E.E.; Parsley, M.E.; Ferguson, M.E.; Jiang, G.; Ammakkanavar, N.R.; Einhorn, L.H.; Cheng, L.; et al. Clinical benefit of a precision medicine based approach for guiding treatment of refractory cancers. Oncotarget 2016, 7, 56491–56500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultova, E.; Westphalen, C.B.; Jung, A.; Kumbrink, J.; Kirchner, T.; Mayr, D.; Rudelius, M.; Ormanns, S.; Heinemann, V.; Metzeler, K.H.; et al. NGS-guided precision oncology in metastatic breast and gynecological cancer: First experiences at the CCC Munich LMU. Arch. Gynecol. Obstet. 2020, 1, 3. [Google Scholar] [CrossRef]
- Stockley, T.L.; Oza, A.M.; Berman, H.K.; Leighl, N.B.; Knox, J.J.; Shepherd, F.A.; Chen, E.X.; Krzyzanowska, M.K.; Dhani, N.; Joshua, A.M.; et al. Molecular profiling of advanced solid tumors and patient outcomes with genotype-matched clinical trials: The Princess Margaret IMPACT/COMPACT trial. Genome Med. 2016, 8, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditsch, N.; Untch, M.; Kolberg-Liedtke, C.; Jackisch, C.; Krug, D.; Friedrich, M.; Janni, W.; Müller, V.; Albert, U.-S.; Banys-Paluchowski, M.; et al. AGO recommendations for the diagnosis and treatment of patients with locally advanced and metastatic breast cancer: Update 2020. Breast Care 2020, 15, 294–309. [Google Scholar] [CrossRef]
- Morash, M.; Mitchell, H.; Beltran, H.; Elemento, O.; Pathak, J. The role of next-generation sequencing in precision medicine: A review of outcomes in oncology. J. Pers. Med. 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoskinson, D.C.; Dubuc, A.M.; Mason-Suares, H. The current state of clinical interpretation of sequence variants. Curr. Opin. Genet. Dev. 2017, 42, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Resta, C.; Galbiati, S.; Carrera, P.; Ferrari, M. Next-generation sequencing approach for the diagnosis of human diseases: Open challenges and new opportunities. Electron. J. Int. Fed. Clin. Chem. Lab. Med. 2018, 29, 4–14. [Google Scholar]
- Patel, J.M.; Knopf, J.; Reiner, E.; Bossuyt, V.; Epstein, L.; DiGiovanna, M.; Chung, G.; Silber, A.; Sanft, T.; Hofstatter, E.; et al. Mutation based treatment recommendations from next generation sequencing data: A comparison of web tools. Oncotarget 2016, 7, 22064–22076. [Google Scholar] [CrossRef] [Green Version]
- Westphalen, B.C.; Bokemeyer, C.; Büttner, R.; Fröhling, S.; Gaidzik, V.I.; Glimm, H.; Hacker, U.T.; Heinemann, V.; Illert, A.L.; Keilholz, U.; et al. Conceptual framework for precision cancer medicine in Germany: Consensus statement of the Deutsche Krebshilfe working group ‘Molecular Diagnostics and Therapy’. Eur. J. Cancer 2020, 135, 1–7. [Google Scholar] [CrossRef]
- Parsons, H.A.; Beaver, J.A.; Cimino-Mathews, A.; Ali, S.M.; Axilbund, J.; Chu, D.; Connolly, R.M.; Cochran, R.L.; Croessmann, S.; Clark, T.A.; et al. Individualized Molecular Analyses Guide Efforts (IMAGE): A prospective study of molecular profiling of tissue and blood in metastatic triple-negative breast cancer. Clin. Cancer Res. 2017, 23, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Federici, G.; Soddu, S. Variants of uncertain significance in the era of high-throughput genome sequencing: A lesson from breast and ovary cancers. J. Exp. Clin. Cancer Res. 2020, 39, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frampton, G.M.; Fichtenholtz, A.; Otto, G.A.; Wang, K.; Downing, S.R.; He, J.; Schnall-Levin, M.; White, J.; Sanford, E.M.; An, P.; et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol. 2013, 31, 1023–1031. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Stephenson, J.J.; Rosen, P.; Loesch, D.M.; Borad, M.J.; Anthony, S.; Jameson, G.; Brown, S.; Cantafio, N.; Richards, D.A.; et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J. Clin. Oncol. 2010, 28, 4877–4883. [Google Scholar] [CrossRef] [PubMed]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Pezo, R.C.; Chen, T.W.; Berman, H.K.; Mulligan, A.M.; Razak, A.A.; Siu, L.L.; Cescon, D.W.; Amir, E.; Elser, C.; Warr, D.G.; et al. Impact of multi-gene mutational profiling on clinical trial outcomes in metastatic breast cancer. Breast Cancer Res. Treat. 2018, 168, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef]
- Yuan, Y.; Yost, S.E.; Yuan, Y.C.; Solomon, N.M.; Mambetsariev, I.; Pal, S.; Frankel, P.; Salgia, R.; Neuhausen, S.L.; Mortimer, J. Genomic mutation-driven metastatic breast cancer therapy: A single center experience. Oncotarget 2017, 8, 26414–26423. [Google Scholar] [CrossRef] [Green Version]
- Jameson, G.S.; Petricoin, E.F.; Sachdev, J.; Liotta, L.A.; Loesch, D.M.; Anthony, S.P.; Chadha, M.K.; Wulfkuhle, J.D.; Gallagher, R.I.; Reeder, K.A.; et al. A pilot study utilizing multi-omic molecular profiling to find potential targets and select individualized treatments for patients with previously treated metastatic breast cancer. Breast Cancer Res. Treat. 2014, 147, 579–588. [Google Scholar] [CrossRef]
- Hlevnjak, M.; Schulze, M.; Elgaafary, S.; Fremd, C.; Michel, L.; Beck, K.; Pfütze, K.; Richter, D.; Wolf, S.; Horak, P.; et al. CATCH: A prospective precision oncology trial in metastatic breast cancer. JCO Precis. Oncol. 2021, 676–686. [Google Scholar] [CrossRef]
- Wheler, J.J.; Atkins, J.T.; Janku, F.; Moulder, S.L.; Stephens, P.J.; Yelensky, R.; Valero, V.; Miller, V.; Kurzrock, R.; Meric-Bernstam, F. Presence of both alterations in FGFR/FGF and PI3K/AKT/mTOR confer improved outcomes for patients with metastatic breast cancer treated with PI3K/AKT/mTOR inhibitors. Oncoscience 2016, 3, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Bryce, A.H.; Egan, J.B.; Borad, M.J.; Keith Stewart, A.; Nowakowski, G.S.; Chanan-Khan, A.; Patnaik, M.M.; Ansell, S.M.; Banck, M.S.; Robinson, S.I.; et al. Experience with precision genomics and tumor board, indicates frequent target identification, but barriers to delivery. Oncotarget 2017, 8, 27145–27154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohal, D.P.S.; Rini, B.I.; Khorana, A.A.; Dreicer, R.; Abraham, J.; Procop, G.W.; Saunthararajah, Y.; Pennell, N.A.; Stevenson, J.P.; Pelley, R.; et al. Prospective clinical study of precision oncology in solid tumors. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toss, A.; Piacentini, F.; Cortesi, L.; Artuso, L.; Bernardis, I.; Parenti, S.; Tenedini, E.; Ficarra, G.; Maiorana, A.; Iannone, A.; et al. Genomic alterations at the basis of treatment resistance in metastatic breast cancer: Clinical applications. Oncotarget 2018, 9, 31606–31619. [Google Scholar] [CrossRef]
- Gerratana, L.; Davis, A.A.; Polano, M.; Zhang, Q.; Shah, A.N.; Lin, C.; Basile, D.; Toffoli, G.; Wehbe, F.; Puglisi, F.; et al. Understanding the organ tropism of metastatic breast cancer through the combination of liquid biopsy tools. Eur. J. Cancer 2021, 143, 147–157. [Google Scholar] [CrossRef] [PubMed]
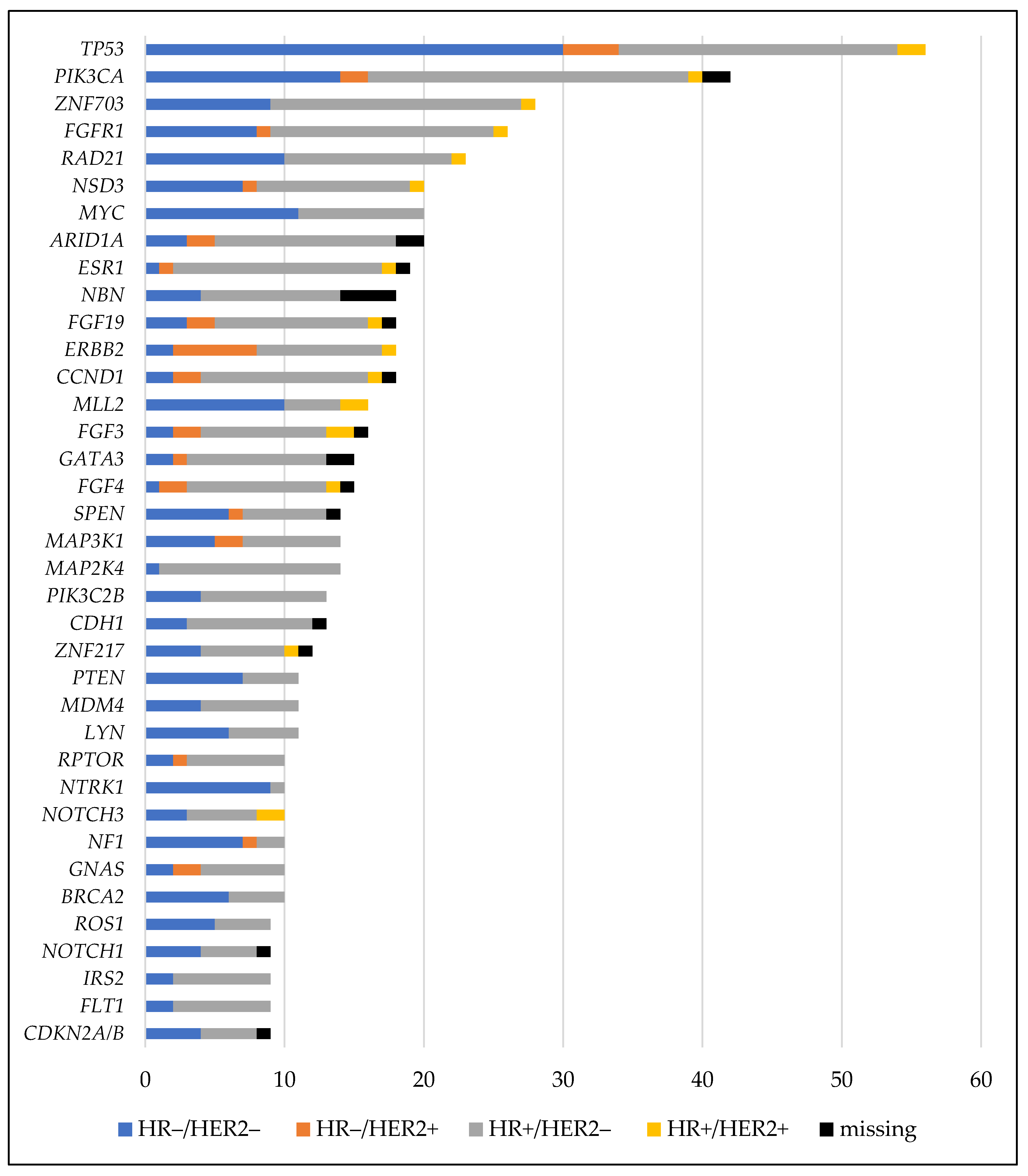
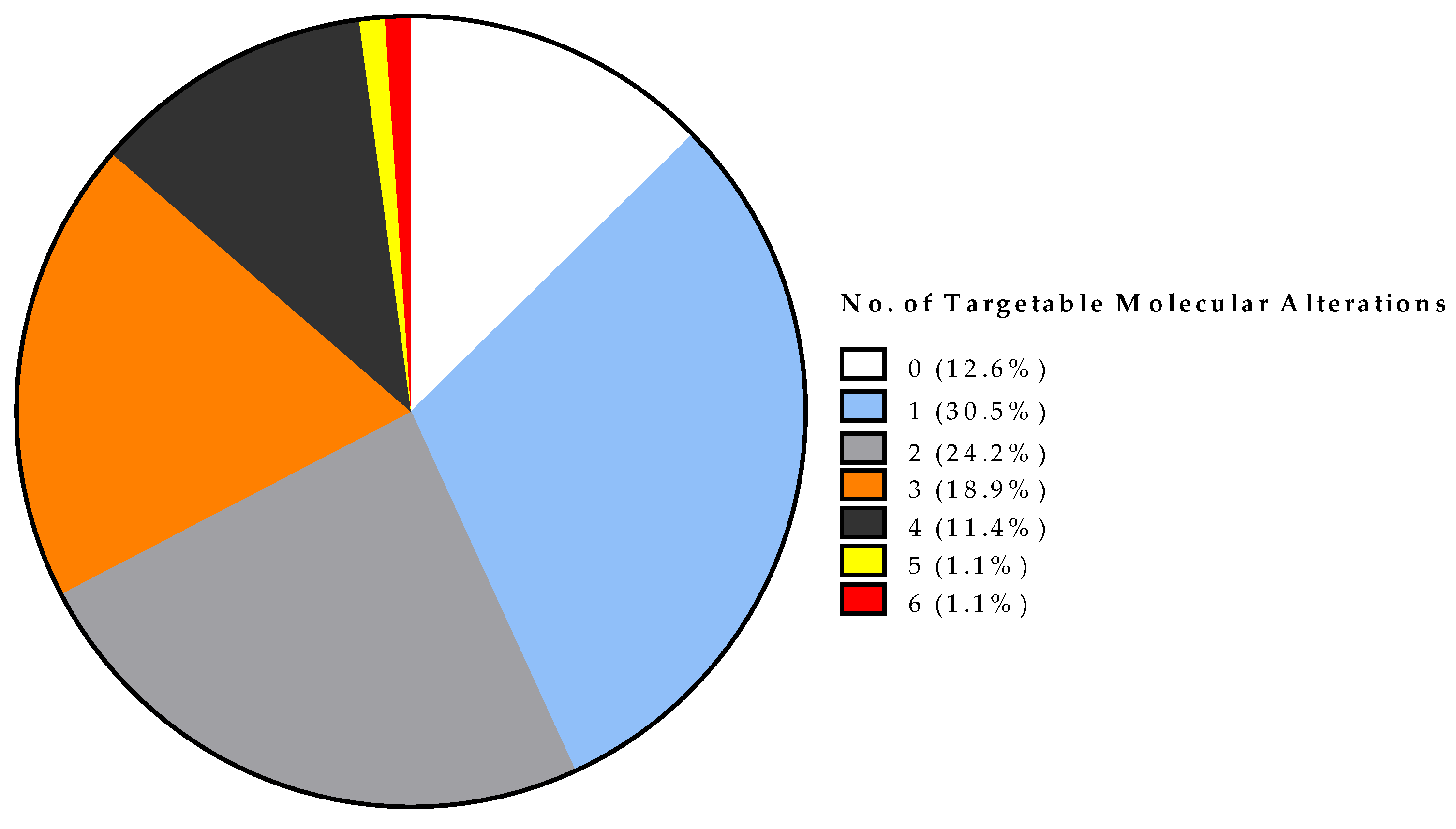



| Variable | No. | % | |
|---|---|---|---|
| Patients | 95 | 100 | |
| Age (years) at initial diagnosis of BC | median (range) | 49 (21–80) | |
| Age (years) when NGS was performed | median (range) | 55 (25–82) | |
| Tumor receptor subtype at diagnosis | HR+/HER2− | 54 | 56.8 |
| HR−/HER2− | 27 | 28.4 | |
| HR+/HER2+ | 7 | 7.4 | |
| HR−/HER2+ | 4 | 4.2 | |
| missing | 3 | 3.2 | |
| Tumor receptor subtype of recurrent tumor site | HR+/HER2− | 48 | 50.5 |
| HR−/HER2− | 38 | 40.0 | |
| HR+/HER2+ | 2 | 2.1 | |
| HR−/HER2+ | 5 | 5.3 | |
| missing | 2 | 2.1 | |
| Number of systemic therapy lines for recurrent disease prior NGS testing | 1 | 27 | 28.4 |
| 2 | 25 | 26.3 | |
| 3 | 13 | 13.7 | |
| 4 | 12 | 12.6 | |
| ≥5 | 18 | 18.9 | |
| Biopsy site for NGS | primary tumor | 35 | 36.8 |
| lymph node | 19 | 20.0 | |
| skin | 9 | 9.5 | |
| liver | 17 | 17.9 | |
| lung/pleura | 5 | 5.3 | |
| other distant sites | 10 | 10.5 | |
| Patient No.1 | Gene | Alteration | Receptor Status of NGS Sample | Previous Lines (No.) of Therapy (M1) | NGS-Based Therapy | Best Response | PFS Ratio |
|---|---|---|---|---|---|---|---|
| 61 | PIK3CA | H1047R | HR+HER2− | 3 | Everolimus + exemestan | PD | 22.00 |
| 57 | ERBB2 | S310F | HR+HER2− | 2 | Docetaxel + trastuzumab + pertuzumab | PD | 6.50 |
| 16 2 | AKT1 | E17K | HR−HER2− 3 | 1 | 1. Everolimus + exemestan | SD | 6.50 |
| FGFR1 | amplification | 2. Pazopanib | SD | 1.63 | |||
| 26 2 | PTEN | loss | HR+HER2+ | 6 | 1. Everolimus + fulvestrant | SD | 6.50 |
| FGFR1 | amplification | 2. Pazopanib | SD | 2.60 | |||
| 5 | ERBB2 | D769Y | HR−HER2+ 3 | 6 | Trastuzumab emtansine | SD | 4.22 |
| 17 | ERBB2 | amplification | HR−HER2+ | 2 | Trastuzumab deruxtecan | PR | 3.89 |
| 82 | PIK3CA | H1047R | HR+HER2− | 4 | Everolimus + exemestan | PD | 3.25 |
| 56 | CD274 (PD-L1), CD273, PDCD1LG2 | amplification | HR−HER2+ | 3 | Pembrolizumab + nab-paclitaxel, after 1 year only pembrolizumab | CR | 2.00 |
| 10 | AKT3 | amplification | HR−HER2− | 2 | Everolimus | PD | 1.63 |
| 58 | CCND1 | amplification | HR−HER2− | 1 | Palbociclib + letrozol | Patient died before response could be evaluated. | 1.50 |
| 20 | PIK3CA | Q546R | HR−HER2− 3 | 3 | Everolimus + exemestan | PD | 1.44 |
| 55 | PALB2 | E830fs * 21 | HR+HER2− 4 | 3 | Olaparib | SD | 1.35 |
| 32 | PIK3CA | H1047R | HR−HER2− 3 | 2 | Everolimus + exemestan | PD | 1.35 |
| 43 | ERBB2 | amplification | HR−HER2+ | 1 | Trastuzumab emtansine | SD | 1.18 |
| 34 | PIK3CA | C420R | HR+HER2− | 4 | Everolimus + exemestan | PD | 1.00 |
| 72 | ERBB2 | T652A | HR+HER2− | 1 | Lapatinib + capecitabine | SD | 0.97 |
| 3 | CCND1 | amplification | HR+HER2− | 2 | Palbociclib + fulvestrant | PR | 0.88 |
| 12 | ESR1 | V422del | HR+HER2− | 2 | Fulvestrant | Patient died before response could be evaluated. | 0.76 |
| 11 | PIK3CAPTEN | N345Kloss | HR−HER2+ 3 | 3 | Everolimus | PD | 0.76 |
| 91 | PIK3CA | N345K | HR−HER2+ | 5 | Everolimus + exemestan | SD | 0.76 |
| 18 | PIK3CA | H1047L | HR−HER2− 3 | 4 | Everolimus + exemestan | PD | 0.75 |
| 86 | ESR1 | D538G | HR+HER2− | 1 | Fulvestrant | SD | 0.67 |
| 76 | ERBB2 | G776>VC | HR+HER2− | 3 | Docetaxel + trastuzumab + pertuzumab | PR | 0.59 |
| 24 | ESR1 | Y537C | HR+HER2− | 4 | Fulvestrant | PD | 0.53 |
| 79 | PIK3CA | L113del | HR+HER2− | 1 | Everolimus + exemestan | PD | 0.38 |
| 60 | FGFR1 | amplification | HR−HER2− 3 | 6 | Pazopanib | Patient died before response could be evaluated. | 0.17 |
| 30 | CCND1 | amplification | HR+HER2− | 4 | CDK4/6 inhibitor + exemestan | SD | 0.17 |
| 14 | AKT1 | E17K | HR−HER2− 3 | 5 | Everolimus + exemestan | PD | 0.13 |
| 49 | PTEN | splice site 165-2A>G | HR+HER2− | 2 | Everolimus + exemestan | Patient died before response could be evaluated. | 0.04 |
| 29 | STK11 | G394_ * 434>? | HR+HER2− | 2 | Everolimus + exemestan | Therapy was discontinued due to poor health status of patient. | |
| Variable | No. | % | |
|---|---|---|---|
| Time from receipt of test results to start of NGS-directed therapy (days) (n = 30) | median (range) | 9 (1–430) | |
| Best response to NGS-directed therapy in patients (n = 30) | complete response | 1 | 3.3 |
| partial response | 3 | 10.0 | |
| stable disease | 9 | 30.0 | |
| progressive disease | 12 | 40.0 | |
| not available | 5 | 16.7 | |
| OS after initial diagnosis (months) (n = 95) | median (95% CI) | 166 (80–251) | |
| Death of patients | yes | 36 | 37.9 |
| no | 59 | 62.1 | |
| OS after test results (weeks) (n = 95) | median (95% CI) | 40.3 (19.8–60.7) | |
| 1-year OS rate (95% CI) | 44.0 (29.6–57.6) | ||
| OS after test results in patients receiving NGS-directed therapy (weeks) (n = 30) | median (95% CI) | 69.1 (17.9–120.4) | |
| 1-year OS rate (95% CI) | 62.9 (41.6–78.2) | ||
| OS after test results in patients not receiving NGS-directed therapy (weeks) (n = 65) | median (95% CI) | 39.7 (12.7–66.7) | |
| 1-year OS rate (95% CI) | 22.7 (6.5–44.4) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruzas, S.; Kuemmel, S.; Harrach, H.; Breit, E.; Ataseven, B.; Traut, A.; Rüland, A.; Kostara, A.; Chiari, O.; Dittmer-Grabowski, C.; et al. Next-Generation Sequencing-Directed Therapy in Patients with Metastatic Breast Cancer in Routine Clinical Practice. Cancers 2021, 13, 4564. https://doi.org/10.3390/cancers13184564
Bruzas S, Kuemmel S, Harrach H, Breit E, Ataseven B, Traut A, Rüland A, Kostara A, Chiari O, Dittmer-Grabowski C, et al. Next-Generation Sequencing-Directed Therapy in Patients with Metastatic Breast Cancer in Routine Clinical Practice. Cancers. 2021; 13(18):4564. https://doi.org/10.3390/cancers13184564
Chicago/Turabian StyleBruzas, Simona, Sherko Kuemmel, Hakima Harrach, Elisabeth Breit, Beyhan Ataseven, Alexander Traut, Anna Rüland, Athina Kostara, Ouafaa Chiari, Christine Dittmer-Grabowski, and et al. 2021. "Next-Generation Sequencing-Directed Therapy in Patients with Metastatic Breast Cancer in Routine Clinical Practice" Cancers 13, no. 18: 4564. https://doi.org/10.3390/cancers13184564





